Abstract
In the literature, there are no data on the effects of copper (Cu) on the development and birth defects of live-bearers freshwater fish embryos. The aim of this study was to investigate the influence of copper sulphate (CuSO4) pentahydrate on the development of embryos in the guppies (Poecilia reticulata). Guppies were exposed to concentration of 0.5, 1.0 and 1.5 mg/L of CuSO4 pentahydrate for 24 h. After 15 days, the female fish was euthanised and the embryos were dissected. No visible lesions were observed in the embryos of guppies exposed to 0.5 mg/L of CuSO4 pentahydrate. In the guppies exposed to 1.0 mg/L CuSO4 pentahydrate, the embryos showed visible abnormalities from blastodisc to middle-eyed stages of development. In the late (very late-eyed and mature embryo) stages embryos, the morphological abnormalities were not observed. The exposure to 1.5 mg/L of CuSO4 pentahydrate caused the death of guppies and their embryos during 24 h. In the light of these results, the 1.0 mg/L and higher dose of CuSO4 pentahydrate is not recommended for the treatment of guppies because this decreases the viability of guppies and causes morphological abnormalities and mortality in their embryos.
Introduction
Fish diseases are one of the causes of monetary loss for industrial pisciculturists and aquarium fish lovers. Fish are persistently bathed in potential pathogens, including bacteria, fungi and parasites. The copper sulphate (CuSO4) is worldwide used as an algaecide and a fungicide in aquaculture and agriculture. It inhibits growth of bacteria such as Salmonella spp., Pasteurella spp., Vibrio spp., Streptococcus spp., Aeromonas spp., Pseudomonas spp. and Edwardsiella spp. Therefore, CuSO4 also is used as a therapeutic medicament for various bacterial and ectoparasitic infections for industrial and aquarium fish (Straus & Tucker Citation1993; Harms Citation1996; Heo Citation1997; Mitchell et al. Citation2008; Straus Citation2008; Park & Heo Citation2009; Bebak et al. Citation2012; Liu et al. Citation2013; Nouh & Selim Citation2013). CuSO4 is used in the control of water plants in fish ponds too (Brown & Rattigan Citation1979; Mal et al. Citation2002). It is used for therapeutic purposes, reducing the incidence of fish parasites such as protozoa, trematodes, and external fungi and bacteria. In this case, recommended concentrations for fish therapeutic purposes usually ranges from 0.05 to 1.1 mg/L (Harms Citation1996; Rowland et al. Citation2008; Straus Citation2008; Hadfield & Clayton Citation2011; de Andrade Waldemarin et al. Citation2012).
CuSO4 can discharge into freshwater ponds where it can affect the freshwater fauna, especially fishes. Above a specific concentration, copper (Cu) is toxic to tilapias, sea breams, salmons, carps, catfishes and various aquarium fish, including the guppies (Straus & Tucker Citation1993; Clearwater et al. Citation2002; Bettini et al. Citation2006; Abdel-Tawwab et al. Citation2007; Varo et al. Citation2007; Park & Heo Citation2009; de Andrade Waldemarin et al. Citation2012; Nouh & Selim Citation2013; Shuhaimi-Othman et al. Citation2015).
In the available literature, we did not find any data on the effects of Cu on the development and birth defects of live-bearers freshwater fish embryos. Therefore, the aim of this study was to investigate the influence of CuSO4 pentahydrate on the development of embryos in the guppies (Poecilia reticulata).
Materials and methods
Twenty healthy female and eight male guppies (about half-year old) were used for this investigation. The research was carried out according to the law of the Republic of Lithuania on Welfare and Protection of Animals and the permission of Bioethical centre of Lithuanian University of Health Sciences (BEC-MF-278; 05-01-2015).
Prior to the experiment, the fishes were placed in the glass tank for adaptation and supplied with aerated tap water for five days. The water pH, hardness and temperature were 7.52, 2.50 mmol/L and 24 ± 1 °C, respectively. The fish were maintained on a 12 h light and 12 h dark photoperiod and water aeration for 12 h. During this time, they were fed ad libitum with commercial flake Tropical® (Tropical, Poland) food twice a day. After adaptation, guppies were divided into four groups. The control group (five females and two males) were kept at the same conditions for 15 days.
Three investigating groups (five female and two male guppies) were used for this study. Guppies of investigating group I were exposed to concentration of 0.5 mg/L, group II – 1.0 mg/L and group III – 1.5 mg/L of CuSO4 pentahydrate (CuSO4.5H2O, 99.9% purity) for 24 h. After 24 and 48 h, the ¼ part of water was renewed. Viability (mobility and appetite) of guppies of investigative groups were observed every day. After 15 days, the females of investigating groups were euthanised. The embryos were dissected from pregnant female guppies of control and investigating groups and placed in 0.9% NaCl solution. Changes in the embryos were analysed and photographed under photomicroscope (Stereo Microscope System, Olympus SZX16, Tokyo, Japan). Embryos of both groups were classified according to Martyn et al. (Citation2006) and Haynes (Citation1995). Results were analysed using the Statistica programme (Statistica Version 5, StatSoft inc., Tulsa, OK).
Results
Viability of guppies
The changes in the appetite and mobility were not observed in the guppies of investigative group I exposed to 0.5 mg/L and control group during 15 days. After exposure to 1.0 mg/L of CuSO4 pentahydrate, the guppies of investigative group II became sluggish, sleepy and had no appetite. After 24 h, when the ¼ part of water was renewed, the fish became agile and voracious again. All male and female fish of both investigative groups survived the entire investigation period (15 days). All female and male guppies exposed to 1.5 mg/L of CuSO4 pentahydrate (group III) died during 24 h.
Embryo development
The exposure to concentration of 0.5 mg/L and 1.0 mg/L of CuSO4 pentahydrate (CuSO4·5H2O) had no significant influence on the number of embryos in the guppies in comparison with control group (p > .05) (Table ).
Table 1. The number of embryos in the control and investigative groups per guppy.
Control group
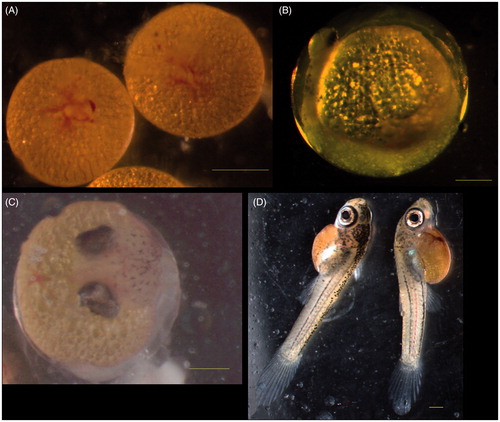
Total of 95 embryos were obtained from female guppies of control group. These embryos were classified according to Martyn et al. (Citation2006) and Haynes (Citation1995). Two female guppies showed synchronous development of a single group of embryos during their reproductive cycle. Three females had embryos of continuous stages of development (blastodisc and shield, optic cup and early–eyed, etc.). No visible lesions were observed in the developing embryos of all stages (Figure ).
Investigating groups
All females in the investigative groups I and II had embryos of continuous stages of development (blastodisc and shield, shield and optic cup, optic cup and early–eyed, etc). But, there were no embryos in the late-eyed stage of development in the investigative group II.
A total of 92 embryos were obtained from female guppies of investigating group I exposed to 0.5 mg/L of CuSO4 pentahydrate. No visible abnormalities were observed in the embryos of this group.
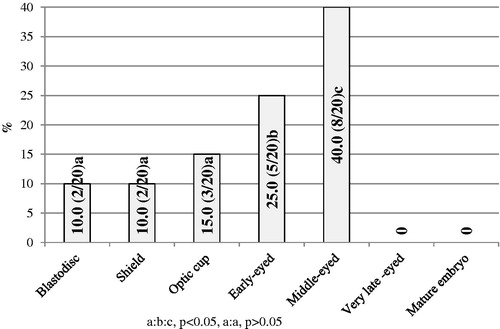
After 15 days, 90 embryos were obtained total from the female guppies exposed to 1.0 mg/L CuSO4 pentahydrate (investigative group II). In all, 20 of them (22.22%) showed visible abnormalities from blastodisc to middle-eyed stages of development. The most number of damaged embryos was found in the early- and middle-eyed stages (25 and 40%, respectively, p < .05). The number of abnormal embryos was similar in the blastodisc, shield and early-eyed stages (10%, 10 and 15%, respectively, p > .05) (Figure ).
Figure 2. The percentage of abnormal embryos in the guppies of investigative group II (abnormal/total with abnormalities).
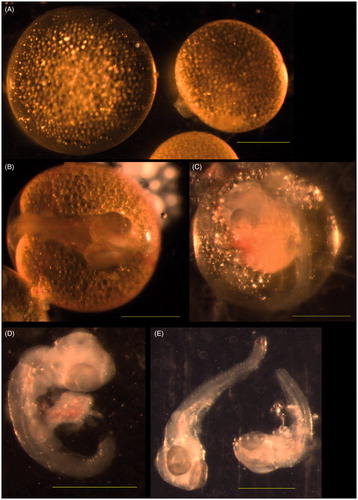
Various abnormalities were observed in the guppies’ embryos of investigating group II exposed to concentration of 1.0 mg/L CuSO4 pentahydrate. There were giant embryos with or without blastodisc (Figure ) in the blastodisc stage. The giant embryos were observed in the shield stage. The embryos in optic cup stage had too big optic cups (Figure ). Non-pigmented skin and yolk droplets in the amniotic cavity (Figure ) were observed in the middle-eyed stage. Died embryos without amnion and yolk sac were found in this stage of development too (Figure ). However, no lesions were observed in the embryos of late (very late-eyed and mature embryo) stages of development.
Figure 3. Abnormal embryos of group II guppies exposed to 1.0 mg/l copper sulphate. (A) Blastodisc stage. No blastodisc was observed in the left giant embryo. Blastodisc was visible in the right embryo of normal size. (B) Optic cup stage. Optic cups were too big. (C) Middle-eyed stage. No skin pigmentation was visible. Yolk droplets in the amnion. (D, E) Middle-eyed stage. Dead embryos without yolk sac and amnion. Bar =500 μm
Discussion
Cu plays an important role in cellular metabolism as a cofactor, participating in respiratory chain, neurotransmitter synthesis, iron metabolism and pigmentation (van den Berghe & Klomp Citation2010; da Silva et al. Citation2014). But, the elevated concentration of Cu in the water has high toxicity to fish. These effects include disturbances in ionic and endocrine regulation (Craig et al. Citation2010; da Silva et al. Citation2014). Acute and subacute toxicity tests for CuSO4 have been conducted using aquarium fish, including the goldfish, zebrafish and guppies (Liu et al. Citation2005; Bettini et al. Citation2006; Park & Heo Citation2009; Garceau et al. Citation2010; Chen et al. Citation2011; Leung et al. Citation2014; Ates et al. Citation2015; Shuhaimi-Othman et al. Citation2015).
There are limited references about the negative influence of Cu on the guppy. In the guppy (Poecilia reticulata), Cu and other heavy metals (Cd, Ni, Pb and Zn) can readily accumulate in the body of fish (Bochenek et al. Citation2008; Yap et al. Citation2008). Severe hyperplasia and exfoliation of the epithelial cells of gill lamellae and obstruction of the internal cavities of renal tubules with necrotised renal epithelial cells sloughed from the basement membrane were observed using 1.17 ppm of CuSO4 (Park & Heo Citation2009).
Bettini et al. (Citation2006) investigated the regeneration in the damaged olfactory mucosa of the Poecilia reticulata when they were returned to dechlorinated tap water after 4-day exposure to 30 μg/L of Cu2+. They estimated that after 10 days, regeneration seems to be complete and integrity of the tissue was restored.
Accumulation of Cu in the body of fish depends on the salinity of water. In the freshwater- acclimated guppies Poecilia vivipara, Cu accumulation was observed in gill and liver. In the saltwater-acclimated guppies, this was observed in the gut only (da Silva et al. Citation2014).
Shuhaimi-Othman et al. (Citation2010, Citation2015) observed that the median lethal concentrations (LC50) values of Cu increased with a decrease in mean exposure times for guppy Poecilia reticulata. The LC50 24 h value for Cu was 0.349 mg/L. The 48, 72 and 96 h LC50 values were 0.145, 0.061 and 0.038 mg/L, respectively.
The results of our study suggest with observation of these authors. In our experiment, all guppies died during 24 h when 1.5 mg of CuSO4 pentahydrate (CuSO4.5H2O) was added to 1 L of water (group III). This concentration of CuSO4 pentahydrate is equivalent to 0.381 mg/L of pure Cu. The supplement of 1.0 mg/L of CuSO4 pentahydrate (equivalent to 0.254 mg/L of pure Cu) (group II) had the negative influence on the guppies. We believe that the significant influence on the survival of the adult fish and their embryos had the renewal of the ¼ part of water after 24 and 48 h. The concentration of 0.5 mg/L of CuSO4 pentahydrate (equivalent to 0.127 mg/L of pure Cu) (group I) had no negative influence on the viability of fish.
Guppies are live-bearer freshwater fish. The male and female become sexually mature on the 56th day after their birth. The synchronously growing diplotene oocytes store nutrients in oil droplets and yolk, before their maturation and fertilisation. Guppies reproduce by internal insemination and fertilisation. Their embryonic development takes place within their mother’s reproductive system. The females give birth to fingerlings by contraction of the ventral muscles and this mechanism generates circular movements that allow the young fish to rapidly reach the free-swimming stage (Martyn et al. Citation2006; Rocha et al. Citation2010; Shahjahan et al. Citation2013). Studying the early development of live bearers is more complicated than that of oviparous species, due to the inaccessibility of developing embryos for experimental manipulation. Perhaps, there is no reliable data about the influence of CuSO4 on the development of guppy embryos.
In the guppies Poecilia reticulata, the gestation period ranged 25–35 days (28.1 ± 2.12 days) (Shahjahan et al. Citation2013). Some females could be pregnant during the beginning of our study (at the moment of exposure to 0.5, 1.0 and 1.5 mg/L of CuSO4 pentahydrate). Perhaps, cannibalism of offspring is observed in the guppies, when the pregnant females are not separated from the other male and female of the group. The offspring were born during the time of study, but we missed the moment to see and save the newborns. After delivery, the females were inseminated and pregnant with a new generation of offspring. Our study lasted for 15 days. At the moment of exposure to 1.0 mg/L of CuSO4 pentahydrate, some females could be pregnant, an average of 10–13 days (it is the likelihood, that the embryos were at optic cup, early-eyed or middle-eyed stages of development). After dissection of these female, we found the embryos without visible morphological abnormalities in very late-eyed and mature embryo stages of development. Therefore, it can be proposed that CuSO4 pentahydrate had no influence on developing embryos at optic cup, early-eyed or middle-eyed stages. However, if fertilisation took place after exposure of guppies to 1.0 mg/L of CuSO4 or females were fertilised at the moment of exposure and after exposure, CuSO4 pentahydrate caused the abnormalities in the developing embryos, such as embryos without blastodisc, giant embryos, embryos with too big optic cups, loss of the skin pigmentation, yolk droplets in the amniotic cavity and mortality of embryos. Therefore, it can be proposed that CuSO4 pentahydrate has a lasting effect.
CuSO4 is used for therapeutic purposes, reducing the incidence of fish parasites, such as protozoa, trematodes, and external fungi and bacteria. In this case, recommended concentration for fish therapeutic purposes usually ranges from 0.05 to 1.1 mg/L (de Andrade Waldemarin et al. Citation2012). However, in the light of the results of this study, we do not recommend to use the 1.0 mg/L and higher dose of CuSO4 pentahydrate for the treatment of guppies.
Conclusion
Our study showed that exposure to 0.5 mg/L and 1.0 mg/L of CuSO4 pentahydrate had no significant influence on the number of embryos in comparison with control group (p > .05). The exposure to 0.5 mg/L of CuSO4 pentahydrate had no influence on the embryo development in the guppies. The exposure to 1.0 mg/L of CuSO4 pentahydrate caused the morphological abnormalities and mortality in guppies’ embryos of early developmental stages, but had no negative influence in the development of the late stage embryos. The exposure to 1.5 mg/L of CuSO4 pentahydrate caused the death of guppies during 24 h. In the light of these results of this study, the 1.0 mg/L and higher dose of CuSO4 pentahydrate is not recommended for the treatment of guppies.
Acknowledgements
The authors wish to thank anonymous reviewer for helpful comments and English correction on an earlier draft of the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This study was funded by the Lithuanian University of Health Sciences.
References
- Abdel-Tawwab M, Mousa MAA, Ahmad MH, Sakr SFM. 2007. The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.). Aquacultur. 264:236–246.
- Ates M, Arslan Z, Demir V, Daniels J, Farah IO. 2015. Accumulation and toxicity of CuO and ZnO nanoparticles through waterborne and dietary exposure of goldfish (Carassius auratus). Environ Toxicol. 30:119–128.
- Bebak J, Garcia JC, Darwish A. 2012. Effect of Copper Sulphate on Aeromonas hydrophila Infection in Channel Catfish Fingerlings. North Am J Aquac. 74:494–498.
- Bettini S, Ciani F, Franceschini V. 2006. Cell proliferation and growth-associated protein 43 expression in the olfactory epithelium in Poecilia reticulata after copper solution exposure. Eur J Histochem. 50:141–146.
- Bochenek I, Protasowicki M, Brucka-Jastrzębska E. 2008. Studies on the bioavailability of heavy metals (Cd, Pb, Cu, Zn) from bottom sediments to guppies, Poecilia reticulata Peters. Arch Pol Fish. 16:155–166.
- Brown BT, Rattigan BM. 1979. Toxicity of soluble copper and other metal ions to Elodea Canadensis. Environ Pollut. 1970. 20:303–314.
- Chen D, Zhang D, Yu JC, Chan KM. 2011. Effects of Cu2O nanoparticle and CuCl2 on zebrafish larvae and a liver cell-line. Aquat Toxicol. 105:344–354.
- Clearwater SJ, Farag AM, Meyer JS. 2002. Bioavailability and toxicity of dietborne copper and zinc to fish. Comp Biochem Physiol C Toxicol Pharmacol. 132:269–313.
- Craig PM, Wood CM, McClelland GB. 2010. Water chemistry alters gene expression and physiological end points of chronic waterborne copper exposure in zebrafish, Danio rerio. Environ Sci Technol. 44:2156–2162.
- Da Silva ES, Abril SIM, Zanette J, Bianchini A. 2014. Salinity-dependent copper accumulation in the guppy Poecilia vivipara is associated with CTR1 and ATP7B transcriptional regulation. Aquat Toxicol. 152:300–307.
- De Andrade Waldemarin KC, Alves RN, Beletti ME, Rantin FT, Kalinin AL. 2012. Copper sulphate affects Nile tilapia (Oreochromis niloticus) cardiomyocytes structure and contractile function. Ecotoxicol. 21:783–794.
- Garceau N, Pichaud N, Couture P. 2010. Inhibition of goldfish mitochondrial metabolism by in vitro exposure to Cd, Cu and Ni. Aquat Toxicol. 98:107–112.
- Hadfield CA, Clayton LA. 2011. Fish quarantine: current practices in public zoos and aquaria. J Zoo Wildl Med. 42:641–650.
- Harms CA. 1996. Treatments for parasitic diseases of aquarium and ornamental fish. Semin Avian Exot Pet Med. 5:54–63.
- Haynes JL. 1995. Standardised classification of Poeciliid development for life-history studies. Copeia. 1995:147–154.
- Heo GJ. 1997. Antibacterial efficacy and safety of copper sulphate pentahydrate to cultured fish. Korean J Vet Res. 37:203–212.
- Leung KP, Chen D, Chan KM. 2014. Understanding copper sensitivity in zebrafish (Danio rerio) through the intracellular localisation of copper transporters in a hepatocyte cell-line ZFL and the tissue expression profiles of copper transporters. Metallomics. 6:1057–1067.
- Liu H, Zhang JF, Shen H, Wang XR, Wang WM. 2005. Impact of copper and its EDTA complex on the glutathione-dependent antioxidant system in freshwater fish (Carassius auratus). Bull Environ Contam Toxicol. 74:1111–1117.
- Liu SS, Wang CL, Zhang J, Zhu XW, Li WY. 2013. Combined toxicity of pesticide mixtures on green algae and photobacteria. Ecotoxicol Environ Saf. 95:98–103.
- Mal TK, Adorjan P, Corbett AL. 2002. Effect of copper on growth of an aquatic macrophyte, Elodea Canadensis. Environ Pollut. 120:307–311.
- Martyn U, Weigel D, Dreyer C. 2006. In vitro culture of embryos of the guppy, Poecilia reticulata. Dev Dyn. 235:617–622.
- Mitchell AJ, Darwish A, Fuller A. 2008. Comparison of tank treatments with copper sulfate and potassium permanganate for sunshine bass with ichthyobodosis. J Aquat Anim Health. 20:202–206.
- Nouh WG, Selim AG. 2013. Toxopathological studies on the effect of formalin and copper sulphate in tilapia as a commonly used disinfectant in aquaculture. J Appl Environ Biol Sci. 3:7–20.
- Park K, Heo G-J. 2009. Acute and subacute toxicity of copper sulphate pentahydrate (CuSO4*5H2O) in the guppy (Poecilia reticulata). J Vet Med Sci. 71:333–336.
- Rocha TL, Carvalho R, Yamada AT, Sabóia-Morais SMT. 2010. Morphologic analysis of developmental phases and gill ontogenesis in neotropical species Poecilia vivipara (Cyprinodontiformes: Poeciliidae) exposed to different salinities. Zool Curitiba. 27:554–562.
- Rowland SJ, Mifsud C, Nixon M, Read P, Landos M. 2008. Use of formalin and copper to control ichthyophthiriosis in the Australian freshwater fish silver perch (Bidyanus bidyanus Mitchell). Aquac Res. 40:44–54.
- Shahjahan R, Ahmed J, Begum RA, Rashid A. 2013. Breeding biology of guppy fish, Poecilia reticulata (Peters, 1859) in the laboratory. J Asiat Soc Bangladesh Sc. 39:259–267.
- Shuhaimi-Othman M, Nadzifah Y, Ahmad AK. 2010. Toxicity of copper and cadmium to freshwater fishes. World Acad Sci Eng Technol. 4:721–723.
- Shuhaimi-Othman M, Yakub N, Ramle NA, Abas A. 2015. Comparative toxicity of eight metals on freshwater fish. Toxicol Ind Health. 31:773–782.
- Straus DL. 2008. Comparison of copper sulphate concentrations to control Ichthyophthiriasis in fingerling channel catfish. J Appl Aquac. 20:272–284.
- Straus DL, Tucker C. 1993. Acute toxicity of copper sulphate and chelated copper to channel catfish Ictalurus punctatus. J World Aquac Soc. 24:390–395.
- van den Berghe PVE, Klomp LWJ. 2010. Posttranslational regulation of copper transporters. J Biol Inorg Chem. 15:37–46.
- Varo I, Nunes B, Amat F, Guilhermino L, Navarro JC. 2007. Effect of sublethal concentrations of copper sulphate on seabream Sparus aurata fingerlings. Aquat Living Resour. 20:263–270.
- Yap CK, Edward FB, Emila RAA, Ainey FI, Ismail A, Tan SG. 2008. Determination of contamination and bioavailabilities of heavy metals (Cu, Cd, Zn, Pb and Ni) in the Serdang urban lake by using guppy fish Poecilia reticulata. Trends Appl Sci Res. 3:69–75.