 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The objective of this study was to evaluate the effects of three fibrolytic enzyme products (cellulase (CEL), xylanase (XYL) and a 1:1 mixture of CEL and XYL (MIX)) at three dose levels (0, 1 and 3 μL/0.5 g DM) on the in vitro fermentation of a diet for growing lambs. Bottles were incubated for 96 h at 39 °C. A mathematical model was used to estimate the parameters describing the gas production (GP) curve (b, c and L). Dry matter degradability (DMD) and fibre (NDFD and ADFD) degradability were determined at the end of the incubation period. Metabolisable energy (ME) and short chain fatty acids (SCFA) were calculated at 24 h of incubation. The asymptotic GP (parameter b) was affected (p < 0.02) by enzyme product and dose level, with a significant linear response (p < 0.05). Dose level affected ME and SCFA with a significant linear (p < 0.05) and quadratic (p < 0.01) response. The interaction between enzyme product and dose level was significant (p < 0.05) for cumulative GP up to 72 and 96 h of incubation, pH, ADFD and DMD. The results suggest that application of exogenous cellulases has the potential to alter asymptotic GP and degradability of ADF and DM of a diet for growing lambs, but most of the results depend on the interaction between enzyme product and dose level. Future studies are required to determine the ideal combination between enzyme product and dose level for optimal degradation of ruminant feeds.
Introduction
Inclusion of higher levels of grain in the diet for ruminants has been associated with declining rates of fibre digestion due to lower fibrolytic activity in the rumen (Martin & Michalet-Doreau Citation1995; Noziere et al. Citation1996) and an apparent microbial preference for non-structural carbohydrates (Mould & Ørskov Citation1983). This may create conditions in which supplementary fibrolytic exogenous enzymes can have beneficial effects on the degradation of the fibre (Beauchemin et al. Citation2001; Mendoza et al. Citation2014). There is a renewed interest in the use of fibrolytic exogenous enzymes for ruminants due to the increase in feeding costs and access to high quality enzymes (Adesogan et al. Citation2014; He et al. Citation2014). However, the effectiveness of enzyme products is highly variable (Colombatto et al. Citation2003a, Citation2003b; Meale et al. Citation2014; Mendoza et al. Citation2014).
Pre-treatment of forages with fibrolytic enzymes can solubilise some fibre and improve the digestibility at short incubation times (Moharrery et al. Citation2009). It appears that effective enzymes work best by removing structural barriers which delay microbial colonisation of digestible fractions (Colombatto et al. Citation2003a, Citation2003b) and increase degradation rate of fibre. Exogenous fibrolytic enzymes also seem to work better at close to neutral ruminal pH (Colombatto et al. Citation2007).
Effect of addition of exogenous enzymes is influenced by factors such as diet composition, type of enzyme preparation, enzyme stability, specific enzyme activities, method of application and dose level (Yang et al. Citation2000; Morgavi et al. Citation2001; Wallace et al. Citation2001; Mendoza et al. Citation2014). Enzyme mixtures of endoglucanase, xylanase, alfa-amylase and protease activity have been reported to improve in vitro gas production (GP) kinetics and ruminal fermentation of fibrous feeds (Elghandour et al. Citation2013) and in vivo nutrient digestibility and ruminal fermentation (Gado et al. Citation2009). Non-linear effects of dose level have been well established in vivo (Lewis et al. Citation1999; Kung et al. Citation2000) and in vitro (Colombatto et al. Citation2003b), the effect of the fibrolytic enzyme addition is reduced as the dose level increases but varies according to the substrate (Yang et al. Citation2000). There are also some inconsistencies on the effects of dose levels on ruminal fermentation kinetics. Some researches have shown that efficiency of forage utilisation was increased at the increasing dose levels of exogenous enzymes (Miller et al. Citation2008) whereas others suggest that exogenous enzymes produced better results at a specific level, rather than showing a dose response (Jalilvand et al. Citation2008).
The objective of this study was to evaluate the effect of three different fibrolytic enzyme products applied at three different dose levels on in vitro GP and fibre degradation of a diet for growing lambs.
Materials and methods
Diet, enzyme product and dose level
A total mixed ration (TMR, 13% CP) was prepared to meet the nutritional requirements (NRC Citation2007) for growing lambs (Table ). Samples from the TMR were dried at 65 °C for 48 h in a forced air oven to constant weight, grounded in a hammer mill to pass a 1 mm sieve and stored in plastic bags for subsequent determination of chemical components and in vitro GP.
Table 1. Ingredients and chemical composition of diet for growing lambs used in the in vitro test.
Two exogenous enzyme products were tested: CEL (Dyadic® Cellulase PLUS, Dyadic Int., Jupiter, FL), XYL (Dyadic® Xylanase PLUS, Dyadic Int., Jupiter, FL) and 1:1 mixture of both enzymes (MIX; CEL + XYL). Dyadic® Cellulase PLUS is a concentrated liquid acid cellulase (E.C. 3.2.1.4) enzyme produced from Trichoderma longibrachiatum (formerly Trichoderma reesei) containing 30,000–36,000 U/g of cellulase and 7500–10,000 U/g of beta-glucanase activity. Dyadic® Xylanase PLUS is a concentrated liquid acid-neutral endo-1,4-β-D-xylanase (E.C. 3.2.1.8) produced by the fermentation of T. longibrachiatum containing 34,000–41,000 U/g of xylanase activity. These activities were provided by the manufacturers and tested in a previous study (Elghandour et al. Citation2015).
The enzyme treatments (CEL, XYL and MIX) were applied at three doses, namely 0, 1 and 3 μL/0.5 g DM. The enzyme dosages were dissolved in distilled water and applied directly onto the substrate inside the bottles 24 h before adding buffer solution and ruminal fluid. Substrates in non-treated bottles (no enzyme) received 1 ml of distilled water without any added enzyme.
In vitro rumen fermentation
Two factors were studied under a factorial arrangement (three enzyme products × three enzyme doses) generating nine treatments (0, 1 and 3 μL/0.5 g DM of CEL; 0, 1 and 3 μL/0.5 g DM of XYL and 0, 1 and 3 μL/0.5 g DM of MIX). Samples of 500 mg of TMR (substrate) were accurately weighed in triplicate into 120-ml serum bottles with appropriate addition of enzyme products and dose levels.
Rumen inoculum was collected from four growing lambs (Pelibuey, 24 ± 0.3 kg live weight) fitted with permanent rumen cannula and fed ad libitum a total mixed ration made up of 50:50 commercial concentrate (PURINA®, Toluca, Mexico) and alfalfa hay formulated to meet all of their nutrient requirements (NRC Citation2007). Fresh water was available to lambs at all time during the rumen inoculum collection phase. Ruminal content of each animal was obtained immediately before the morning feeding, mixed and strained through four layers of muslin and then kept at 39 °C under a continuous CO2 stream. Ten millilitres of particle-free ruminal fluid and 40 ml buffer medium (containing micro- and macro-elements, a reducing agent and a reduction indicator of resazurin; Mauricio et al. Citation1999) were added to each bottle. Negative controls containing buffered rumen fluid with or without enzyme but no substrate, were also included in triplicate for correction of gas produced from small particles present in the ruminal fluid or sugars present in the enzyme products. Cumulative GP (ml/0.5 g DM) was recorded with a pressure transducer fitted with a microprocessor (HD 8804, Delta Ohm, Italy) at 6, 12, 19, 24, 48, 72 and 96 h post incubation at 39 °C. Volume of gas (ml/0.5 g DM) produced after 24 h of incubation (GP24) was used as an index of energy feed value.
At the end of incubation (96 h) bottles were uncapped, pH was measured using a pH metre (Conductronic pH 15, Puebla, Mexico) and contents of each serum bottle were filtered using filter bags (ANKOM F-57, ANKOM, Macedon, NY) under vacuum. Fermentation residues in the bags were dried at 105 °C overnight to estimate the DM disappearance.
Analytical procedures and degradability determination
Samples of TMR were analysed for dry matter (DM), ash and N content according to the AOAC methods (AOAC Citation1999). Neutral detergent fibre (NDF) and acid detergent fibre (ADF) content (Van Soest et al. Citation1991) were analysed using the ANKOM200 Fibre Analyser unit (ANKOM Technology, Macedon, NY). For NDF analysis, samples were treated with α-amylase (Sigma A-3403 Sigma-Aldrich® Co., St. Louis, MO), and the neutral detergent solution contained sodium sulphite and the residues were not corrected for residual ash. Hemicellulose content was calculated from the difference between NDF and ADF. The residue of 48 h of incubation was analysed for NDF and ADF to calculate fibre degradability (NDFD and ADFD, respectively).
Calculations
Estimates of kinetics parameters of GP (ml/0.5 g DM) were obtained using the NLIN procedure (version 9.2; SAS Institute, Cary, NC) according to France et al. (Citation2000) using the following model:
where GPt is the volume of GP at time t; b is the asymptotic GP (ml/0.5 g DM); c is the rate of GP (/h) and L (h) is the discrete lag time prior GP.
Metabolisable energy value of the TMR at 24 h (ME, MJ/kg DM) was estimated according to Menke and Steingass (Citation1988) as:
where GP24 is the volume of gas produced up to 24 h expressed as ml/0.5 g DM and CP is the percentage of crude protein on DM basis.
Short chain fatty acid (SCFA, mmol) concentrations were calculated according to Getachew et al. (Citation2002) as:
where GP24 is the 24-h net GP (ml/200 mg DM).
Statistical analysis
Data for in vitro ruminal fermentation were analysed using a randomised complete design with three enzyme products (CEL, XYL and MIX)×three dose levels (0, 1 and 3 μl/0.5 g DM) in a factorial arrangement with three repetitions (version 9.2; SAS Institute, Cary, NC). The linear model was:
where y = the dependent variable; μ = overall mean; E = effect of i-enzyme product; D = effect of j-dose level; ED = interaction between the i-enzyme product and j-dose level; and e = the residual error.
Significant interactions at p < 0.05 were detected for in vitro ruminal parameters. Linear and quadratic polynomial contrasts were used to determine effect of dose level on all dependent variables.
Results
Curves of cumulative GP modelled with the kinetic model of France et al. (Citation2000) for each of the enzyme product and dose level are shown in Figure . Estimated parameters of this model are presented in Table . Least squares and standard errors of GP at different times are also shown in Table . Curves of cumulative GP modelled with the kinetic model for enzymes products are shown in Figure .
Figure 1. Cumulative gas production (ml/0.5 g DM) from in vitro fermentation of a diet for growing lambs with different dose levels (0, 1 and 3 μL/0.5 g DM) of exogenous liquid enzyme products. CEL: cellulase; XYL: xylanase; MIX: mixture 1:1 of both products.
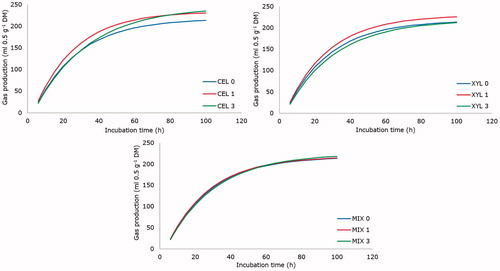
Figure 2. Cumulative gas production (ml 0.5/g DM) from in vitro fermentation of a diet for growing lambs with different exogenous liquid enzyme products. CEL: cellulase; XYL: xylanase; MIX: mixture 1:1 of both products.
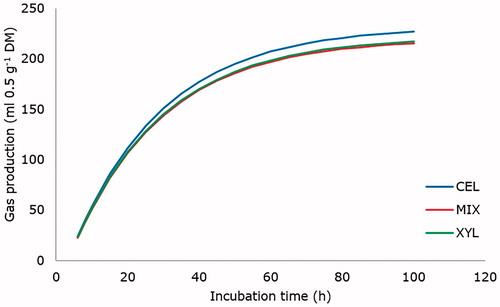
Table 2. In vitro rumen gas kinetics and cumulative gas production of a diet for growing lambs with different dose levels (0, 1 and 3 μL/0.5 g DM) of different exogenous liquid enzyme products.
A significant enzyme product by dose level interaction (p < 0.05) was found for cumulative GP up to 72 and 96 h of incubation. The asymptotic GP was affected (p < 0.02) by enzyme product and dose level, with a significant linear response (p < 0.05). The rate of GP (parameter c) and cumulative GP up to 6, 12, 19, 24 and 48 h after incubation was affected (p < 0.05) by dose level, but lag time (parameter L) was not affected. Dose level affected (p < 0.05) the rate of GP with a quadratic response (p < 0.01). Dose level also affected (p < 0.05) cumulative GP up to 6 h, 12 h, 19 h, 24 h and 48 h after addition of the enzyme product with a quadratic effect.
In vitro rumen fermentation profile (pH, ME and SCFA), DMD, NDFD and ADFD of the diet for growing lambs are shown in Table . Enzyme product affected (p < 0.05) pH, ADFD and DMD. Dose level affected ME and SCFA with a linear (p < 0.05) and quadratic (p < 0.01) response. The enzyme product by dose level interaction was significant (p < 0.05) for pH, DMD and ADFD.
Table 3. In vitro rumen fermentation profile, dry matter and fibre degradability of a diet for growing lambs with different dose levels (0, 1 and 3 μL/0.5 g DM) of different exogenous liquid enzyme products.
Discussion
Gas production in vitro appears related to the chemical composition of the feed, in particular to the fibre content and its structural polysaccharides (Jalilvand et al. Citation2008). Musco et al. (Citation2016) reported negative significant correlations between content of NDF in grass and in vitro organic matter degradability (r = −0.70) and fermentation rate of GP (r = −0.70). The fermentation of high concentrate diets, in ruminal fluid is generally enzyme-limited. In the present study, the addition of fibrolytic enzymes to the diet for growing lambs increased GP during the final period (GP72 and GP96) of fermentation, which may be a reflection of an increase in bacterial numbers, and hence, hydrolytic capacity of the ruminal fluid. This view is similar to previous hypotheses that exogenous enzymes increase fibrolytic activity due to the increased number of ruminal microbes (Colombatto et al. Citation2003b), and increased bacterial attachment and synergistic effects with hydrolysis of ruminal microorganisms. Another report (Nsereko et al. Citation2002) showed that the addition of a fibrolytic enzyme preparation increased the number of cellobiose-utilising, xylanolytic and amylolytic bacteria, but had no effect on the number of cellulolytic bacteria; the population density of Ruminobacter amylophilus was increased also by the addition of the fibrolytic enzyme. Moreover, Chung et al. (Citation2012) reported that Selenomonas ruminantium tended to increase linearly with increasing dose levels of a fibrolytic enzyme.
In this study, dose level had a significant effect on ME, SCFA and GP up to 6, 12, 19, 24 and 48 h of incubation after adding the enzyme. Togtokhbayar et al. (Citation2015) demonstrated that the addition of low and moderate dose levels (1.0–1.5 μL/g) of a commercial enzyme product with xylanase activity increases the GP at all the incubation times, using wheat straw as a substrate. However, in our study the highest dose level of xylanase (3 μL/0.5 g of growing lamb diet) may have prevented binding of enzymes to substrate receptors, which might have reduced proportional attachment by ruminal microorganism to fibre (Beauchemin et al. Citation2001). Colombatto et al. (Citation2003b) suggest that increasing the dose level of an enzyme from 1× to 5× increased the rate of GP, but dose levels of 10× were ineffective.
Enzymes produced by a variety of microbes are capable of degrading lignocellulosic materials to SCFA, but require substantive rumen retention time (Kumar et al. Citation2009). In our study, lag time of the GP curve was not affected by the enzyme product and also by the dose level. However, Colombatto et al. (Citation2003a, Citation2003b) mentioned that enzymes could degrade complex substrates to simpler forms at early stages of fermentation to allow faster ruminal microbial colonisation and fermentation. Some authors have suggested that pre-treatment of feed with enzymes could create a stable enzyme–feed complex (Kung et al. Citation2000), but others have indicated an alteration in fibre structure, which would stimulate microbial colonisation (Nsereko et al. Citation2000).
The major fibrolytic enzymes are cellulases and xylanases which degrade cellulose and hemicellulose, respectively. Some authors mention that the combination of products with different enzymatic activities (Eun & Beauchemin Citation2007) or the use of multi-enzyme (Gado et al. Citation2011; López et al. Citation2013) products may be more effective in degrading forage compared with enzymes of single activity. In our study the GP kinetics was better for CEL compared with XYL and MIX. These results clearly show that enzymes had different effects on diet components, and therefore, diet characteristics influenced the response. However, other mechanisms of action of enzymes than direct effects on cell wall should be involved, because CEL was effective in increasing degradation rate of substrate. It is possible that enzymes altered or weakened the cell wall structure, thus allowing earlier access of ruminal micro-organisms to cell contents and increasing the degradation rate of the diet (Nsereko et al. Citation2002; Morgavi et al. Citation2004; Giraldo et al. Citation2008), but these modifications were not reflected in changes with MIX and XYL. In contrast, Dıaz et al. (Citation2015) reported that the use of xylanase product from ruminal microorganism produced only subtle effects on GP of certain forages.
The interaction effect between enzyme product and dose level was significant for DMD and ADFD suggesting there is an optimal combination between enzyme product and dose level for a maximum value of DMD and ADFD. Addition of 1 μl/0.5 g DM resulted in the highest values of DMD and ADFD. Direct-fed enzymes have been shown to enhance microbial colonisation of feeds by increasing the numbers of ruminal fibrolytic microbes (Nsereko et al. Citation2002; Morgavi et al. Citation2004) resulting in an increased degradation rate of ruminal fibre (Giraldo et al. Citation2008), increased synthesis of ruminal microbial protein (Yang et al. Citation1999; Nsereko et al. Citation2002) and increased total tract digestibility (Gado et al. Citation2009, Citation2011). Responses in grain-fed sheep to enzyme supplementation have been variable with no changes in DM intake (Rojo et al. Citation2005) or total tract digestibility (McAllister et al. Citation1999), with no improvements in sheep live weight or feed conversion efficiency (McAllister et al. Citation2000; Mora-Jaimes et al. Citation2002).
Mendoza et al. (Citation2014) suggested that the response to exogenous enzymes depends on the quality of feed, particularly the proportion of the NDF that is potentially digestible in the rumen. They indicated that NDF contains three fractions classified according to the kinetics of digestion of the rumen: digestible, potentially digestible and indigestible. The potentially digestible fraction disappears from the rumen by passage and digestion. The indigestible fraction does not supply nutrients to the ruminant, passing undigested to appear in the faeces. These authors hypothesised that the addition of exogenous enzymes will improve the digestibility of forages that have high proportion of potentially digestible fraction. This hypothesis may explain well the observed positive effect of the enzyme product on the degradability of DM and ADF evaluated in this study, but apparently contradicts the not observed positive effect on the degradability of NDF. However, the diet for growing lambs formulated in this study had a low level of NDF (36.7%) with a low level corn stover (250 g/kg DM), suggesting that this diet had low level of potentially digestible NDF and therefore the effect of enzyme product on the degradability of NDF was not significant.
Conclusions
In conclusion, the effect of adding exogenous enzymes is influenced by factors such as type of enzyme product and dose level. The results found in this study suggest that application of exogenous cellulases has the potential to alter asymptotic GP and degradability of DM and ADF of the diet, but most of the results depend on enzyme product by dose level interaction. Future studies are required to find the ideal combination between enzyme product and dose level for optimising the degradation of ruminant feeds.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adesogan AT, Ma ZX, Romero JJ, Arriola KG. 2014. Ruminant nutrition symposium: improving cell wall digestion and animal performance with fibrolytic enzymes. J Anim Sci. 92:1317–1330.
- AOAC. 1999. Official methods of analysis. 16th ed. Gaithersburg (MD): Association of Official Analytical Chemists International.
- Beauchemin KA, Morgavi DP, McAllister TA, Yang WZ, Rode LM. 2001. The use of enzymes in ruminant diets. In: Garnsworthy PC, Wiseman J, editors. Recent advances in animal nutrition. Loughborough (UK): Nottingham University Press. p. 297–322.
- Chung YH, Zhou M, Holtshausen L, Alexander TW, McAllister TA, Guan LL, Oba M, Beauchemin KA. 2012. A fibrolytic enzyme additive for lactating Holstein cow diets: ruminal fermentation, rumen microbial populations, and enteric methane emissions. J Dairy Sci. 95:1419–1427.
- Colombatto D, Morgavi DP, Furtado AF, Beauchemin KA. 2003a. Screening of exogenous enzymes for ruminant diets: relationship between biochemical characteristics and in vitro ruminal degradation. J Anim Sci. 81:2628–2638.
- Colombatto D, Mould FL, Bhat MK, Morgavi DP, Beauchemin KA, Owen E. 2003b. Influence of fibrolytic enzymes on the hydrolysis and fermentation of pure cellulose and xylan by mixed ruminal microorganisms in vitro. J Anim Sci. 81:1040–1050.
- Colombatto D, Mould FL, Bhat MK, Owen E. 2007. Influence of exogenous fibrolytic enzyme level and incubation pH on the in vitro ruminal fermentation of alfalfa stems. Anim Feed Sci Technol. 137:150–162.
- Dıaz A, Ranilla MJ, Giraldo LA, Tejido ML, Carro MD. 2015. Treatment of tropical forages with exogenous fibrolytic enzymes: effects on chemical composition and in vitro rumen fermentation. J Anim Physiol Anim Nutr. 99:345–355.
- Elghandour MMY, Kholif Márquez-Molina O, Vázquez-Armijo JF, Puniya AK Salem AZM. 2015. Influence of individual or mixed cellulase and xylanase mixture on in vitro rumen gas production kinetics of total mixed rations with different maize silage and concentrate ratios. Turk J Vet Anim Sci. 39:435–442.
- Elghandour MMY, Salem AZM, Gonzalez-Ronquillo M, Bórquez JL, Gado HM, Odongo NE, Peñuelas CG. 2013. Effects of exogenous enzymes on in vitro gas production kinetics and ruminal fermentation of four fibrous feeds. Anim Feed Sci Technol. 179:46–53.
- Eun JS, Beauchemin KA. 2007. Assessment of the efficacy of varying experimental exogenous fibrolytic enzymes using in vitro fermentation characteristics. Anim Feed Sci Technol. 132:298–315.
- France J, Dijkstra J, Dhanoa MS, López S, Bannink A. 2000. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr. 83:143–150.
- Gado HM, Salem AZM, Odongo NE, Borhami BE. 2011. Effect of exogenous enzymes ensiled with orange pulp on digestion, blood metabolites and growth performance in lambs. Anim Feed Sci Technol. 165:131–136.
- Gado HM, Salem AZM, Robinson PH, Hassan M. 2009. Influence of exogenous enzymes on nutrient digestibility, extent of ruminal fermentation as well as milk production and composition in dairy cows. Anim Feed Sci Technol. 154:36–46.
- Getachew G, Makkar HPS, Becker K. 2002. Tropical browses: contents of phenolics compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid an in vitro gas production. J Agric Sci. 139:341–352.
- Giraldo LA, Tejido ML, Ranilla MJ, Carro MD. 2008. Effects of exogenous fibrolytic enzymes on in vitro ruminal fermentation of substrates with different forage:concentrate ratios. Anim Feed Sci Technol. 141:306–325.
- He ZX, He ML, Walker ND, McAllister TA, Yang WZ. 2014. Using a fibrolytic enzyme in barley-based diets containing wheat dried distillers grains with solubles: ruminal fermentation, digestibility, and growth performance of feedlot steers. J Anim Sci. 92:3978–3987.
- Jalilvand G, Odongo NE, López S, Naserian A, Valizadeh R, Eftekhar Shahrodi F, Kebreab E, France J. 2008. Effects of different levels of an enzyme mixture on in vitro gas production parameters of contrasting forages. Anim Feed Sci Technol. 146:289–301.
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P. 2009. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res. 48:3713–3729.
- Kung L, Jr, Treacher RJ, Nauman GA, Smagala AM, Endres KM, Cohen MA. 2000. The effect of treating forages with fibrolytic enzymes on its nutritive value and lactation performance of dairy cows. J Dairy Sci. 83:115–122.
- Lewis GE, Sanchez WK, Hunt CW, Guy MA, Pritchard GT, Swanson BI, Treacher RJ. 1999. Effect of direct-fed fibrolytic enzymes on the lactational performance of dairy cows. J Dairy Sci. 82:611–617.
- López D, Elghandour MMY, Salem AZM, Vázquez-Armijo JF, Salazar MC, Gado HM. 2013. Influence of exogenous enzymes on in vitro gas production kinetics and dry matter degradability of a high concentrate diet. Anim Nutr Feed Technol. 13:527–536.
- Martin C, Michalet-Doreau B. 1995. Variations in mass and enzyme activity of rumen microorganisms: effect of barley and buffer supplements. J Sci Food Agric. 67:407–413.
- Mauricio RM, Mould FL, Dhanoa MS, Owen E, Channa KS, Theodorou MK. 1999. Semi automated in vitro gas production technique for ruminant feedstuff evaluation. Anim Feed Sci Technol. 79:321–330.
- McAllister TA, Oosting SJ, Popp JD, Mir Z, Yanke LJ, Hristov AN, Treacher RJ, Cheng K-J. 1999. Effect of exogenous enzymes on digestibility of barley silage and growth performance of feedlot cattle. Can J Anim Sci. 79:353–360.
- McAllister TA, Stanford K, Bae HD, Treacher RJ, Hristov AN, Baah J, Shelford JA, Cheng K-J. 2000. Effect of a surfactant and exogenous enzymes on digestibility of feed and on growth performance and carcass traits of lambs. Can J Anim Sci. 80:35–44.
- Meale SJ, Beauchemin KA, Hristov AN, Chaves AV, McAllister TA. 2014. Board-invited review: opportunities and challenges in using exogenous enzymes to improve ruminant production. J Anim Sci. 92:427–442.
- Mendoza GD, Loera-Corral O, Plata-Pérez F, Hernández-García P, Ramírez-Mella M. 2014. Considerations on the use of exogenous fibrolytic enzymes to improve forage utilization. Sci World J. doi: 10.1155/2014/247437.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 28:47–55.
- Miller DR, Elliott R, Norton BW. 2008. Effects of an exogenous enzyme, Roxazyme® G2 liquid, on digestion and utilisation of barley and sorghum grain-based diets by ewe lambs. Anim Feed Sci Technol. 140:90–109.
- Moharrery A, Hvelplund T, Weisbjerg MR. 2009. Effect of forage type, harvesting time and exogenous enzyme application on degradation characteristics measured using in vitro technique. Anim Feed Sci Technol. 153:178–192.
- Mora-Jaimes G, Bárcena-Gama R, Mendoza-Martínez GD, González-Muñoz SS, Herrera-Haro J. 2002. Performance and ruminal fermentation in lambs fed sorghum grain treated with amylases. Agrociencia. 36:31–38.
- Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, McAllister TA, Iwaasa AD, Wang Y, Yang WZ. 2001. Resistance of feed enzymes to proteolytic inactivation by rumen micro organisms and gastrointestinal proteases. J Anim Sci. 79:1621–1630.
- Morgavi DP, Beauchemin KA, Nsereko VL, Rode LM, McAllister TA, Wang Y. 2004. Trichoderma enzymes promote Fibrobacter succinogenes S85 adhesion to, and degradation of, complex substrates but not pure cellulose. J Sci Food Agric. 84:1083–1090.
- Mould FL, Ørskov ER. 1983. Manipulation of rumen fluid pH and its influence on cellulolysis in sacco, dry matter degradation and the rumen microflora of sheep offered either hay or concentrate. Anim Feed Sci Technol. 10:1–14.
- Musco N, Koura IB, Tudisco R, Awadjihè G, Adjolohoun S, Cutrignelli MI, Mollica MP, Houinato M, Infascelli F, Calabrò S. 2016. Nutritional characteristics of forage grown in south of Benin. Asian-Australas J Anim Sci. 29:51–61.
- Noziere P, Besle JM, Martin C, Michalet-Doreau B. 1996. Effect of barley supplement on microbial fibrolytic enzyme activities and cell wall degradation rate in the rumen. J Sci Food Agric. 72:235–242.
- NRC. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington (DC): National Academy Press.
- Nsereko VL, Beauchemin KA, Morgavi DP, Rode LM, Furtado AF, McAllister TA, Iwaasa AD, Yang WZ, Wang Y. 2002. Effect of a fibrolytic enzyme preparation from Trichoderma longibrachiatum on the rumen microbial population of dairy cows. Can J Microbiol. 48:14–20.
- Nsereko VL, Morgavi DP, Rode LM, Beauchemin KA, McAllister TA. 2000. Effects of fungal enzyme preparations on hydrolysis and subsequent degradation of alfalfa hay fiber by mixed rumen microorganisms in vitro. Anim Feed Sci Technol. 88:153–170.
- Rojo R, Mendoza GD, González SS, Landois L, Bárcena R, Crosby MM. 2005. Effects of exogenous amylases from Bacillus licheniformis and Aspergillus niger on ruminal starch digestion and lamb performance. Anim Feed Sci Technol. 123–124:655–665.
- Togtokhbayar N, Cerrillo MA, Rodriguez GB, Elghadour MMY, Salem AZM, Urankhaich C, Jigjidpurev S, Odongo NE, Kholif AD. 2015. Effect of exogenous xylanase on rumen in vitro gas production and degradability of wheat straw. Anim Sci J. 86:765–771.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Wallace RJ, Wallace SJA, McKain N, Nsereko VL, Hartnell GF. 2001. Influence of supplementary fibrolytic enzymes on the fermentation of corn and grass silages by mixed ruminal microorganisms in vitro. J Anim Sci. 79:1905–1916.
- Yang WZ, Beauchemin KA, Rode LM. 1999. Effects of an enzyme feed additive on extent of digestion and milk production of lactating dairy cows. J Dairy Sci. 82:391–403.
- Yang WZ, Beauchemin KA, Rode LM. 2000. A comparison of methods of adding fibrolytic enzymes to lactating cow diets. J Dairy Sci. 83:2512–2520.
