ABSTRACT
Objectives: Currently, there is no standard treatment for patients with acute myeloid leukaemia (AML) ineligible for standard induction chemotherapy (IC). This study aimed to report real-world evidence data on the efficacy and safety of decitabine in this patient group.
Methods: This study was a Belgian, retrospective, non-interventional, multicentre registry of patients ≥ 65 years, with newly-diagnosed de novo or secondary AML ineligible for IC. Patients were treated according to routine clinical practice. Overall survival (OS), progression-free survival (PFS) and transfusion independence for ≥8 consecutive weeks were evaluated.
Results: Forty-five patients were enrolled, including 67% (n = 30) with secondary AML. Median OS and PFS were 7.3 months (95% CI: 2.2–11.1) and 4.1 months (95% CI: 2.1–7.6) respectively. A subpopulation analysis showed that patients treated with ≥4 cycles (n = 21) had significantly better outcomes compared to patients receiving <4 cycles (median OS 17.5 vs 1.6 months; median PFS 17.5 vs. 1.4 months). Twenty-five percent and 58% of patients that were respectively RBC or platelet transfusion-dependent at baseline became transfusion independent during treatment.
Conclusion: This real-world data confirms that decitabine can lead to transfusion independence and longer OS in AML patients, particularly after administering ≥4 cycles, as indicated in the summary of product characteristics.
1. Introduction
Acute myeloid leukaemia (AML) is characterized by the malignant transformation of myeloid stem cells in the bone marrow, which become incapable of normal differentiation and maturation, resulting in ‘blast’ cells. Due to the accumulation of blast cells, having an impact on the number and function of erythrocytes, granulocytes and platelets, patients with AML are often prone to anaemia, thrombocytopenia and/or neutropenia [Citation1].
AML is one of the most common myeloid malignancies [Citation2,Citation3], but remains an orphan disease [Citation2], with an incidence rate of 3.7 per 100,000 in Europe [Citation3]. AML has a median age at presentation of 64 years [Citation4], with approximately 18,000 new patients diagnosed in Europe each year [Citation3]. This represents 0.6% of all cancers in Europe [Citation4]. In Belgium, most recent incidence data show an annual incidence of 4.59 per 100,000 (2016), or 517 new patients diagnosed with AML per year; 318 patients ≥ 65 years and 199 patients between 18 and 65 years (2016) [Citation5].
Classical standard treatment for fit adult AML patients includes a combination of induction chemotherapy (anthracycline for 3 days and cytarabine for 7 days) with the aim of achieving a complete remission (CR). This is followed by post-remission therapy (consolidation therapy) [Citation6] which consists of chemotherapy (usually intermediate-dose cytarabine or a second cycle of induction) and/or haematopoietic stem cell transplant (HSCT). The classical induction chemotherapy, however, is limited to patients who can tolerate this treatment. An accurate assessment of fitness for standard induction chemotherapy is central to the management of AML. Several systems to quantify morbidities and/or risk of treatment-related mortality (TRM) have been proposed and are based on patient-specific and disease-specific factors guiding the choice of intensive or alternative treatments [Citation7]. Recent national/international guidelines of AML [Citation7,Citation8] therefore recommend to assess treatment alternatives for unfit patients.
Currently, no single standard of care exists for patients with AML ineligible for standard induction chemotherapy. For a long period, best supportive care (BSC) was the only treatment option, including blood product support and antibiotic treatment as required, with periodic treatment with hydroxyurea to control the peripheral white blood count [Citation9]. Alternative treatment options currently available for these patients are limited to low-intensity treatment or clinical trials with investigational drugs. Low-intensity options are either low-dose cytarabine (LDAC) or therapy with hypomethylating agents (HMAs).
In recent years, the HMAs azacitidine and decitabine have been licensed for the treatment of adult patients with AML ineligible for standard induction chemotherapy. The additional value of decitabine over BSC or LDAC has been demonstrated in 2 randomized clinical trials (RCTs) [Citation10,Citation11]. Kantarjian et al. [Citation11] (2012) conducted a multicentre, randomized, open-label, phase III trial (DACO-016) to compare the efficacy and safety of decitabine with treatment of choice (TC) in older patients with newly diagnosed AML and poor- or intermediate-risk cytogenetics. Four hundred eighty-five patients aged ≥ 65 years were randomly assigned 1:1 to receive decitabine 20mg/m2 per day for 5 consecutive days every 4 weeks or TC (supportive care or cytarabine 20mg/m2 per day as for 10 consecutive days every 4 weeks). The primary analysis with 396 deaths (81.6%) suggested an increase in median overall survival (OS) with decitabine vs. TC (7.7 months versus 5.0 months; HR 0.85; P = 0.108) which was confirmed by the mature analysis with 446 deaths (HR 0.82, P = 0.037). The complete remission (CR) rate plus complete remission with incomplete platelet recovery (CRp) was 17.8% with decitabine vs 7.8% with TC (P = 0.001). Patients received a median of 4 cycles of decitabine (range: 1–29). Cashen et al. (2010) [Citation10] performed a multicentre, open-label phase II study (DACO-017) of decitabine for the first line treatment of older patients with AML (similar patient population as in the DACO-016 trial). This study enrolled 55 patients (mean age: 74 years) who were treated with a median of 3 cycles of decitabine. The overall response rate was 25% (CR 24%). The median OS of patients in DACO-017 was 7.7 months, as in DACO-016.
For both HMAs, in most patients, a minimal number of cycles is needed in order to achieve a complete or partial remission (PR): 4 cycles for decitabine and 6 cycles for azacitidine. Additionally, treatment with azacitidine and decitabine is continued in the absence of disease progression, i.e. as long as the patient shows a response, continues to benefit from the therapy or exhibits stable disease. This was confirmed by a retrospective study conducted in a single centre in the US [Citation12] and evaluating the efficacy of azacitidine and decitabine in 75 patients followed up in a real-world setting. The authors found that multiple courses of HMAs were needed (median: 3.5 cycles) in most patients to achieve a CR or complete remission with incomplete count recovery (CRi), 1 patient even requiring 15 cycles.
While the above described studies have demonstrated efficacy and safety in patients unfit for intensive therapy according to the pivotal trial criteria, the efficacy, optimal treatment duration and outcomes of patients receiving decitabine still need to be further demonstrated in a real-world setting, more particularly in patients who did not meet the eligibility criteria of DACO-016 and DACO-017.
The purpose of this registry was to evaluate effects of decitabine in Belgian AML patients not eligible for standard induction therapy with regards to OS, response rate and transfusion need.
2. Methods
2.1. Study design
This registry was set up in Belgium, as a retrospective, non-interventional, multicentre (5 centres), observational study including adult patients (≥ 65 years) with newly diagnosed de novo or secondary AML and who were not eligible to receive intensive induction chemotherapy and hence received decitabine. Each patient was treated according to routine clinical practice.
2.2. Patient selection
To be eligible for enrolment in the registry, patients were to be 65 years or older, with newly diagnosed de novo or secondary AML according to WHO classification (2008) [Citation13] and ineligible for treatment with standard induction chemotherapy because of 1 or more of the following reasons: 1) ECOG (Eastern Cooperative Oncology Group) performance status ≥ 2; 2) Presence of significant comorbidities as described in the HCT-CI (Haematopoietic cell transplantation – specific comorbidity index) [Citation14]; 3) Presence of secondary AML; 4) Unfavorable cytogenetic or molecular markers. These reasons reflected the reimbursement criteria for decitabine in Belgium during the inclusion period of the registry.
The enrollment period extended from October 2011 (patients sourced from the Medical Need Program – MNP – which included patients until November 2013) until October 2015 (first 22 months following reimbursement).
No specific exclusion criteria were applicable.
2.3. Study procedures
The inclusion target was 55 patients, distributed over 5 participating centres, with each centre aiming for a target proportion of 30% patients from MNP and 70% of patients having started decitabine after reimbursement was granted. The start of the observation period was the diagnosis of AML and the end of the observation period was the last contact date, date of last follow-up visit, or date of death. Only data available from patient’s medical records were collected and entered into a Case Report Form (CRF) by the participating physician or delegate using electronic data capture (eDC) via an internet browser-based interface. Centres were trained on the use of the eDC system. Data were collected retrospectively at predefined time points during the course of decitabine treatment in routine clinical practice. Patient- and disease-related characteristics (including clinical laboratory assessments and cytogenic/molecular profile) were documented at diagnosis. Efficacy assessment and relevant medical events like adverse events (AEs) (see below 2.4 Outcomes) were assessed during each administration cycle from treatment initiation. Available Information on concomitant medication was also collected at these time-points. In addition, end of treatment information was collected (if available at data cut-off time), as well as data on last follow-up visit or date of death (if available at data cut-off time).
2.4. Outcomes
During the study period, OS, as well as PFS were assessed. OS was measured from the first administration of decitabine until death. PFS was calculated as the interval from the date of the first administration of decitabine to the date of disease progression or date of death from any cause, whichever occurred first.
As reported before, obtaining a CR or PR may take longer than 4 cycles and the summary of product characteristics (SmPC) recommends pursuing treatment until disease progression. In order to understand the impact of this recommendation in a real-world setting, patients having received at least 4 cycles of therapy and patients having received less than 4 cycles of therapy were analysed as distinct subgroups.
Treatment response information (CR, PR, non-response, stable disease, progressive disease) was to be assessed as per National Comprehensive Cancer Network (NCCN) guidelines (2016), by measuring peripheral and bone marrow blast count (%), haemoglobin concentration (g/dL), white blood cell count (x109/L) and absolute neutrophil count (x109/L). The assessment was scheduled at two different time points (after 4 cycles, and at the end of treatment).
Transfusion independence ((red blood cell (RBC-TI) and platelet (PLT-TI)) for at least 8 consecutive weeks was also evaluated.
During the study period, AEs were classified and recorded based on the annotated CRF and using the MedDRA standardized classification; the grading of AEs was determined using the NCI-CTC (National Cancer Institute Common Toxicity Criteria) version 4.3.
2.5. Statistical methods
As a purely descriptive study, there were no primary or secondary hypotheses tested. Demographic and primary analyses were performed across all treated patients. Descriptive statistics, such as a number of observations, mean, standard deviation, minimum, median, and maximum were provided for continuous variables. Frequency counts and percentages were presented for categorical variables. All analyses were stratified by prognostic patient characteristics as available in the data. All time-to-event endpoints were analysed using appropriated statistical techniques: descriptive univariate and stratified analyses were performed using non-parametric Kaplan-Meier techniques, and graphically presented with Kaplan–Meier graphs. Multivariate time-to-event analyses were performed using Cox proportional hazards regressions in order to explore the association between baseline characteristics and the time-to-event endpoints. Hazard ratio’s (including 95% confidence intervals (CI)) were estimated. Descriptive analyses were also provided for safety data. The statistical program used was SAS (version 9.4).
3. Results
3.1. Study population and baseline characteristics
A total of 45 patients meeting the eligibility criteria were enrolled across the 5 study sites. Thirty-eight percent (n = 17) of the enrolled patients were included via the MNP, 62% (n = 28) were enrolled after reimbursement of decitabine in Belgium. The reasons why the enrolled patients had been found ineligible for standard induction chemotherapy (as reported by the participating physicians) – and therefore eligible for treatment with decitabine – were the presence of secondary AML, an ECOG score of 2 or more, at least one co-morbidity following HCT-CI or unfavorable cytogenic or molecular markers (see supplementary materials). Remarkably, 67% of the study population had secondary AML, a known poor prognostic factor. Mean age of the patients was 76.5 years (SD 5.9), 60% (n = 27) were transfusion-dependent at baseline. Baseline characteristics are presented in .
Table 1. Baseline characteristics study population (n = 45)
3.2. Efficacy results
3.2.1. Treatment duration
The average treatment duration observed in the registry was 6 cycles (median: 3). Decitabine treatment was still ongoing in 4 patients at the time of database closure. The data showed that a high proportion of patients (n = 24; 53.3%) received ≤ 3 cycles of decitabine while 25% of the patients received ≥10 cycles of decitabine. An overview of the number of cycles received per patient is presented as supplementary material.
The reasons for discontinuing treatment can be found in . Most patients stopped treatment due to progression of the disease. Another frequent reason for stopping treatment was death, which occurred mostly after cycle 1. It can be suggested that the discontinuation of treatment is only caused to a very limited extent by the toxicity of the treatment itself.
Table 2. Reasons for discontinuing decitabine treatment
3.2.2. Overall survival
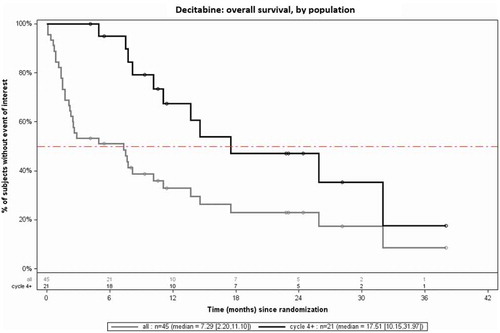
The median OS of all patients (N = 45) treated with decitabine was 7.3 months (Confidence Interval (CI): 2.2–11.1), vs. 17.5 months (CI: 10.2–32.0) in the subpopulation treated with at least 4 cycles (n = 21), thus suggesting a trend towards a better OS in the latter subgroup. No remarkable differences were observed in the baseline characteristics associated to the patients treated with ≥ 4 cycles versus the complete study population. presents the comparison of the median OS as observed within the full population vs. the subpopulation of patients who received at least 4 cycles.
3.2.3. Progression-free survival
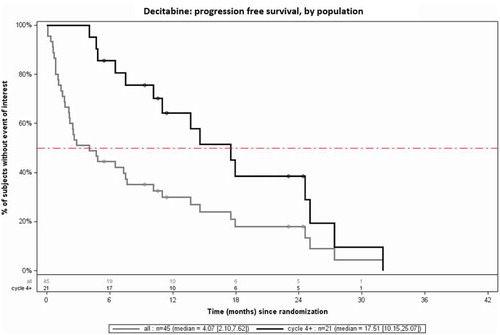
The median PFS in the total study population (n = 45) was 4.1 months (CI: 2.1–7.6), vs. 17.5 months (CI: 10.2–25.1) in the subpopulation receiving at 4 four cycles (n = 21), thus clearly showing a significantly higher PFS in patients treated with at least 4 cycles. A comparison of median PFS between the 2 populations is presented in .
3.2.4. Response
The clinical response could not be systematically measured as per protocol, due to the fact real-life clinical practice in Belgium deviates from the NCCN guidelines; in particular, bone marrow count was not routinely controlled as it is an invasive procedure. It was only performed in cases where peripheral blast count, haemoglobin concentration, white blood cell count and absolute neutrophil count were inconclusive to continue treatment.
3.2.5. Transfusion independency
Within those patients who reached RBC-TI for at least 8 consecutive weeks during decitabine treatment (25%), RBC-TI was maintained in average for 37.9 weeks (SD 27.1, median 24). In patients achieving PLT-TI for at least 8 consecutive weeks during decitabine treatment (58%), PLT-TI was maintained for an average of 23.1 weeks (SD 25.8, median 14). The evolution of RBC-TI/PLT-TI over the decitabine treatment period is summarized in .
Table 3. Evolution of transfusion dependency (RBC/PLT) in the study population (N = 45)
3.3 Safety
Twenty-nine percent of the patients (n = 13) experienced at least one decitabine-related AE. Most of these were of haematological nature, which is to be expected in this disease area. Due to underreporting, these results are not displayed because of a potential limited reliability. The other decitabine-related AEs were of non-haematological nature and their frequency can be found in , together with the maximum grade observed during the study period.
Table 4. All non-haematological decitabine-related adverse events (AEs) in the study population (MedDRA standardized classification; NCI-CTC grading)
No additional decitabine-related AEs were reported in clinical practice, compared to those reported in the pivotal studies [Citation10,Citation11]. The impact of treatment-related AEs on decitabine dosing was low; dose-adaptation, dose delay and treatment cessation were observed respectively in 1 (2.2%), 5 (11.1%), and 2 patients (4.4%), which is in line with the fact that a dose delay is recommended rather than dose-adaptation.
4. Discussion
This registry, performed in Belgium in a sample of 45 AML patients not eligible for standard induction therapy, showed that the median OS of patients treated with decitabine in a real-life setting (7.3 months) was comparable to the OS observed in the two clinical trials DACO-016/DACO-017 (median OS: 7.7 months)). Of note, the Belgian registry had a higher proportion of patients with secondary AML (67%) compared to the DACO-016 trial population (36%). Moreover, the patients included in the Belgian registry were in general a very poor risk group as governed by the reimbursement criteria, which predicts a worse prognosis of the real-world patient group compared to the study group.
A minimal treatment duration is required before HMAs reach their optimal clinical efficacy. In the DACO-016 [Citation11] and DACO-017 [Citation10] studies, the median time from the first dose to achieving CR was respectively 4 and 4.5 cycles, with one patient even receiving 29 cycles. In a post hoc analysis of the DACO-016 data, it was observed that patients having received at least 4 cycles of decitabine had a significantly higher median OS (12.5 months; 95% CI 10.3–16.0) than patients treated for less than 4 cycles (2.4 months; 95% CI: 1.9–2.7), resulting in a hazard ratio (HR) of 0.23 (p < 0.0001). The Belgian registry confirms this observation in a real-life setting: patients treated with at least 4 cycles of decitabine (n = 21; 46.7%) demonstrated a significantly higher median OS, namely 17.5 months vs. 1.6 months in patients treated for less than 4 cycles (and 7.3 months across the total study population). These data support the recommendation found in the SmPC of decitabine that treatment should not be discontinued at an early stage in the absence of disease progression or patient intolerance to treatment. Notably, in our registry, 47% of the patients (n = 21) received ≥ 4 cycles, and a quarter of the patients received ≥ 10 cycles of decitabine. This is in line with the DACO-016 study, with approximately 25% of the patients receiving at least 9 cycles of decitabine. This highlights, despite how well tolerated decitabine is, still >50% patients today are not treated with the recommended minimum of 4 cycles of decitabine, which is critical in ensuring optimum outcomes for these patients.
Treatment with HMAs is not with a curative intent. The goal of these kind of treatments is to prolong survival and, to improve the quality of life. Depth of response (CR) is therefore not the only meaningful goal for this type of treatment. Transfusion independence has been reported in the literature as an important treatment outcome (He et al., 2015). It is also a strong prognostic factor for prolonged survival and is likely to significantly improve quality of life (QoL) based on patient-reported outcomes [Citation15,Citation16]. Therefore, the transfusion independence ((red blood cell (RBC-TI) and platelet (PLT-TI)) for at least 8 consecutive weeks was evaluated in our study. In our registry, 25% of the patients who were RBC-transfusion dependent became RBC-independent for at least 8 consecutive weeks while on decitabine treatment. Also, treatment with decitabine resulted in PLT transfusion independence for at least eight consecutive weeks in 58% of the patients.
In a post hoc analysis of the DACO-016 study, He et al. [Citation17] evaluated the impact of decitabine on transfusion dependence and survival in 485 elderly patients with newly diagnosed AML, by measuring RBC-TI and PLT-TI in both decitabine (n = 242) and TC arm (n = 243). More RBC-transfusion dependent (RBC-TD) patients at baseline became transfusion independent with decitabine than with TC (26% versus 13%; p = 0.0026). Similar results were obtained for patients who were PLT TD (transfusion dependent) at baseline (31% versus 13%, p = 0.0069). These authors concluded that even in the absence of CR, TI was associated with an improved OS, and that decitabine achieved a greater degree of TI than TC.
The impact of transfusion independence on QoL was also studied by Balducci [Citation18] in a population of patients with myelodysplastic syndrome (MDS), who may progress in a later stage to AML. The author observed, based on his study, that transfusion independence in patients with MDS significantly improved both OS and health-related QoL (HRQOL). Similarly, in a systematic literature search including 10 studies, Platzbecker et al. [Citation16] evaluated the impact of transfusion dependence on QoL in MDS patients and showed that a significantly better score for QoL-measurements was observed in transfusion independent patients in all the included studies. The fact that 25% and 58% of patients achieved RBC and PLT-transfusion independence respectively in our registry therefore suggests better QoL outcomes in these patients treated with decitabine.
The safety profile of decitabine was also confirmed in our study. No additional decitabine-related AEs were reported in clinical practice as compared to those reported in the DACO-016 and DACO-017 pivotal studies.
One of the limitations of the data is inherent to its observational nature, resulting in missing information. This method of record review is by essence dependent on the quality of the record keeping. As an example, in our study, the reason for discontinuation of treatment was not documented in approximately 9% of the patients. These missing data can consequently hide important information; in this specific case, it was not possible to establish whether early treatment discontinuation was due to toxicity or to disease progression. Similarly, the unavailability of clinical response data prevented from performing a comparison with the pivotal studies with regard to this important endpoint. This second limitation was somehow mitigated by the availability of information on transfusion independency.
Patients were included from 5 hospitals from different geographical regions, so the data can be considered representative for a considerable portion of the total Belgian population eligible for decitabine treatment. This however remains a relatively small sample size (n = 45) which might not be fully representative of the whole AML population treated with decitabine in Belgium.
Despite these limitations, some important conclusions can be drawn from this study. First, despite the fact that the included population was associated with poorer prognosis, the study provided results that are completely in line with the DACO-016 and DACO-017 pivotal studies as far as the clinical benefit and safety profile of decitabine are concerned. Secondly, the study supported the claim that longer treatment duration (≥ 4 cycles) is associated with a better survival and, therefore, that the optimal clinical benefit can be obtained when the patient is treated until disease progression.
Supplemental Material
Download MS Word (90.3 KB)Disclosure statement
IQVIA Consulting Solutions, employer of Caekelbergh K. and Chevalier P received consulting fees from Janssen-Cilag NV. Meers S., Bailly B and Dierickx D. reported grants from Janssen-Cilag NV during the conduct of the study. Vande Broek I reported grants from Janssen-Cilag NV, outside the submitted work. Malfait B., Geers J., Diels J, and Van Hoorenbeeck S are employees of Janssen-Cilag NV. Van Kouwenhove M and Braakman J are employees of Janssen-Cilag BV. Doyle M is a Janssen Sciences Ireland UC employee.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999 Sep 30;341(14):1051–1062. PubMed PMID: 10502596; eng.
- Nieto M, Demolis P, Behanzin E, et al. The European medicines agency review of decitabine (dacogen) for the treatment of adult patients with acute myeloid leukemia: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2016 Jun;21(6):692–700. PubMed PMID: 27091416; PubMed Central PMCID: PMCPMC4912358. eng.
- Visser O, Trama A, Maynadie M, et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur J Cancer. 2012 Nov;48(17):3257–3266. ( Oxford, England: 1990). PubMed PMID: 22770878; eng.
- Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematologic malignancies. Ann Oncol. 2007;18:i3–i8.
- Belgian Cancer Registry 2018. Belgian cancer registry. [cited 15 Dec 2018]. Available from: https://kankerregister.org/
- De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016 Jul 1;6(7):e441. PubMed PMID: 27367478; PubMed Central PMCID: PMCPMC5030376. eng.
- Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017 Jan 26;129(4):424–447. PubMed PMID: 27895058; PubMed Central PMCID: PMCPMC5291965. eng.
- Network NCC. NCCN guidelines version 1. 2016 acute myeloid leukemia 2016 [cited 2018 Dec 15]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx
- Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007 Mar 15;109(6):1114–1124. PubMed PMID: 17315155; eng.
- Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010 Feb 1;28(4):556–561. PubMed PMID: 20026803; eng.
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012 Jul 20;30(21):2670–2677. PubMed PMID: 22689805; PubMed Central PMCID: PMCPMC4874148. eng.
- Tawfik B, Sliesoraitis S, Lyerly S. Efficacy of hypomethylating agens as frontline, salvage, or consolidation therapy in adults with acute myeloid leukaemia (AML). Ann Hematol. 2014;93:47–55.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391–2405. PubMed PMID: 27069254; eng.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct 15;106(8):2912–2919. PubMed PMID: 15994282; PubMed Central PMCID: PMCPMC1895304. eng.
- Gavillet M, Noetzli J, Blum S, et al. Transfusion independence and survival in patients with acute myeloïed leukemia treated with 5-azacytidine. Haematologica. 2012;97:1929–1931.
- Platzbecker U, Hofbauer LC, Ehninger G, et al. The clinical, quality of life, and economic consequences of chronic anemia and transfusion support in patients with myelodysplastic syndromes. Leuk Res. 2012 May;36(5):525–536. PubMed PMID: 22300879; eng.
- He J, Xiu L, De Porre P, et al. Decitabine reduces transfusion dependence in older patients with acute myeloid leukemia: results from a post hoc analysis of a randomized phase III study. Leuk Lymphoma. 2015 Apr;56(4):1033–1042. PubMed PMID: 25098427; eng.
- Balducci L. Transfusion independence in patients with myelodysplastic syndromes: impact on outcomes and quality of life. Cancer. 2006 May 15;106(10):2087–2094. PubMed PMID: 16607649; eng.