Abstract
Lipid oxidation is a very complex and important event threatening the quality of foods especially of those containing highly unsaturated fats. Fish are the main source of polyunsaturated fatty acids that, unfortunately, are highly susceptible to degradation process, such as oxidation. Fish supply chain generally involves many steps and each of them together with their interaction might play a central role in muscle quality maintenance. From this review emerged that antioxidants supplementation diet can play a central role to limit the detrimental effects of stress (pre-slaughter or at killing) and storage. In this sense, lycopene shows the best antioxidant activity during stressful conditions while α-tocopherol acts preferentially in long-term frozen storage. Stress just before or at slaughter can greatly threaten flesh quality both immediately and after storage by inducing numerous metabolic pathways, that often involve the production of very reactive molecular species, such as hydroperoxides. A common operation such as bleeding can significantly reduce both reactive molecules and haemoglobin (Hb), which is recognised as a great pro-oxidant. Temperature and duration are two critical points of storage phase which has to be considered even by consumers. Frozen storage at very low temperatures (−30 °C, −40 °C) confirms to be the best storage practise. Finally, cooking can compromise aromatic profile of cooking fillets. Thus, feeding antioxidant, reducing stress both during pre-slaughter practise and at killing, good storage practises, if associated with an appropriate cooking method (low temperature, short time) seems to be the clues for preserving the fragile lipid fraction from farm to fork.
Introduction
Lipid oxidation has been deeply studied in the course of the past recent decades, and its complex mechanisms, kinetics and products are now to a large degree well established. As reported by Schaich (Citation1992), mechanisms frequently proposed are based on kinetics, usually prerequisite of either oxygen consumption or appearance of peroxides (indicated as peroxide value, PV), malondialdehyde (MDA, expressed as thiobarbituric acid reactive substances, TBARS), free fatty acids (FFAs) and/or volatile compounds, therein assuming standard radical chain reaction sequences. However, when the above-mentioned side reactions are either ignored or reactions proceed by a pathway different from that being measured, erroneous conclusions can be easily drawn. Thus, these various pathways and or reaction tracks need to be evaluated simultaneously to reflect a near-to-realistic picture of the most likely pathway of lipid oxidation in either of the model systems, foods or biological tissues. The complexity of this phenomenon can be seen by a large number of studies reporting lipid oxidation and how best it either resembled or contrasted by comparisons within this subject (Ramanathan & Das Citation1992; Niki et al. Citation2005; Azhar & Nisa Citation2006; Okpala et al. Citation2014).
Lipid oxidation indeed is a very important event leading to the quality of foods, especially of those containing highly unsaturated fats. Quality losses, production of unpalatable flavour and odour, shortening of shelf life, losses of nutritional values (e.g. loss of polyunsaturated fatty acids, PUFAs) and possible production of unhealthy molecules are some of the extensive consequences of lipid oxidation in foods.
Fish lipid differs from mammalian lipid. The main difference is that fish lipids include up to 40% of long-chain fatty acids (14–22 carbon atoms) which are highly unsaturated. Mammalian fat will rarely contain more than two double bonds per fatty acid molecule while the depot fats of fish contain several fatty acids with five or six double bonds. Moreover, fish oils contain other PUFAs which are considered as ‘essential’ such as eicosapentaenoic (EPA, C20:5n3) and docosahexaenoic (DHA, C22:6n3) acids. Indeed, EFSA (Citation2010) reported that a daily intake of 250–500 mg of EPA + DHA decreases the risk of mortality from coronary heart disease and sudden cardiac death. This supports the previous funding that EPA in blood is an extremely potent antithrombotic factor (Simopoulos Citation1991).
Nonetheless, long-chain fatty acids are as important as their high susceptibility to degradation, such as oxidation. It has been proved (German & Kinsella Citation1985; Richards et al. Citation2002; Azhar & Nisa Citation2006; Maqsood & Benjakul Citation2011; Maqsood et al. Citation2012) that the lipid oxidation of food, especially of PUFA contained in fish, is rather linked to the formation of off-flavour components, less of quality during different storage conditions, loss of nutritional value and even formation of anti-nutritional molecules.
Fish supply chain generally involves many steps and each of them might play a central role in the maintenance of muscle quality. Indeed, farming, killing, handling and storage are only some of the steps between farm and consumers’ kitchens and plate. For this reason, in order to prevent possible waste of nutrient value, it is important to briefly review the main factors affecting lipid oxidation of fish from farm to fork. The structure of this contribution is schematically organised such that at subsequent sections, the antioxidant in the feed is presented, thereafter, pre-slaughter procedures of starvation and crowding, then followed by the killing activity, then, handling up to cooking.
Antioxidant in feed
Fish lipids are rich in n-3 fatty acids that are essential to human health. Lipid oxidation is a major concern during processing and storage of fish because it contributes to quality deterioration and decreases marketability of fish products. Fillet accumulation of antioxidant, e.g. vitamin E (vit. E) or astaxanthin, during feeding, may prevent quality deterioration associated with lipid oxidation following processing and storage.
reports the results of some studies concerning the effect of dietary antioxidant on lipid oxidation. The role of vit. E is clearly discerned from Stéphan et al. (Citation1995) who demonstrated that TBARS level of low tocopherol diet is almost 100 times more than that of the highest tocopherol level diet in turbot (Scophthahus maximus). In addition, looking at the results immediately after death (data not shown) is possible to find slightly higher TBARS level (0.029 mg MDA/kg) in fish fed low dietary α-tocopherol (20 mg/kg feed), than in fish fed high antioxidant (320 mg/kg feed) for which 0.016 mg MDA/kg was measured. Hence, the antioxidant properties of tocopherol seem accentuated by long-term frozen storage (6 months storage at −20 °C). Interestingly, the same authors performed in parallel an in vitro study on the antioxidant ability of α-tocopherol. Uncertain patterns might be discerned. On one hand, α-tocopherol antioxidant activity is increased by increasing concentrations. On the other hand, the extent of antioxidant effect seemed to be strictly dependent on lipid content and composition of the matrix, especially PUFAn3 content. So the higher lipid and PUFAn3 content, the higher vit. E antioxidant activity. That fact seemed to be confirmed by Chaiyapechara et al. (Citation2003) who found the antioxidant activity of vit. E higher in fat rainbow trout (9.60% fat) than in fish containing 8.4% lipid. Furthermore, the action was expressed preferentially in long-term frozen storage (24 weeks at −30 °C) than in a short refrigerated one (4 °C). Unfortunately, authors did not analyse the α-tocopherol content during the storage, so it is not possible to unevenly establish the antioxidant role of α-tocopherol.
Table 1. Effect of antioxidant supplementation diet on secondary lipid oxidation products (TBARS, mg MDA/kg fillet) of different species.
Confirming the antioxidant action of α-tocopherol, Huang et al. (Citation2003) investigating hybrid tilapia (Oreochromis niloticus ×O. aureus) found that different diet vit. E supplementation levels could influence lag phase (initiation phase) of lipid peroxidation with an apparent increase during either refrigerated (7 d at 4 °C) and frozen storage (8 weeks at −40 °C). As shown in , TBARS are affected by the dietary vit. E supplementation and fish fed with high vit. E levels show to be less prone to be oxidised than the low fed ones. Similar results were found by Zhang et al. (Citation2007) in Sparus macrocephalus fillets. Even in this case, fillets of fish fed with high tocopherol (553 or 1069 mg/kg) diet for 8 weeks exhibited significantly low (1.44 g/kg fillet) levels of oxidation products during 9 d of ice storage thanks to their high tocopherol muscle content.
In summary, it is possible to assert that α-tocopherol antioxidant activity is increased by increasing concentration levels in feed; α-tocopherol performs better in high-fat substrates; α-tocopherol acts preferentially in long-term frozen storage.
Jensen et al. (Citation1998) fed rainbow trout (Oncorhynchus mykiss) with different astaxanthin and α-tocopherol levels for 6 months in order to understand the role of feeding antioxidant on lipid stability of the raw fish during frozen storage (−28 °C, 12 or 18 months). Globally, storage reduced both astaxanthin and tocopherol content in fish fillet, although the highest decrease was observed for astaxanthin content. Thus, results suggest that astaxanthin might protect against lipid oxidation during the early stages of oxidative deterioration, where α-tocopherol has little effects, thanks to carotenoids’ role as scavengers of free radicals during the initiation of lipid oxidation. Such an ability is confirmed by the funding of Choubert et al. (Citation2011), that found that carotenoid-supplemented diets (100 mg astaxanthin/kg feed or 80 mg canthaxanthin/kg feed) did not significantly reduce TBARS content in rainbow trout during long-term (18 months) frozen storage (−20 °C).
Recently, new natural antioxidants (as thymol, carvacrol and lycopene) have been utilising in feed supplementation. As reported by Giannenas et al. (Citation2012) it appears that a less unique pattern for carvacrol and thymol might be discerned. Indeed, the authors found that feeding rainbow trout with thymol (6 mg/kg) for 8 weeks improved oxidative stability during a short refrigerated storage (5 d at 4 °C) more than carvacrol supplementation (12 mg/kg). TBARS content of fish treated with thymol remains unaltered for the entire trial, at 2.25 μg/g protein, while the carvacrol group raised up 2.78 μg/g protein. Interestingly, Girao et al. (Citation2012) tested the antioxidant ability of feed supplementation with lycopene (600 mg/kg) on Nile tilapia (O. niloticus) undergone stress confinement. Two main effects of lycopene may be discerned. First, no alteration of TBARS content accompanied by unaltered enzymatic antioxidant activity (catalase, glutathione reductase, lactate dehydrogenase) in not stressed fish fed lycopene suggests that lycopene should have an antioxidant role by removing reactive oxygen species (ROS) generated by cellular metabolism. Latter, lycopene abolished the effect of stress during confinement, remaining unchanged both enzymatic activities and TBARS (stick at 0.32 μg/kg), thus confirming it played an important role during the initiation phase of lipid oxidation. More recently, Sahin et al. (Citation2014) studied the effect of lycopene supplementation at different concentration (0, 200 or 400 mg/kg) on stressed rainbow trout quality performance. They found that dietary supplementation of lycopene to fish reduces the detrimental effects of stress (high stocking density) on growth performance and modulates oxidative status via activating host defence system at cellular level. It appears that lycopene can be added up to 400 mg/kg to rainbow trout diets to improve flesh quality.
During the last years, rosemary extract has been utilising in fish feeding, however, contrasting results were found. Data from Hernández et al. (Citation2014) show that animals fed diets containing high dose rosemary extract (1200, 1800 and 2400 mg/kg) have a significantly lower TBARS index than the control group (no added group) or the group fed with low dose (600 mg/kg) over the first 7 d of storage (average 0.11, 0.13 and 0.22 mg MDA/kg, respectively). However, on day 21, a certain tendency emerged towards an increase in the TBARS index as the dose increased, possibly due to a pro-oxidant effect of the rosemary extract at high doses. The lowest rosemary dose raised up to 0.49 mg/kg, while the other groups achieved at maximum 0.71 mg/kg, even if any statistical differences emerged. While comparing rosemary, thymol, carvacrol or a synthetic antioxidant as BHT feed supplementation for their capability of preventing lipid oxidation, Álvarez et al. (Citation2012) found the following increasing stability order: carvacrol > rosemary = BHT > thymol. Thus, during 14 d of refrigerated storage, fillets from fish fed diet with carvacrol (500 mg/kg, 18 weeks) showed the lowest TBARS content (0.2 mg MDA/kg fillet), while the maximum was reached by thymol group with 0.4 mg MDA/kg.
Pre-slaughter procedure: starvation and crowding
Food quality is perceived as a global concept. Food should be primarily safe, tasty and healthy. However, food safety and ethics is increasing of global interest. In this context, commonly pre-slaughter practises that may be responsible for animal stress are starvation and crowding. Starving the fish for some days prior to slaughter is a common practise in the case of farmed fish, with the scope to delay spoilage by reducing the amount of faeces in the intestine. During the last decades, many authors have investigated the influence of starvation on flesh quality in different fish species such as S. aurata (Ginés et al. Citation2002; Álvarez et al. Citation2008), Dentex dentex (Suárez & Cervera Citation2010), O. mykiss and Salmo trutta (Bayir et al. Citation2014) but only a few of them focused on the induced oxidative stress.
Álvarez et al. (Citation2008) exposed S. aurata to 24, 48 or 72 h of starvation and among others parameters they evaluated TBARS on fillets. Although no significant differences between starvation periods emerged, a trend may be discerned. Particularly, it seemed that the TBARS values increased with starvation time. Indeed, 2.50 ± 0.90, 3.63 ± 1.62 and 4.57 ± 1.75 mg MDA/kg were found in S. aurata starved for 24, 48 and 72 h, respectively.
Interestingly, Bayir et al. (Citation2014) measured oxidative stress indicators, such as ROS in liver and muscle samples from O. mykiss and S. trutta exposed to a 45-d starvation period at low water temperature. They found that in both species lipid peroxidation increased with starvation length, even if the metabolic response to food deprivation in the muscle of each species was different.
Crowding is a temporary status immediately before killing when fish can be collected in very high density. As reported by Pérez-Sánchez et al. (Citation2013), crowding causes a complex stress by affecting hepatic gene expression, antioxidant defence system, cell–tissue repair mechanism, xenobiotic metabolism and stress transcriptional regulation. This response, similar to the one described by Bayir et al. (Citation2014) for starvation, may explain the funding of Bagni et al. (Citation2007), that monitored the effect of crowding (density >70 kg/m3) on the oxidative stress of two common Mediterranean species (gilthead sea bream, S. aurata, and European sea bass, Dicentrarchus labrax). Oxidative stress was determined in terms of increment of the reactive oxygen metabolites (ROMs) and of antioxidant power (AOP). From the data emerged that in the case of stress conditions, the ROMs production can be counteracted by an adaptive response, such as the activation of the AOP mechanism. However, the stress extent may greatly affect this response, by shifting from a positive response (high AOP, low ROMs) to a negative one (low AOP, high ROMs). The former is the case of uncrowded fish, the latter of crowded fish. Furthermore, stress response seemed to strictly depend on species. Indeed, gilthead sea bream showed to be less affected by the application of stress than European sea bass (no significant differences between two stress groups were found for AOP and ROMs). Gilthead sea bream as well showed a lower survival time than European sea bass. Nathanailides et al. (Citation2011) supported the hypothesis that increased levels of stress can lead to increased lipid oxidation in European sea bass fillets. In detail, fish were processed with a high stress method (the water was lowered and the fish were captured using a net, then killed by immersion in an ice-cold bath) or with a lower stressful one (the level of water was lowered and fish were anaesthetised moderately by immersion in a 30 mg/l clove oil bath for 5 min, then slaughtered by immersion in ice-cold sea water). Results showed that the handling stress prior to slaughtering affects significantly TBARS contents, which were 1.04 and 1.16 mg MDA/kg in no stressed and stressed fish, respectively.
The above-cited studies of this subsection let emerge that pre-slaughter stress may induce complex metabolic responses: rapid ATP depletion may generate various pro-oxidant substances, which in turn may induce an activation of AOP mechanism for ROS and ROMs depletion. Unfortunately, high stressful conditions or stress length may cause the adaptive response to be useless resulting in an increase of lipid oxidation.
Killing
Stunning/killing procedures applied in aquaculture are different and fish species vary in their response to the different methods utilised. Mediterranean aquaculture species are usually killed by asphyxiation in air, immersion in ice/water slurry or by percussive stunning. Ice killing is usually used in selective fisheries. Recent alternative stunning/killing processes have been experimentally investigated for Mediterranean fish species in an effort to develop and optimise commercial methods by assuring both high standards of fish welfare and product quality (EFSA Citation2008; Poli Citation2009). It has been widely reviewed that pre-slaughter (as anaesthesia) and slaughter stressful practises could have an important effect on the flesh quality in fish (Poli et al. Citation2005). A clear effect emerged mostly on the physical properties of flesh, because severe stress at slaughter time exhausts muscular energies, produces more lactic acid, reduces muscular pH and increases the rate of rigor mortis onset (Poli et al. Citation2005). In this way, these practises could have significant negative effects on fish technological traits and in their flesh quality.
According to Hultin (Citation1992), anaerobiosis influences the conversion of xanthine dehydrogenase to xanthine oxidase. The latter enzyme transfers electrons directly to molecular oxygen producing superoxide and hydrogen peroxide, which can produce hydroxyl radicals in the presence of redox iron. These compounds have been proposed as among the principal initiators of lipid oxidation in biological tissues. Thus, the rapid conversion of ATP to hypoxanthine and of xanthine dehydrogenase to xanthine oxidase could influence lipid oxidation time of fresh and semi-preserved fish, especially when molecular oxygen is reintroduced during post-mortem processing.
Tejada and Huidobro (Citation2002) found out that slaughter method (percussion, ice salt-water slurry bath and asphyxia) has no clear influence on the oxidative stability of gilthead sea bream (S. aurata), probably due to the interaction of many factors such as stress, handling speed after death and lipid content of flesh.
Morzel and van de Vis (Citation2003) studied the effect of killing methods on lipid oxidation of eel (Anguilla anguilla L.). Particularly, electricity and oxygen removal (new killing method) resulted in higher quality of eels in comparison with the dry-salt technique, by reducing stress and improving freshness. Furthermore, the less stressful practise seemed to reduce the extent of lipid oxidation. In detail, authors pointed out that enhanced lipid oxidation in salt-bath eels can be partially explained by the physical damage to the muscle, thereby increasing the cell ruptures and the consequent accessibility to the catalytic enzymes. In addition, the presence of salt may be considered in some extent a slight pro-oxidant.
Results from Giuffrida et al. (Citation2007) were in agreement with this explanation. Particularly, ice slurry slaughtered gilthead sea bream (S. aurata) showed higher (and then better) ATP/IMP levels (an indicator of less stressed fish) and to be less prone to lipid oxidation, as revealed by the MDA values, 0.158 mg MDA/kg flesh against 0.227 mg MDA/kg flesh in CO2-slaughtered fish. The same pattern was found in electrically stunned rainbow trout (O. mykiss) whilst compared electricity with anoxia and bleeding as killing methods. TBARS values for these groups were 0.68, 1.09 and 1.03 mg MDA/kg flesh, respectively. Sakai and Terayama (Citation2008) studied the effect of bleeding as killing method on chub mackerel (Scomber japonicus) lipid oxidation. Struggling death in iced sea water was utilised as a control. The MDA content in the muscles of the bleeding fish samples were significantly higher than those of the control after 119 h of storage at 0 °C, with 0.367 and 0.184 mg/kg, respectively. On the contrary, no differences were found in the 4-hydroxyhexanal content of the samples. These results confirmed that fish subjected to stressful conditions were more prone to be oxidised and suggested that bleeding can be considered as a stressing killing method.
On the contrary, Duran et al. (Citation2008) found that slaughter method (asphyxia or percussion) had no effect on the MDA values of carp (Cyprinus carpio). However, when considering rainbow trout (O. mykiss) the MDA content of flesh from fish slaughtered by asphyxiation was significantly higher than that of specimens slaughtered by percussion (4 and 3 mg MDA/kg flesh, respectively). It is important to note that the fat level of trout was higher than that of carp (5% against 1%), which led to an observed difference in the MDA contents of trout slaughtered by different methods.
The effects of different stunning/killing procedures (anaesthesia with clove oil, anaesthesia with 2-phenoxyethanol, percussive stunning, immersion in ice/water slurry, chilling on ice and anaesthesia with clove oil followed by immersion in ice/water slurry) on flesh quality of European sea bass (D. labrax) were investigated by Simitzis et al. (Citation2014). Globally, MDA ranged between 29.9 and 95 mg/kg flesh in chilling on ice and percussion slaughtered sea bass. Despite such large range of values, authors did not find any significant difference among the tested killing methods, suggesting no killing effects on lipid oxidation.
Interestingly, in contrast, to the results seen previously, in a recent study Secci et al. (Citation2016) found out the link between stress during slaughter and lipid oxidation. Their results revealed the presence of very high level of reactive molecules, such as hydroperoxides, in stressed rainbow trout whilst they were not detected in the not-stressed group. Thus, probably as a consequence of the greater enzymatic activity under stress condition, the presence of lipid oxygenated products affected the development of lipid oxidation during post-mortem storage.
Handling
It is widely reviewed that any process causing disruption of the muscle membrane system (such as grinding, freezing and cooking) results in exposure the lipid fraction to oxygen, and thus accelerates the development of the oxidative damage. However, one of the first processes after stunning and killing procedures in the fish industry is the blood removal. Although it is not a kind of handling altering the lipid structure, blood removal is strictly linked to the quality deterioration of fish muscle, especially to the lipid oxidation. Richards and Hultin (Citation2002) studied the contribution of blood and blood components to lipid oxidation in rainbow trout (O. mykiss) and Atlantic mackerel (S. scombrus). They performed a complex project, finding out three main points: bleeding significantly reduced the probability of rancidity (expressed both as sensory score and TBARS value) development during storage; this probability strictly depended on species and type of muscle considered (rainbow trout versus mackerel, light versus dark muscle); the extent of lipid oxidation was more pronounced in minced muscle as compared to the intact one.
The first point was confirmed by many authors. Tejada and Huidobro (Citation2002) recognised the greater extent of lipid oxidation of ungutted gilthead sea bream (S. aurata) when comparing with gutted samples at day 11 of refrigerated storage (+2 °C), reaching as high as 8 mg MDA/kg flesh, a value commonly utilised as rancidity threshold. However, according to the same authors, such an increase seemed not to be significant.
Sakai et al. (Citation2006) attempted to measure Hb content in bled skipjack tuna (Katsuwonus pelamis) flesh, and they analysed MDA contents and 4-hydroxyhexenal (HHE) in the muscle as indicators of the lipid oxidation level. First, Hb content was lower in bleeding samples than in the control ones, containing 0.07 and 1.01 mg/g, respectively. Concerning lipid oxidation, Sakai et al. (Citation2006) did not find significant differences in MDA content in samples while bleeding fish showed lower level of HHE than the control samples both immediately after death (not detected versus 0.20 nmol/kg) and after 2 d of storage at 0 °C (0.07 and 0.43 nmol/kg, respectively).
More recently, Maqsood & Benjakul (Citation2011) confirmed that bleeding decreases Hb content and consequently lipid oxidation in Asian sea bass muscle (Lates calcarifer). Their results indicate that lipid oxidation (measured as PV, TBARS and volatiles) was more pronounced in the un-bled samples during 15 d of refrigerated storage (2 °C). Particularly, blood contains a high amount of Hb which action as pro-oxidant is still discussed. However, the extent of lipid oxidation is affected not only by Hb concentration but also by the presence of different type of Hbs in fish muscle (Richards & Hultin Citation2002) and their breakdown during storage, resulting in the release of non-haeme iron (Maqsood & Benjakul Citation2011).
At this point, it is easy to understand that different species, as well as different kind of muscle, may greatly differ in term of Hb content and composition, so causing a different susceptibility of the muscle to be oxidised. This is the case of muscle that contains a large amount of blood, such as dark muscle, which is found to be more prone to be oxidised (Richards & Hultin Citation2002). In addition, Hb concentration might explain the higher values of lipid oxidation in minced muscle than in whole/intact one (Richards & Hultin Citation2002). Indeed, the mechanical action of mincing can provoke rupture blood vessels, erythrocytes and some other cells and so cause Hb release. As stated, that release can promote lipid oxidation.
At the same time, grounding increases the exposition area of muscle to atmosphere oxygen, moving to a real pro-oxidant factor. According to them, Thiansilakul et al. (Citation2011) confirmed that myoglobin (Mb) was able to catalyse lipid oxidation in washed Asian sea bass (L. calcarifer) minced intensively. Primary and secondary oxidation products, as well as off-odour development, were significantly higher in Mb addicted samples than in the control ones (no Mb addicted). At day 8 of storage (4 °C), volatiles were mainly composed by 11.54%, 11% and 7.08% of 1-octen-3-ol, hexanal and 2-pentyl furan, respectively, whilst hexanal and 2-pentyl furan applied globally for 3.7% in the control samples. These changes were more likely associated with metmyoglobin formation occurring in washed mince, as a consequence of the increase in storage time. Moreover, lipid oxidation in washed mince with added Mb was mainly governed by pH. Specifically, lowest the pH (6) highest the lipid oxidation extent was.
Recently, it was also found that the higher the haeme affinity of Mb, the lower the Mb-mediated lipid oxidation was obtained (Richards et al. Citation2009). Therefore, low pH was not only associated with Mb oxidation, but also weakened the haeme-globin complex, leading to a release of haeme group, which was able to induce the lipid oxidation.
Storage
The problem of the quality deterioration during storage is well known and it is related to both temperature and storage time. As well, the quality lowering rate depends on species of fish. Nishimoto et al. (Citation1985) found out that the highest temperature of storage the fastest deterioration of fish freshness. To date, many authors have focused on quality changes during storage by studying separately ice or chilling storage and the frozen one.
The concentration of TBARS in good quality frozen and chilled fish or in fish stored on ice is typically between 5 and 8 mg MDA/kg whereas levels of 8 mg MDA/kg are generally regarded as the limit of acceptability for most species (Schormüller Citation1968). More strictly, Ke et al. (Citation1984) proposed that TBARS values for fish products below 0.58 mg/kg were perceived as not rancid; 0.58–1.51 mg/kg as slightly rancid, but acceptable; and values above 1.51 mg/kg were perceived as rancid.
Özyurt et al. (Citation2009) studied red mullet (Mullus barbatus), and goldband goatfish (Upeneus moluccensis), both belong to the Mullidae family, funding different shelf-life and lipid oxidation levels when stored 11 d at 2 °C. The authors analysed both primary (PV) and secondary (TBARS) lipid oxidation products. PV significantly raised from 0.64 and 0.83 meq (peroxide oxygen/kg fat) to 2.26 and 4.82 meq/kg at the end of the trial, in red mullet and goldband goatfish, respectively. TBARS values were found stable around 0.51 and 0.57 mg MDA/kg flesh for both species during the whole storage. Similar PV values were obtained by Timm-Heinrich et al. (Citation2013) studying the oxidative changes of rainbow trout during ice storage (12 d at 2 °C) by following PV and volatile content. PV were below 0.5 meq O2/kg flesh during the first 5 d of storage. After, PV had a slightly increase up to 0.56 meq O2/kg on day 7, whilst they started raising significantly up to 6 meq O2/kg on day 12. Similarly, the volatile fraction started to increase significantly from day 5 onwards, especially 1-penten-3-ol, 1-penten-3-one and 2-pentenal. Globally, volatiles were found in low concentration (ng/kg), confirming a little oxidation during 12 d of storage on ice. Concerning TBARS levels, Etemadian and Shabanpour (Citation2014) found their increase from an initial value of 0.56 mg MDA/kg of muscle to 2.92, 2.67 and 2.31 mg MDA/kg of Rutilus frisii kutum slices during 15 d of iced storage.
Probably related to bacterial growth at positive temperature, the 7th d appeared to be critical even in other papers. For example, Hernández et al. (Citation2009) studied lipid oxidation of aquacultured meagre (Argyrosomus regius) fillets during 18 d of storage at 4 °C, finding significant differences from day 7 onwards. Particularly, TBARS gradually increased from 0.10 to 2.55 mg MDA/kg flesh. In the agreement, Simitzis et al. (Citation2014) found that, in general, positive temperature (4 °C) increased MDA levels around 3.6 times in 7 d of storage. In the same paper, Simitzis et al. (Citation2014) looked for TBARS level even in frozen (−20°C) samples, finding that freezing raised up TBARS level around 2.7 times after 90 d. An interesting connection between refrigerated and frozen storage was found some years before by Huang et al. (Citation2003) who measured the same MDA value (7.2 μg) in hybrid tilapia fillets (O. niloticus × O. aureus) stored for 7 d at 4 °C or for 8 weeks at −40 °C.
When meat and meat products are stored under frozen conditions, microbial spoilage may be delayed, but fat deterioration occurs and the meat constituents may be oxidised (Ojagh et al. Citation2014). Interestingly, the main cause of lipid oxidation during frozen storage seemed to be due to the enzymatic lipolysis activity. Indeed, Karlsdottir et al. (Citation2014a) indicated that enzymatic lipolysis was the driving factor influencing the fillet quality over storage and it mostly affects long chain polyunsaturated lipids in the light muscles.
However, previous studies had confirmed that low storage temperatures were optimal for preserving fish from oxidative deterioration. Refsgaard et al. (Citation1998) compared lipid oxidation of Atlantic salmon fillets (S. salar) stored at −10 or −20 °C for 34 weeks. The content of lipid hydroperoxides and FFAs increased during storage as affected by a significant time-temperature interaction, and the changes were fastest in salmon stored at −10 °C. Specifically, hydroperoxides raised from 0 to 10 meq O2/kg, while FFA increased from 1 to 8.7% in 34 weeks of storage. Such as oxidative product increase was associated with a decrease in highly unsaturated fatty acid content (C20:5n3, C22:5n3 and C22:6n3) in Atlantic salmon stored at −10 or −20 °C. Also for the PUFAs, significant time-temperature interaction effects were found, confirming the fastest decrease at −10 °C. Concerning to volatile products, aldehydes and ketones were identified. For hexanal, heptanal, (E)-2-hexenal, (E,E)-2,4-heptadienal and nonanal significant time effects were found due to increasing concentrations during storage, independently of storage temperature, while temperature influenced significantly hexanal and 2-hexanal levels. A small increase in the amount of secondary lipid oxidation products was also observed by Jensen et al. (Citation1998). The significant and preservative action of negative storage temperature was confirmed by Choubert et al. (Citation2011), that determined lipid oxidation (TBARS) in packed rainbow trout stored for 18 months at −20 °C. Results showed that TBARS significantly increased after the first month of storage, but not other changes occurred during the 5 later months.
Baron et al. (Citation2007) studied the lipid oxidation during frozen storage of rainbow trout fillets, stored for 13 months at −20, −30 or −80 °C. Lipid oxidation was followed by measuring lipid hydroperoxides (PV), as well as secondary oxidation products (volatiles). There was a significant increase in the level of lipid hydroperoxides after 8 months of frozen storage for fish stored at −20 °C, which was even more pronounced after 13 months, reaching 6.6 meq/kg of oil, indicating on-going oxidation. In contrast, samples stored at −80 and −30 °C did not show any significant increase in peroxides during the entire storage period (with p=0.26 and p=0.07, respectively). Measurement of secondary oxidation products was followed for 13 months, and the development of hexanal (an oxidation product of linoleic acid), and 1-penten-3-one and t,t-2,4-heptadienal, both oxidation products of n-3 fatty acids. Other volatiles were also measured during storage (1-penten-3-ol, heptanal, 1-octen-3-ol, t-2-octenal, nonanal, t,c-2,6-nonadienal, decanal), and their development was generally in agreement with what is reported here for hexanal, 1-penten-3-one, and t,t-2,4- heptadienal. Volatile patterns indicated that fish stored at −20 °C was the most oxidised and that little difference was observed between −80 and −30 °C. On the basis of their observations, the ranking order −20 °C> −30 °C> −80 °C was obtained for the development of oxidation products in fish stored at freezing temperatures. Likewise, both Indergård et al. (Citation2014) and Karlsdottir et al. (2014a) recently confirmed that pattern. The first authors examined lipid oxidation, by PV and TBARS, in Atlantic salmon during a long-term frozen storage at –25, –45 and –60 °C. After 1 year of storage at –25 °C, the concentration of PV in red and white fish muscles increased from 1.26 to 1.82 and from 1.08 to 1.76 meq O2/kg fat, respectively. Formation of TBARS was higher in the red muscles than in the white ones and reached a value of 14.04 mg MDA/kg fish after 1 year of storage at –25 °C. Decreasing the temperature to –45 °C inhibited PV and TBARS formation. In the latter paper, the authors studied the lipid deterioration of two lean fish species, i.e. saithe (Pollachius virens) and hoki (Macruronus novaezelandiae), during frozen storage at −20 and −30 °C (up to 18 months). As even in the previous case, Karlsdottir et al. (Citation2014a) analysed both light and dark muscles. Results showed significant lipid deterioration with the extended storage time, but lower storage temperature showed significantly more preservative effects. The formation of hydroperoxides as well appeared to be strongly influenced by species. Saithe was very stable during the first 12 months of storage regardless to storage temperature. After 18 months of frozen storage, however, a slight, yet significant, increase of peroxides (up to 50 mmol/kg muscle) was observed in light and dark muscle types. On the other hand, hydroperoxide formation in hoki showed a much more pronounced and more progressive peroxide formation over time at both storage temperatures. However, as previously shown for handling, the extent of oxidation rate was showed to be strictly connected with the type of muscle considered. Indeed, dark muscle showed to be the most prone to be oxidised regardless to storage temperature, by ranging up to 325 and 250 mmol/kg at −20 °C and −30 °C, respectively. The light muscle presented a pattern similar to saithe, by remaining almost unaltered for the first 12 months. As for saithe, at the end of storage, a significant increase of peroxide was observed (50 and 100 mmol/kg muscle at −30 and −20 °C, respectively) in hoki samples. It has to be noted that the marked difference in peroxide content between hoki dark and light muscle is likely due to the considerably higher lipid content in dark muscle than in the light one (7.6% versus 0.6%). The difference in fat content might explain either the difference between oxidation susceptibility of saithe when compared with hoki, since saithe fat content ranged from 0.6% to 1.1%, whilst hoki has from 0.6% to 7.6% in light and dark muscle, respectively. TBARS results for both the saithe dark and light muscle showed low/no formation of secondary oxidation products up to month 6, followed by a sharp increase up to month 12, after which only the dark muscle values continued to increase. As well as PVs, low temperature protected against oxidation as revealed by the significantly higher increase of TBARS in −20 °C stored samples than the −30 °C stored ones.
Cooking
Prior to consumption, fresh and frozen fish usually undergo different preserving treatments or different cooking processes, while the consumption of raw fish is not considered a traditional custom in the Western society. Boiling, frying, pan-frying, grilling, roasting, baking and microwaving are the most popular cooking methods. However, despite making food safer and tastier, the temperatures reached during cooking process may affect radically the characteristics and composition of food by enhancing lipid oxidation on behalf the other processes.
In general, the cooked saithe fillets exhibited an increase in hydroperoxide levels after steaming (Karlsdottir et al. Citation2014b) which is a characteristic sign that thermally catalysed oxidation has taken place. TBARS were in line with peroxide content, by showing an increase after cooking. That pattern was more evident in samples previously frozen stored (6 months) in which TBARS raised from less than 0.72 to 5 mg MDA/kg muscle. Even concerning the volatile fraction, storage time seems to negatively affect aroma of cooked fillets. Particularly, Aro et al. (Citation2002) noted that short chain acids, deriving from aldehydes oxidation and microbial fermentation and partially causing an unpleasant odour, increased in herring when baked after storage (48 h).
High temperature and medium of cooking may be the main responsible for the different effect on cooked meat. This fact is mostly evident for frying which seems to lead a wide variety of changes. , as an example, reports data from Al-Saghir et al. (Citation2004) for Atlantic salmon and rainbow trout, respectively. Salmon fried with olive or corn oil at 180 °C increase its PV 3 or 2 times, respectively, whilst sunflower frying doubled PV in rainbow trout. The significant differences in peroxide content in both cases seem to depend on the first oxidation state and degree of unsaturation of the frying oils. Indeed, as reported by Al-Saghir et al. (Citation2004) olive oil has a high initial PV (11.8 ± 0.03 meq O2/kg), which provides the explanation for the increased PV of salmon after frying with olive oil.
Figure 1. Peroxides accumulation on rainbow trout (Al-Saghir et al. Citation2004) and Atlantic salmon (Tokur Citation2007) cooked by different methods.
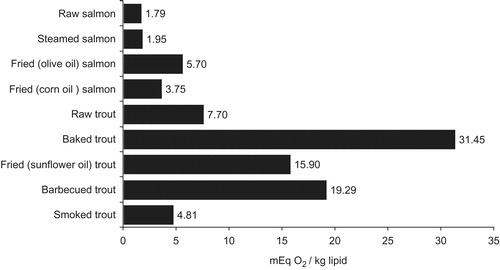
Concerning the oxidative effects of frying, it has to be underlined that is very hard to follow clearly lipid oxidation, especially its primary products. In effect, high temperatures accelerate oxidation but oil dissolves oxidation products, reducing their concentration in fillet and making the oxidative damage difficult to assess (Weber et al. Citation2008). The low levels of conjugated dienes (CD), peroxides and MDA, if apparently could lead to think of a smaller oxidation, actually seem to depend on the more rapid evolution of the oxidative mechanism taking place in this kind of cooking method. shows that higher amount of peroxides accumulates in baking (31.45 ± 1.17 mEq O2/kg lipid) and barbecuing rainbow trout fillets when compared with the frying ones. Furthermore, when compared with grilled, boiled, oven and microwave baked ones, the CD and peroxides values decreased for all fried (215–220 °C) samples of silver catfish (Rhamdia quelen), probably because of their decomposition into secondary oxidation products which might have been lost in the frying oil or transformed in protein adducts (Weber et al. Citation2008).
The interaction between lipid oxidation products and proteins is hypothesised even by Talab (Citation2014) for explaining the decreasing effect of the cooking method on carp lipid oxidation. Evaluating the effects of different cooking methods on PV and TBARS of raw, fried, microwave and halogen cooked carp fish cutlets, the author found that cooking decreased both primary and secondary oxidation products. Particularly, PV recorded values were 3.69, 2.98, 2.80 and 2.60 meq O2/kg for raw, fried, microwave and halogen-cooked samples while TBARS of raw, fried, microwave and halogen-cooked carp cutlets were 1.20, 1.18, 1.09 and 1.01 mg MDA/kg, respectively.
Similarly, Wu and Mao (Citation2008) supposed that the high temperatures accompanying drying processes during microwaving could speed up the breakdown of peroxides into their carbonyl components, and thus the PV, and more generally, the primary oxidation products, may remain low. For this reason, CD and peroxides did not differ from both raw silver catfish (Weber et al. Citation2008) and raw grass carp fillets (Ctenopharyngodon idellus) (Wu & Mao Citation2008), while a significant increase in secondary oxidation products was observed both in catfish and in carp baked in microwave and conventional ovens. These data were supported by previous research (Tokur Citation2007) in which baking and barbecuing significantly increase TBARS levels (5.78 ± 0.94 and 8.40 ± 0.51 mg MDA/kg muscle) in rainbow trout fillets in comparison with the smoking process (1.82 ± 0.16 mg MDA/kg muscle).
The oxidative impact of cooking and its fast develop can be noted by the abundance of aldehydes and chetons (volatiles), mainly arising from unsaturated fatty acid degradation. Several volatile molecules belonging to the main odourant categories (furanones, pyrazines, aldehydes, chetons and other intermediate products from Maillard reactions, such as 5-methylfurfural), can be detected in cooked fish or be loose in the cooking medium, as in the case of boiled fish (Morita et al. Citation2003). Interestingly, in spite of having a poor organoleptic profile, steam-cooked bighead carp (Hypophthalmichthys nobilis) was not rejected by consumers. Strangely, hedonic score resulted higher for steamed carp than for the oven-baked one, so that 64% of the panellists liked very much or moderately the appearance and flavour of the steamed carp (Freeman Citation1999).
It is nevertheless true that some volatile compounds from lipid oxidation, like (Z)-4-heptenal and hexanal (Prost et al. Citation1998), above odour threshold, could be responsible for off-flavours and consequently can deteriorate the organoleptic profile of cooked fillets.
By the way, it is important to remember that many other molecules, such as proteins, are responsible for the aromatic profile. The weak odour of steamed horse mackerel for example, when compared with grilled and fried samples could be explained by the concentration at lower levels of alanine, aspartic acid, glutamic acid, glycine and proline which are responsible for flavour and taste in seafood and seafood products (Ruiz-Capillas & Moral Citation2004). For this reason, it is not possible to strictly link the organoleptic profile exclusively to lipid oxidation.
Anyway, the lipid stability of baked fish can be affected by the presence of antioxidative agents in dietary treatment. Jittinandana et al. (Citation2006) compared the results between rainbow trout fed with low and high dietary vit. E supplementation showing a significant difference in TBA after baking (0.67 versus 1.20 mg MDA/kg).
Conclusions
The complexity of lipid oxidation is reflected on the large variety of molecules that can be generated and on the various factors which affect it. Hence, every step in fish supply chain seems to be important for preserving lipid integrity and native quality. A feeding with antioxidants is a recent practise to arise AOP in muscle. However, some issues emerge from this review. First, it is quite clear that the main antioxidants such as α-tocopherol and astaxanthin act during the initiation phase of lipid oxidation by acting as scavengers of ROS and ROMs species. Thanks to this ability, the antioxidants can play a central role to limit the detrimental effects of stress (pre-slaughter or at killing) and storage. In this sense, lycopene modulates oxidative status via activating host defence system during stressful conditions while α-tocopherol acts preferentially in long-term frozen storage. The growing efficiency carvacrol > rosemary > thymol has to be taken into consideration while using new antioxidants such as essential oils (thymol, carvacrol, rosemary extract). Finally, it has to consider that, in some cases increasing the concentration of the antioxidants in the diet is not always associated with increasing lipid stability. Further investigations on feeding field are recommended. It could be of interest to evaluate the role of new alternative protein and/or lipid sources (such as insect meals) on lipid composition and stability of the fillets. Even the utilisation of agriculture by-products rich in polyphenols (for example, those derived from olive oil production chain) may be considered both for their role as potential antioxidants and an environmental point of view.
Stress just before or at slaughter can greatly threaten flesh quality both immediately and after storage. Stress induces the activation of numerous metabolic pathways, that often involve the production of very reactive molecular species, such as hydroperoxides. Their presence can be the main cause of increasing level of lipid oxidation products both immediately after death or during storage. A common operation such as bleeding can significantly reduce both reactive molecules and Hb, which is recognised as a great pro-oxidant. However, stress response seems to be strictly species-specific, so different species of fish can react differently to the same killing method and show a different pattern in lipid degradation. Future outlooks on the connection between stress at slaughter and oxidative stability, as well as the evaluation of the effects of new killing procedures on both animal welfare and fish quality should be conducted.
Temperature and length are two critical points of storage phase which has to be considered even by consumers. Frozen storage at very low temperatures (−30, −40 °C) confirms to be the best storage practise, while the refrigerated one shows some limit. Particularly, considering lipid oxidation, it has to be underlined that 7 d of refrigerated storage is accepted as the maximum storage length for many species of fish.
Finally, if it is true that cooking can make safer a product regardless its storing age, it is also true that age can compromise aromatic profile of cooking fillets. However, on one hand, it could be interesting to evaluate the effect of new cooking methods, such as under vacuum cook or air-frying on lipid oxidation. On the other, it should be deepened the connections between oxidation, volatiles composition and consumers perception and acceptance. Perhaps the creation of acceptance predictive models based on lipid stability could even be useful.
In conclusion, many steps long the production-supply chain of fish can lead lipid deterioration and many interactions between them may contribute to alter flesh quality. Thus, feeding antioxidant, reducing stress, good storage practises, if associated with an appropriate cooking method (low temperature, short time), seem to preserve such a fragile and extremely important lipid fraction from farm to fork.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Al-Saghir S, Thurner K, Wagner K, Frisch G, Luf W, Razzazi-Fazeli E, Elmadfa I. 2004. Effects of different cooking procedures on lipid quality and cholesterol oxidation of farmed salmon fish (Salmo salar). J Agric Food Chem. 52:5290–5296.
- Álvarez A, García García B, Garrido MD, Hernández MD. 2008. The influence of starvation time prior to slaughter on the quality of commercial-sized gilthead seabream (Sparus aurata) during ice storage. Aquaculture 284:106–114.
- Álvarez A, García García B, Jordán MJ, Martínez-Conesa C, Hernández MD. 2012. The effect of diets supplemented with thyme essential oils and rosemary extract on the deterioration of farmed gilthead seabream (Sparus aurata) during storage on ice. Food Chem. 132:1395–1405.
- Aro T, Brede C, Manninen P, Kallio H. 2002. Determination of semivolatile compounds in Baltic herring (Clupea harengus membras) by supercritical fluid extraction, supercritical fluid chromatography-gas chromatography-mass spectrometry. J Agric Food Chem. 50:1970–1975.
- Azhar KF, Nisa K. 2006. Lipids and their oxidation in seafood. J Chem Soc Pak. 28:298–305.
- Bagni M, Civitareale C, Priori A, Ballerini A, Finoia M, Brambilla G, Marino G. 2007. Pre-slaughter crowding stress and killing procedures affecting quality and welfare in sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata). Aquaculture 263:52–60.
- Bayir M, Bayir A, Aras NM. 2014. A comparison of the effect of long-term starvation on responses to low-temperature stress by juvenile rainbow (Oncorhynchus mykiss) and brown (Salmo trutta) trout reveal different responses in the two species. Mar Freshw Behav Physiol. 47:239–251.
- Baron CP, Kjaersgard IVH, Jessen F, Jacobsen C. 2007. Protein and lipid oxidation during frozen storage of rainbow trout (Oncorhynchus mykiss). J Agric Food Chem. 55:8118–8125.
- Chaiyapechara S, Casten MT, Hardy RW, Dong FM. 2003. Fish performance, fillet characteristics, and health assessment index of rainbow trout (Oncorhynchus mykiss) fed diets containing adequate and high concentrations of lipid and vitamin E. Aquaculture 219:715–738.
- Choubert G, Brisbarre F, Baccaunaud M. 2011. Impact of dietary carotenoid and packaging during frozen storage on the quality of rainbow trout (Oncorhynchus mykiss) fed carotenoids. J Sci Food Agric. 91:1075–1082.
- Duran A, Erdemli U, Karakaya M, Yilmaz MT. 2008. Effects of slaughter methods on physical, biochemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) and mirror carp (Cyprinus carpio) filleted in pre-, in- or post-rigor periods. Fish Sci. 74:1146–1156.
- EFSA. 2008. Scientific opinion of the panel on animal health and welfare on a request from the European Commission on animal welfare aspects of husbandry systems for farmed European seabass and gilthead seabream. EFSA J. 844:1–21.
- EFSA. 2010. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 8:1461:1–107.
- Etemadian Y, Shabanpour B. 2014. Changes in physicochemical properties and shelf life ability of kutum (Rutilus frisii kutum) slices during packaging and storage in ice. J Food Process Preserv. 38:159–168.
- Freeman DW. 1999. Comparison of moist and dry cooking on sensory quality, consumer acceptance and marketability of canned bighead carp. J Aquat Food Prod Technol. 8:33–44.
- German JB, Kinsella JE. 1985. Lipid oxidation in fish tissue. Enzymatic initiation via lipoxygenase. J Agric Food Chem. 33:680–683.
- Giannenas I, Triantafillou E, Stavrakakis S, Margaroni M, Mavridis S, Steiner T, Karagouni E. 2012. Assessment of dietary supplementation with carvacrol or thymol containing feed additives on performance, intestinal microbiota and antioxidant status of rainbow trout (Oncorhynchus mykiss). Aquaculture 350–353:26–32.
- Ginés R, Palicio M, Zamorano MJ, Argüello A, López JL, Afonso JM. 2002. Starvation before slaughtering as a tool to keep freshness attributes in gilthead sea bream (Sparus aurata). Aquacult Int. 10:379–389.
- Girao PM, Pereira da Silva EM, de Melo MP. 2012. Dietary lycopene supplementation on Nile tilapia (Oreochromis niloticus) juveniles submitted to confinement: effects on cortisol level and antioxidant response. Aquaculture 43:789–798.
- Giuffrida A, Pennisi L, Ziino G, Fortino L, Valvo G, Marino S, Panebianco A. 2007. Influence of slaughtering method on some aspects of quality of gilthead seabream and smoked rainbow trout. Vet Res Commun. 31:437–446.
- Hernández MD, López MB, Álvarez A, Ferrandini E, García García B, Garrido MD. 2009. Sensory, physical, chemical and microbiological changes in aquacultured meagre (Argyrosomus regius) fillets during ice storage. Food Chem. 114:237–245.
- Hernández A, García García B, Jordán MJ, Hernández MD. 2014. Improved conservation of gilthead seabream (Sparus aurata) in ice storage. The influence of doses of rosemary extract added to feed. Aquaculture 486–487:31–40.
- Huang S, Weng Y, Huang C. 2003. Lipid peroxidation in sarcoplasmic reticulum and muscle of tilapia is inhibited by dietary vitamin E supplementation. J Food Biochem. 28:101–111.
- Hultin HO. 1992. Quality assurance in the fish industry. In: Huss HH, Jakobsen M, Liston J, editors. Biochemical deterioration of fish muscle. Amsterdam, The Netherlands: Elsevier; p. 125–138.
- Indergård E, Tolstorebrov I, Larsen H, Eikevik TM. 2014. The influence of long-term storage, temperature and type of packaging materials on the quality characteristics of frozen farmed Atlantic salmon (Salmo salar). Int J Refrig. 41:27–36.
- Jensen C, Birk E, Jokumsen A, Skibsted LH, Bertelsen G. 1998. Effect of dietary levels of fat, α-tocopherol and astaxanthin on colour and lipid oxidation during storage of frozen rainbow trout (Oncorhynchus mykiss) and during chill storage of smoked trout. Z Lebensm Unters Forsch A. 207:189–196.
- Jittinandana S, Brett Kenney P, Slider SD, Hankins JA. 2006. Effect of high dietary vitamin E on lipid stability of oven-cooked and hot-smoked trout fillets. J Food Sci. 71: 130–136.
- Karlsdottir MG, Sveinsdottir K, Kristinsson HG, Villot D, Craft BD, Arason S. 2014a. Effects of temperature during frozen storage on lipid deterioration of saithe (Pollachius virens) and hoki (Macruronus novaezelandiae) muscles. Food Chem. 156:234–242.
- Karlsdottir MG, Sveinsdottir K, Kristinsson HG, Villot D, Craft BD, Arason S. 2014b. Effect of thermal treatment and frozen storage on lipid decomposition of light and dark muscles of saithe (Pollachius virens). Food Chem. 164: 476–484.
- Ke PJ, Cervantes E, Robles-Martinez C. 1984. Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation spectrophotometric method. J Sci Food Agric. 35:1248–1254.
- Maqsood S, Benjakul S. 2011. Effect of bleeding on lipid oxidation and quality changes of Asian seabass (Lates calcarifer) muscle during iced storage. Food Chem. 124:459–467.
- Maqsood S, Benjakul S, Kamal-Eldin A. 2012. Haemoglobin-mediated lipid oxidation in the fish muscle: a review. Trends Food Sci Technol. 28:33–43.
- Morita K, Kubota K, Aishima T. 2003. Comparison of aroma characteristics of 16 fish species by sensory evaluation and gas chromatography analysis. J Sci Food Agric. 83:289–297.
- Morzel M, van de Vis H. 2003. Effect of the slaughter method on the quality of raw and smoked eels (Anguilla anguilla L.). Aquacult Res. 34:1–11.
- Nathanailides C, Panopoulos S, Kakali F, Karipoglou C, Lenas D. 2011. Antemortem and postmortem biochemistry, drip loss and lipid oxidation of European sea bass muscle tissue. Procedia Food Sci. 1:1099–1104.
- Niki E, Yoshida Y, Saito Y, Noguchi N. 2005. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 338:668–676.
- Nishimoto JI, Suwetja IK, Miki H. 1985. Estimation of keeping freshness period and practical storage life of mackerel muscle during storage at low temperatures. Mem Fac Fish Kagoshima Univ. 34:89–96.
- Ojagh SM, Rezaei M, Razavi SH. 2014. Improvement of the storage quality of frozen rainbow trout by chitosan coating incorporated with cinnamon oil. J Aquat Food Prod Technol. 23:146–154.
- Okpala COR, Choo WS, Dykes GA. 2014. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. Food Sci Technol. 55:110–116.
- Özyurt G, Kuley E, Özkütük S, Özogul F. 2009. Sensory, microbiological and chemical assessment of the freshness of red mullet (Mullus barbatus) and goldband goatfish (Upeneus moluccensis) during storage in ice. Food Chem. 114: 505–510.
- Pérez-Sánchez J, Borrel M, Bermejo-Nogales A, Benedito-Palos L, Saera-Vila A, Calduch-Giner JA, Kaushik S. 2013. Dietary oils mediate cortisol kinetics and the hepatic mRNA expression profile of stress-responsive genes in gilthead sea bream (Sparus aurata) exposed to crowding stress. Implications on energy homeostasis and stress susceptibility. Comp Biochem Physiol Part D Genomics Proteomics. 8:123–130.
- Poli BM. 2009. Farmed fish welfare-suffering assessment and impact on product quality. Ital. J Anim Sci. 8:139–160.
- Poli BM, Parisi G, Scappini F, Zampacavallo G. 2005. Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquacult Int. 13:29–49.
- Prost C, Serot T, Demaimay M. 1998. Identification of the most potent odorants in wild and farmed cooked turbot (Scophtalmus maximus L.). J Agric Food Chem. 46: 3214–3219.
- Ramanathan L, Das NP. 1992. Studies on the control of lipid oxidation in ground fish by some polyphenolic natural products. J Agric Food Chem. 40:17–21.
- Refsgaard HHF, Brockhoff PB, Jensen B. 1998. Sensory and chemical changes in farmed Atlantic salmon (Salmo salar) during frozen storage. J Agric Food Chem. 46:3473–3479.
- Richards MP, Cai H, Grunwald EW. 2009. Phenylalanine substitution at site B10 (L29F) inhibits metmyoglobin formation and myoglobin-mediated lipid oxidation in washed fish muscle: mechanistic implications. J Agric Food Chem. 57:7997–8002.
- Richards MP, Hultin HO. 2002. Contributions of blood and blood components to lipid oxidation in fish muscle. J Agric Food Chem. 50:555–564.
- Richards MP, Modra AM, Li R. 2002. Role of deoxyhemoglobin in lipid oxidation of washed cod muscle mediated by trout, poultry and beef hemoglobins. Meat Sci. 62:157–163.
- Ruiz-Capillas C, Moral A. 2004. Free amino acids in muscle of Norway lobster (Nephrops novergicus (L.)) in controlled and modified atmospheres during chilled storage. Food Chem. 86:85–91.
- Sahin K, Yazlak H, Orhan C, Tuzcu M, Akdemir F, Sahin N. 2014. The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture 418–419:132–138.
- Sakai T, Ohtsubo S, Minami T, Terayama M. 2006. Effect of bleeding on hemoglobin contents and lipid oxidation in the skipjack muscle. Biosci Biotechnol Biochem. 70:1006–1008.
- Sakai T, Terayama M. 2008. Effect of bleeding on lipid oxidation in the chub mackerel muscle. Biosci Biotechnol Biochem. 72:1948–1950.
- Schaich KM. 1992. Metals and lipid oxidation. Contemporary issues. Lipids 27:209–218.
- Schormüller J. 1968. Handbuch der Lebensmittelchemie (BandIII/2). Berlin-Heidelberg; New York: Springer Verlag.
- Secci G, Parisi G, Dasilva G, Medina I. 2016. Stress during slaughter increases lipid metabolites and decreases oxidative stability of farmed rainbow trout (Oncorhynchus mykiss) during frozen storage. Food Chem. 190:5–11.
- Simitzis PE, Tsopelakos A, Charismiadou MA, Batzina A, Deligeorgis SG, Miliou H. 2014 . Comparison of the effects of six stunning/killing procedures on flesh quality of sea bass (Dicentrarchus labrax, Linnaeus 1758) and evaluation of clove oil anaesthesia followed by chilling on ice/water slurry for potential implementation in aquaculture. Aquacult Res. 45:1759–1770.
- Simopoulos AP. 1991. Omega-3 fatty acids in health and disease and in growth and development, a review. Am J Clin Nutr. 54:438–463.
- Stéphan G, Guillaume J, Lamour F. 1995. Lipid peroxidation in turbot (Scophthahus maximus) tissue: effect of dietary vitamin E and dietary n-6 or n-3 polyunsaturated fatty acids. Aquaculture 130:251–268.
- Suárez MD, Cervera MAR. 2010. Influence of starvation on flesh quality of farmed dentex (Dentex dentex). J World Aquacult Soc. 41:490–505.
- Talab AS. 2014. Effect of cooking methods and freezing storage on the quality characteristics of fish cutlets. Adv J Food Sci Technol. 6:468–479.
- Tejada M, Huidobro A. 2002. Quality of farmed gilthead seabream (Sparus aurata) during ice storage related to the slaughter method and gutting. Eur Food Res Technol. 215:1–7.
- Thiansilakul Y, Benjakul S, Richards MP. 2011. Effect of myoglobin from Eastern little tuna muscle on lipid oxidation of washed Asian seabass mince at different pH conditions. J Food Sci. 76:C242–C249.
- Timm-Heinrich M, Eymard S, Baron CP, Nielsen HH, Jacobsen C. 2013. Oxidative changes during ice storage of rainbow trout (Oncorhynchus mykiss) fed different ratios of marine and vegetable feed ingredients. Food Chem. 136:1220–1230.
- Tokur B. 2007. The effect of different cooking methods on proximate composition and lipid quality of rainbow trout (Oncorhynchus mykiss). Int J Food Sci Technol. 42:874–879.
- Weber J, Bochi VC, Ribeiro CP, de M. Victorio A, Emanuelli T. 2008. Effect of different cooking methods on the oxidation, proximate and fatty acid composition of silver catfish (Rhamdia quelen) fillets. Food Chem. 106:140–146.
- Wu T, Mao L. 2008. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 110:647–653.
- Zhang X, Wu T, Cai L, Zhu Y. 2007. Effects of α-tocopheryl acetate supplementation in preslaughter diet on antioxidant enzyme activities and fillet quality of commercial-size Sparus macrocephalus. J Zhejiang Univ Sci B. 8:680–685.
