Abstract
Pidotimod is a synthetic dipeptide with immunomodulatory activity, but the capacity of pidotimod as an immunologic adjuvant to improve vaccine immunogenicity remains unclear. This study evaluated the effectiveness of pidotimod on immune responses of chickens to Newcastle disease (ND) vaccine, and its efficiency to improve protection against highly virulent ND virus (NDV). A total of 300 one-day-old broilers were divided into five groups (numbered I to V). Group I were unvaccinated and served as blank control group. Chickens in Group II to V were immunised with ND vaccine and supplemented with 0, 0.5, 1, 2 g/L of pidotimod in drinking water. The results showed that treatment with pidotimod (1 g/L) remarkably improved (p < .05) the potency of ND vaccine in chickens, as demonstrated by increased immune organ index (organ weight/body weight) and elevated antibody production. Meanwhile, no adverse effect on growth performance was observed. Moreover, as an adjuvant to ND vaccine, pidotimod (1 g/L) also dramatically promoted (p < .05) cell-mediated immunity, as characterised by increased lymphocytes proliferation and elevated ratio of CD4+ to CD8+ cells following NDV stimulation. Furthermore, administration of pidotimod (1 g/L) was efficient in inducing effective protection against lethal NDV challenge. Collectively, these data indicate that pidotimod may have the potential to be used as an effective vaccine adjuvant and the immunopotentiating effects deserve further study.
Introduction
Newcastle disease (ND) is a widespread, highly contagious viral disease, and causes severe economic losses to poultry industry (Ahmed et al. Citation2007; Miller et al. Citation2010; Ganapathy et al. Citation2014). Over the past decades, intensive vaccination programmes against ND have been practised widely (Xiao et al. Citation2009). To elicit potent protective immune responses against infection, these ND vaccines may require the use of adjuvants for optimal efficacy. Therefore, immunostimulants have been widely used as adjuvants to induce robust, long-lasting humoral and cellular immune responses, and thus improving the efficacy of vaccines (Onuigbo et al. Citation2012; Yu et al. Citation2015; Hou et al. Citation2016).
Pidotimod (3-l-pyroglutamyl-l-thiaziolidine-4-carboxylic acid) is a synthetic dipeptide with biological and immunological activity on innate and adaptive immune responses (Riboldi et al. Citation2009; Carta et al. Citation2013). Studies show that pidotimod is able to stimulate protective effects against bacterial and viral infections without any interaction with therapeutics in mice and humans (Coppi et al. Citation1994; Manzardo et al. Citation1994). In particular, administration of pidotimod has been shown to increase resistance to viral infections and make the antiviral drugs more effective for recurrent respiratory infection in children (Careddu et al. Citation1994). In a previous study, we have demonstrated that pidotimod is sufficient to facilitate murine macrophage polarisation and improves its function in vitro (Hu et al. Citation2014). Moreover, applications of pidotimod can also stimulate human dendritic cells to release high amounts of pro-inflammatory cytokines, thereby driving T cell proliferation and differentiation (Giagulli et al. Citation2009). The ability of pidotimod to promote strong humoral and cellular immune response has also been evaluated in different mouse models (Giagulli et al. Citation2009; Zhao et al. Citation2013). Despite this knowledge, there is little information about the effect of pidotimod as an immunologic adjuvant to improve vaccine immunogenicity. Therefore, this study was designed to evaluate the immune-potentiating effect of pidotimod as an adjuvant on immune responses of chickens to ND vaccine, and its efficiency to improve protection against highly virulent ND virus (NDV).
Materials and methods
Animals and ethics statement
One-day-old Arbour Acres commercial male broilers (purchased from Hewei Co., Ltd., Anhui, China) were housed in wire cages in air-conditioned room at 37 °C and lighted for 24 h at the beginning of pre-trial period. Then, temperature was gradually decreased to room temperature and the light time was reduced to 12 h/day, both of which were kept constant during the experiment. Commercial feed were purchased from Hangzhou Fujia Feed Co., Ltd. The diets supplied all essential nutrients required by Chinese Feeding Standard of Chicken (Ministry of Agriculture of China Citation2004) and NRC (Citation1994). Chickens were provided with food and water ad libitum. Efforts were made to minimise suffering. Animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee of Zhejiang University (ZJU2015-480-12). All procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of China (1988).
Experimental design
A total of 300 one-day-old broilers were randomly divided into five groups (numbered I to V), each consisting of 60 chickens. The 6-day-old chickens (Day 6) received pidotimod (Qilu Animal Health Products Co., Ltd. Jinan) in drinking water at low (Group III, 0.5 g/L), medium (Group IV, 1 g/L) and high (Group V, 2 g/L) dose, for five successive days. On Day 7, chickens with pidotimod treatment (Group III, IV, V) or without pidotimod treatment (Group II) were vaccinated with live attenuated Newcastle disease vaccine IV (LaSota strain, purchased from Beijing Veterinary Biological Product Factory, China) by the nose and eye dropping method (1 dose/chicken). To improve the potency of ND vaccine, chickens received pidotimod again on Day 21, 22, 28 and 29, respectively. Group I served as blank control group, and was not subjected to any treatment. Groups I, II, III, IV and V were named Control, Vac, Vac + PT-Low, Vac + PT-Med, and Vac + PT-High, respectively.
Growth performance
Body weight of the chickens from each group (n = 60 broilers/group, Group I to V) was measured on Day 14, 21, 28, and 35 using a digital balance.
Immune organ index
Chickens randomly selected from each group (n = 6/group, Group I to V) were weighed and humanely euthanized by cervical dislocation on Day 14 and 28, respectively. Spleen, thymus and bursa of Fabricius were immediately excised and weighed. The immune organ index was calculated: organo-somatic index = organ weight (g)/body weight (kg).
Serum haemagglutinin inhibition (HI) test
Blood sample of chickens were randomly collected from the brachial vein (n = 6/group, Group II to V) on Day 14, 21 and 28, and serum HI antibody assay was performed as previously reported (Nguyen et al. Citation2012). The highest dilution of serum causing complete inhibition was considered the end point. Titres were expressed as reciprocal log2 values of the highest dilution that displayed HI.
ND-specific IgG in chicken
Blood samples (n = 6/group, Group II to V) were randomly collected on Day 14, 21 and 28, respectively. Serum samples were prepared and analysed for IgG antibodies by IDEXX® ELISA kit (IDEXX Laboratories) according to the manufacturer’s instructions. The optical density (OD) was read with an ELISA plate reader at 630 nm.
Lymphocyte proliferation assay
Blood samples of chickens were collected randomly from each group (n = 6/group, Group I to V) on Day 28, and peripheral blood lymphocytes were isolated using Lymphocyte Separation Medium (Dakewe, Shenzhen, China). Cell proliferation was assessed by a colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Xiao et al. Citation2009). Briefly, 3 mL of blood samples was diluted with the same volume of sterilised Hank’s solution. Diluted blood was carefully layered over an equal volume of lymphocyte separation medium in another sterile test tube, followed by centrifugation at 2000 × g for 10–15 min. The lymphocyte layer formed was pipetted carefully to another tube and rinsed twice with Hank’s solution. Cells were seeded into 96-well plates (Costar, Shanghai, China) at a density of 5 × 106/mL. Cells were then left untreated or stimulated with formalin-inactivated NDV (1 multiplicity of infection (MOI)), or concanavalin A (ConA, 10 μg/mL). After 44 h of incubation in 5% CO2 at 41 °C, MTT (5 mg/mL) was added for another 4 h of incubation. Then, dimethylsulphoxide (DMSO) was added to dissolve the formazan crystals. Optical density (OD) was measured at 570 nm. The stimulation index was calculated as the OD value of stimulated culture divided by the OD of non-stimulated cultures.
Ratio of CD4+ and CD8+ T lymphocytes
Lymphocytes were prepared from peripheral blood samples (n = 6/group, Group I to VI) on Day 14, 21 and 28, respectively. Cells were incubated with formalin-inactivated NDV (1 MOI) for 24 h. Then, cells were collected and stained with fluorescent-conjugated antibodies against chicken CD3, CD4 and CD8. The ratio of CD4+ to CD8+ T-cell was determined by flow cytometry analysis with an Accuri C6® FACS machine (Zhao et al. Citation2016). Gates set on forward and side angle light scatter was used to exclude dead cells and debris.
Challenge experiment
Chickens (n = 20/group, Group I to Group V) were challenged by oculonasal injection of 106 EID50 (median egg infectious doses) of a virulent NDV strain (provided by College of Veterinary Medicine, China Agricultural University) at 35 days of age (Day 35). Following challenge, birds were monitored for survival and clinical signs twice daily for 7 days. Serum samples and lymphocytes (n = 6/group, Group II to Group V) were obtained 7 days after challenge (Day 42) for analysis of NDV-specific antibody response and ratio of CD4+ to CD8+ cells as previously described.
Statistical analysis
Statistical analysis was performed using GraphPad Prism® (GraphPad Software, San Diego, CA). Survival curves were compared using Kaplan–Meier analysis followed by log-rank test. One-way ANOVA with Tukey’s post hoc test was used to compare the parameters between groups. Data are expressed as means and SEM. Differences were considered significant at p < .05.
Results
Effect on growth performance
To investigate any adverse effect on growth performance of broilers, live body weight of chickens was recorded during the experimental period. As depicted in Figure , no significant differences were found in the average body weight of birds among groups. These results reveal no additive adverse effects of pidotimod on broiler growth performance.
Figure 1. Growth performance of chickens. Body weight of chickens from each group (n = 60 broilers/group, Group I to V) was measured on Day 14, 21, 28, and 35 using a digital balance. Data are expressed as means and SEM. Unless otherwise noted, significance was determined by one-way ANOVA with Tukey’s post hoc test.
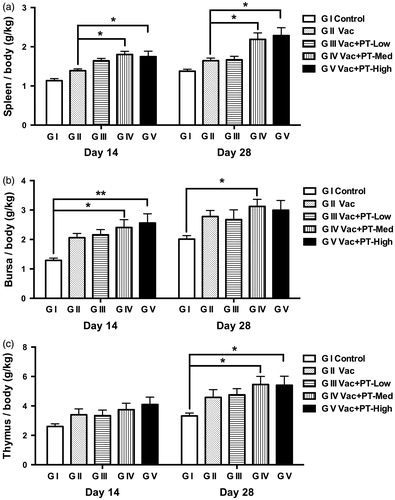
Changes in immune organ index
Spleen, bursa of Fabricius and thymus were weighed at slaughter, and the immune organ index was calculated. As illustrated in Figure , the spleen index of chickens in the medium- and high-dosage group was higher (p < .05) than those of vaccinated group (G II) on Day 14 and 28, respectively. However, the index of bursa (Figure ) and thymus (Figure ) was not changed by treatment with pidotimod compared with vaccinated group (G II). Notably, when compared with blank control group (G I), administration of medium- and high-dosage of pidotimod increased (p < .05) the bursa index (Figure ) on Day 14 and thymus index (Figure ) on Day 28, respectively. Furthermore, on Day 28, elevated bursa index was also observed in medium-dose group compared to non-vaccinated control group (p < .05) (Figure ).
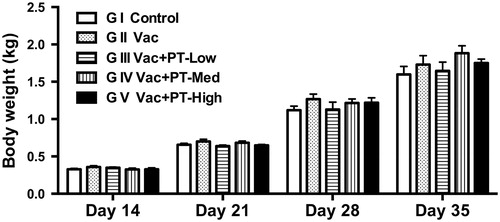
Changes in serum antibody response
In this study, no significant differences were observed in antibody levels against NDV between groups (Group I to V) before pidotimod administration (data not shown). Serum samples collected from each group after vaccination were analysed for antibody responses using HI test (Figure ) and ELISA assay (Figure ). On Day 21, serum antibody titres (Figure ) and concentrations (Figure ) of chickens receiving medium-dose (1 g/L) of pidotimod were higher than that of vaccinated group (G II) (p < .05 and p < .01, respectively). Additionally, the antibody levels in medium- and high-dose group were numerically higher than those in vaccinated group (G II) on Day 28 without significance. However, on Day 14, no change in antibody concentrations among groups was observed after vaccination.
Figure 3. Changes in serum antibody response against NDV in chickens. Blood were collected from each group on Day 14, 21 and 28, respectively. Serum samples were then analysed for antibody responses using HI test (a) and ELISA assay (b). Results are expressed as means and SEM (n = 6/group; *p < .05; **p < .01). NDV: Newcastle disease virus; OD: optical density.
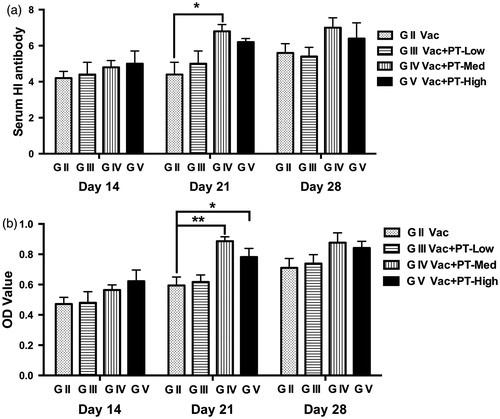
Changes of lymphocytes proliferation
To study the potential influence of pidotimod treatment on antigen- and mitogen-induced lymphocytes proliferation, peripheral blood lymphocytes were isolated and stimulated with formalin-inactivated NDV or ConA in vitro. As shown in Figure , lymphocytes proliferation in response to inactivated NDV or ConA was higher (p < .05) in PT-medium group compared with vaccinated (G II) and blank control group (G I). Moreover, a greater response was also observed in lymphocytes proliferation in high-dose group compared with vaccinated or blank control group, but did not reach significance. Additionally, lymphocytes isolated from control (G I), vaccinated (G II) or low-dose group (G III) showed similar proliferative responses after stimulation.
Figure 4. Proliferative responses of lymphocytes isolated from peripheral blood in chickens. Blood samples were collected on Day 28. The stimulation index was calculated as the optical density value of stimulated culture divided by the optical density of non-stimulated cultures. Results are expressed as means and SEM (n = 6/group; *p < .05; **p < .01). NDV: Newcastle disease virus; ConA: concanavalin A.
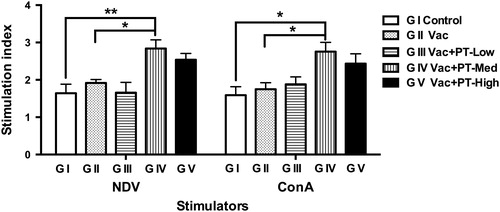
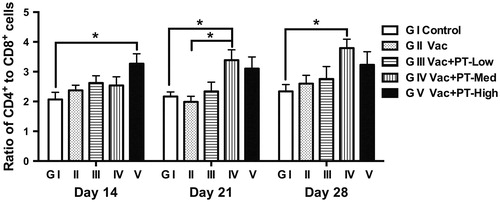
Changes in the ratio of CD4+ to CD8+ T cells
Peripheral blood lymphocytes were stimulated with inactivated NDV in vitro and the ratio of CD4+ to CD8+ T cells was determined by flow cytometry analysis. As shown in Figure , a higher (p < .05) ratio of CD4+ to CD8+ cells was observed in PT-high-dose group (G V) on Day 14 in comparison with the ratio in control group (G I). On Day 21, the ratio in PT-medium-dose group (G IV) was greater (p < .05) than those in blank (G I) or vaccinated control group (G II). Moreover, increased (p < .05) ratio in PT-medium-dosage group (G IV) was also noted on Day 28 compared to control group (G I). Further, lymphocytes without inactivated NDV stimulation showed a similar pattern of changes in the ratio of CD4+ to CD8+ cells, whereas there was no significant difference between groups (data not shown).
Figure 5. Ratio of CD4+ and CD8+ T lymphocytes. Lymphocytes were prepared from peripheral blood samples on Day 14, 21 and 28, respectively. Cells were stimulated with formalin-inactivated NDV (1 MOI) for 24 h. The ratio of CD4+ to CD8+ T cells was determined by flow cytometry analysis. Data are expressed as means and SEM (n = 6/group; *p < .05).
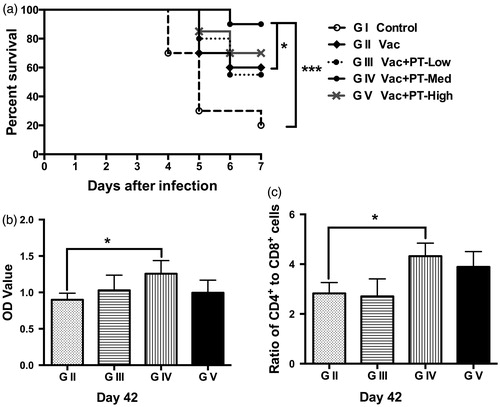
Protection against NDV challenge
As shown in Figure , unimmunised chickens infected with NDV exhibited early mortality, starting at 4 days post infection and reached 80% mortality (survival rate = 20%) by 7 days after inoculation. In contrast, vaccination delayed infection and increased survival rate to 60% at day 7 after the virus inoculation. Notably, chickens receiving medium-dose (1 g/L) of pidotimod exhibited the highest survival rate (90%), which was notably higher than vaccinated (G II) (p < .05) and control group (G I) (p < .001) (Figure ). Consistent with this observation, serum antibody response against NDV in PT-medium group was also greater (p < .05) than that in vaccinated group (G II) at day 7 after the virus inoculation (Day 42) (Figure 6). Furthermore, application of medium-dose of pidotimod also increased (p < .05) the ratio of CD4+ to CD8+ cells after stimulation with inactivated NDV on Day 42 (Figure ).
Figure 6. Protective effect of pidotimod against lethal NDV challenge. Chickens (n = 20/group) were challenged with 106 EID50 of a virulent NDV strain at 35 days of age. (a) Chickens were monitored twice daily for survival. Survival curves were compared using Kaplan–Meier analysis followed by log-rank test. (b) Antibody level and (c) the ratio of CD4+ to CD8+ T cells was analysed. Data are expressed as means and SEM (n = 6/group; *p < .05; ***p < .001). NDV: Newcastle disease virus.
Discussion
Immunologic adjuvants are often used to induce a more robust immune response to vaccine antigens, and thus providing increased immunity to a particular disease (Aguilar & Rodríguez Citation2007). However, many currently available adjuvants suffer from side toxicity or poor efficacy. Pidotimod, a synthetic immunostimulant, has been shown to improve the responses to inflammatory stimuli acting on different immunological pathways (Ferrario et al. Citation2015). Studies conducted in vivo and in vitro have shown its immune-modulation efficacy and safety in patients (Di Renzo et al. Citation1997; Namazova-Baranova et al. Citation2014). Therefore, the present study was conducted to evaluate the potentiating effect of pidotimod as a candidate adjuvant on immune responses of chickens to NDV.
In the current study, no adverse effect of pidotimod on growth performance of broilers was observed. The weight of central (bursa of Fabricius and thymus) and peripheral (spleen) lymphoid organs, which relatively reflects the immune function and disease resistance of chickens (Al-Khalifa et al. Citation2012; Rajput et al. Citation2013; Zhao et al. Citation2016), was also recorded during the experiment. Our results demonstrated that the indexes of immune organs in chickens receiving medium- or high-dose of pidotimod were significantly higher than those in vaccinated or non-vaccinated control group. These data are in accordance with the findings of Huo et al. (Citation2013), who showed that pidotimod restored spleen organ indexes in immunocompromised mice, suggesting that administration of pidotimod may contribute to immune system development.
Given that the dynamic changes of serum antibody levels reflect the state of humoral immunity in chicken (Khalifeh et al. Citation2009; Miller et al. Citation2013; Wang et al. Citation2013; Zhu et al. Citation2016), chicken serum samples were collected and analysed for antibody responses using HI test and ELISA assay in the current study. Our findings manifested that oral administration of a medium (1 g/L) or high dosage (2 g/L) of pidotimod could effectively improve the humoral response to ND vaccine when compared with the controls (Day 21). Noticeably, a trend of increase in antibody response was also observed in medium- and high-dose groups on Day 28. A previous study has described that, vaccinated mice treated with pidotimod had increased level of anti-toxoplasma IgG antibody, suggesting that pidotimod could stimulate strong specific humoral immunity in toxoplasma-infected mice (Zhao et al. Citation2013). In another study, Giagulli et al. (Citation2009) demonstrated that immunisation with ovalbumin co-administered with pidotimod resulted in strong humoral immune responses and enhanced antigen-specific antibody secretion in serum of mice. Consistent with these findings, our results confirm that addition of pidotimod can significantly promote antigen-specific antibody response, and thus improve the immune efficacy of the vaccine.
T lymphocyte proliferation is considered an important index to reflect cellular immunity and to evaluate the immune-enhancing activity of immunomodulators (Kong et al. Citation2004; Zhao et al. Citation2013). In a previous study, pidotimod-treated dendritic cells were able to drive T cell proliferation and stimulate naive T cell differentiation towards a Th1 phenotype (Giagulli et al. Citation2009). In our study, a significant increase in the T lymphocytes proliferation was observed in pidotimod (1 g/L) group in response to stimulation when compared with controls. This might indicate that, as an immunostimulant, pidotimod is able to promote T cell proliferation after stimulation. We also explored the effects of pidotimod on facilitating the differentiation and proliferation of CD4+ T helper cells and CD8+ cytotoxic T lymphocytes in peripheral blood stimulated by inactive NDV in vitro. It is well known that effective CD4+ and CD8+ T-cell responses are essential for eradicating acute viral infection and preventing viral persistence (Kapczynski et al. Citation2013; Uyangaa et al. Citation2015). Thus, the ratio of CD4+ to CD8+ cells has been identified as an evaluation of the immune system status and correlates strongly with immune responsiveness (Zhu & Paul Citation2010). The presented results showed that, lymphocytes of chickens treated with pidotimod had a remarkably higher ratio of CD4+ to CD8+ T cells compared to the controls, especially the PT-Medium (1 g/L) group. Huo et al. (Citation2013) further analysed CD4+ T cells subpopulation, showing that the percentage of CD4 + IFN-γ+ cells was remarkably recovered in immunocompromised mice co-administered with pidotimod. Collectively, these data suggest that pidotimod supplementation may contribute to the enhancement of cell-mediated immunity by promoting T lymphocytes proliferation and elevating the ratio of CD4+ to CD8+ T cells following NDV infection.
We further evaluated the protective effect of pidotimod treatment against lethal NDV challenge. As expected, unvaccinated birds infected with NDV exhibited early mortality and high mortality whereas vaccination delayed infection and increased survival rate after virus inoculation. In contrast, chickens receiving medium-dose of pidotimod exhibited the highest survival rate (90%), significantly higher than the controls. Previous report using immunosuppressed mouse model also showed that pidotimod treatment significantly increased survival rate and extended survival time in immunocompromised mice (Huo et al. Citation2013). Meanwhile, Zhao et al. (Citation2013) also reported that immunised mice co-administered with pidotimod had significant higher survival rate and longer survival time after challenged with viable parasites. Consistent with high survival rate, greater serum antibody response against NDV and increased ratio of CD4+ to CD8+ T-cell was also observed in chickens receiving medium-dose of pidotimod at day 7 after the virus inoculation. These results indicate that application of pidotimod (1 g/L) can enhance the potency of ND vaccine and induce effective protection against lethal NDV challenge.
Conclusions
From the present study it can be concluded that combination of dipeptide pidotimod and ND vaccine are synergistic for improving humoral and cellular immune responses, which may provide a better protection against viral infection. The immune-enhancing activity of pidotimod makes possible to use it as adjuvant molecule to modulate protective immune responses.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This work was supported by the grants from the planning subject of ‘the twelfth five-year-plan’ in national science and technology for the rural development in China [No. 2013BAD10B03].
References
- Aguilar JC, Rodríguez EG. 2007. Vaccine adjuvants revisited. Vaccine. 25:3752–3762.
- Ahmed KA, Saxena VK, Ara A, Singh KB, Sundaresan NR, Saxena M, Rasool TJ. 2007. Immune response to Newcastle disease virus in chicken lines divergently selected for cutaneous hypersensitivity. Int J Immunogenet. 34:445–455.
- Al-Khalifa H, Givens DI, Rymer C, Yaqoob P. 2012. Effect of n-3 fatty acids on immune function in broiler chickens. Poult Sci. 91:74–88.
- Careddu P, Mei V, Venturoli V, Corsini A. 1994. Pidotimod in the treatment of recurrent respiratory infections in paediatric patients. Arzneimittelforschung. 44:1485–1489.
- Carta S, Silvestri M, Rossi GA. 2013. Modulation of airway epithelial cell functions by pidotimod: NF-kB cytoplasmatic expression and its nuclear translocation are associated with an increased TLR-2 expression. Ital J Pediatr. 39:29.
- Coppi G, Falcone A, Manzardo S. 1994. Protective effects of pidotimod against experimental bacterial infections in mice. Arzneimittelforschung. 44:1417–1421.
- Di Renzo M, Pasqui AL, Bruni F, Saletti M, Bova G, Chiarion C, Girardello R, Ferrì P, Auteri A. 1997. The in vitro effect of pidotimod on some immune functions in cancer patients. Immunopharmacol Immunotoxicol. 19:37–51.
- Ferrario BE, Garuti S, Braido F, Canonica GW. 2015. Pidotimod: the state of art. Clin Mol Allergy. 13:8.
- Ganapathy K, Catelli E, Lemiere S, Montiel E, Jones RC. 2014. Protection conferred by a live avian Metapneumovirus vaccine when co-administered with live LaSota Newcastle disease vaccine in chicks. Ital J Anim Sci. 13:3227. doi: 10.4081/ijas.2014.3227.
- Giagulli C, Noerder M, Avolio M, Becker PD, Fiorentini S, Guzman CA, Caruso A. 2009. Pidotimod promotes functional maturation of dendritic cells and displays adjuvant properties at the nasal mucosa level. Int Immunopharmacol. 9:1366–1373.
- Hou R, Chen J, Yue C, Li X, Liu J, Gao Z, Liu C, Lu Y, Wang D, Li H, et al. 2016. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohydr Polym. 142:73–81.
- Hu S, Fu X, Fu A, Du W, Ji J, Li W. 2014. The regulatory peptide pidotimod facilitates M2 macrophage polarization and its function. Amino Acids. 46:1177–1185.
- Huo XX, Wang L, Chen ZW, Chen H, Xu XC, Zhang AM, Song XR, Luo QL, Xu YH, Fu Y, et al. 2013. Preventive effect of pidotimod on reactivated toxoplasmosis in mice. Parasitol Res. 112:3041–3051.
- Kapczynski DR, Afonso CL, Miller PJ. 2013. Immune responses of poultry to Newcastle disease virus. Dev Comp Immunol. 41:447–453.
- Khalifeh MS, Amawi MM, Abu-Basha EA, Yonis IB. 2009. Assessment of humoral and cellular-mediated immune response in chickens treated with tilmicosin, florfenicol, or enrofloxacin at the time of Newcastle disease vaccination. Poult Sci. 88:2118–2124.
- Kong X, Hu Y, Rui R, Wang D, Li X. 2004. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol. 4:975–982.
- Manzardo S, Falcone A, Pinzetta A, Ieva G, Coppi G. 1994. General pharmacology of pidotimod and testing for drug interactions. Arzneimittelforschung. 44:1441–1447.
- Miller PJ, Afonso CL, El Attrache J, Dorsey KM, Courtney SC, Guo Z, Kapczynski DR. 2013. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev Comp Immunol. 41:505–513.
- Miller PJ, Decanini EL, Afonso CL. 2010. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect Genet Evol. 10:26–35.
- Ministry of Agriculture of China. 2004. Feeding standard of chicken. Beijing, China: Standards Press of China.
- Namazova-Baranova LS, Alekseeva AA, SKharit SM, Kozhevnikova TN, Taranushenko TE, Tuzankina IA, Scarci F. 2014. Efficacy and safety of pidotimod in the prevention of recurrent respiratory infections in children: a multicentre study. Int J Immunopathol Pharmacol. 27:413–419.
- Nguyen TL, Wang D, Hu Y, Fan Y, Wang J, Abula S, Guo L, Zhang J, Khakame SK, Dang BK. 2012. Immuno-enhancing activity of sulfated Auricularia auricula polysaccharides. Carbohydr Polym. 89:1117–1122.
- NRC. 1994. Nutrient requirements of poultry. 9th ed. Washington (DC): National Academy Press.
- Onuigbo EB, Okore VC, Ofokansi KC, Okoye JO, Nworu CS, Esimone CO, Attama AA. 2012. Preliminary evaluation of the immunoenhancement potential of Newcastle disease vaccine formulated as a cationic liposome. Avian Pathol. 41:355–360.
- Rajput IR, Li LY, Xin X, Wu BB, Juan ZL, Cui ZW, Yu DY, Li WF. 2013. Effect of Saccharomyces boulardii and Bacillus subtilis B10 on intestinal ultrastructure modulation and mucosal immunity development mechanism in broiler chickens. Poult Sci. 92:956–965.
- Riboldi P, Gerosa M, Meroni PL. 2009. Pidotimod: a reappraisal. Int J Immunopathol Pharmacol. 22:255–262.
- Uyangaa E, Choi JY, Patil AM, Kim JH, Kim SB, Kim K, Ryu HW, Oh SR, Eo SK. 2015. Functional restoration of exhausted CD4(+) and CD8(+) T cells in chronic viral infection by vinegar-processed flos of Daphne genkwa. Comp Immunol Microbiol Infect Dis. 39:25–37.
- Wang M, Meng X, Yang R, Qin T, Li Y, Zhang L, Fei C, Zhen W, Zhang K, Wang X, et al. 2013. Cordyceps militaris polysaccharides can improve the immune efficacy of Newcastle disease vaccine in chicken. Int J Biol Macromol. 59:178–183.
- Xiao C, Bao G, Hu S. 2009. Enhancement of immune responses to Newcastle disease vaccine by a supplement of extract of Momordica cochinchinensis (Lour.) Spreng. seeds. Poult Sci. 88:2293–2297.
- Yu J, Shi FS, Hu S. 2015. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet Immunol Immunopathol. 167:147–155.
- Zhao X, Hu Y, Wang D, Liu J, Guo L. 2013. The comparison of immune-enhancing activity of sulfated polysaccharidses from Tremella and Condonpsis pilosula. Carbohydr Polym. 98:438–443.
- Zhao Y, Huang B, Huang S, Zheng H, Li YQ, Lun ZR, Shen J, Wang Y, Kasper LH, Lu F. 2013. Evaluation of the adjuvant effect of pidotimod on the immune protection induced by UV-attenuated Toxoplasma gondii in mouse models. Parasitol Res. 112:3151–3160.
- Zhao X, Sun W, Zhang S, Meng G, Qi C, Fan W, Wang Y, Liu J. 2016. The immune adjuvant response of polysaccharides from Atractylodis macrocephalae Koidz in chickens vaccinated against Newcastle disease (ND). Carbohydr Polym. 141:190–196.
- Zhu F, Liu X, Sun Z, Yu C, Liu L, Yang S, Li B, Wei K, Zhu R. 2016. Immune-enhancing effects of Taishan Pinus massoniana pollen polysaccharides on DNA vaccine expressing Bordetella avium ompA. Front Microbiol. 7:66.
- Zhu J, Paul WE. 2010. Heterogeneity and plasticity of T helper cells. Cell Res. 20:4–12.