Abstract
This study aimed to investigate the preservability and viability of the rabbit spermatozoa diluted in a new semen extender Formula® in comparison with Lepus® at 17 °C of storage. The main characteristic of the new extender formulation is the use of an enzymatic agent associated to a polysaccharide as energy source precursor, added with gentamycin. During eight trials, ejaculates from 70 bucks were collected and diluted at 1:10 ratio with both the extenders, after 24 h of storage the semen doses were used for the artificial insemination (AI). Aliquots of the semen doses for each trial were stored at 17 °C, the total and progressive motility were checked at 0, 4, 12, 18, 24, 36, 48, 60, 72, 84, 96, 108 h of storage. A total of 1267 and 1525 does were inseminated, respectively with Formula® and Lepus®. During storage the mean total and progressive motility (77.23% and 72.854%, respectively) were significantly higher for Formula® (p < .01) and the progressive motility at almost 70% was maintained for at least 60 h vs the 24 h of storage for Lepus® with significant differences after 12 h of storage (p < .05). The new extender reported a higher pregnancy rate (p < .05) and an average of 9.25 rabbits born per litter vs 8.83 for the traditional extender (p < .05), while the mean of the newborn alive was 9.08 using Formula® vs 8.51 with Lepus® (p < .05). In conclusion, the use of Formula® is recommended for rabbit semen AI programmes.
Introduction
The artificial insemination (AI) has been practiced in the rabbit intensive breeding for over 30 years (Sinkovicks et al. Citation1983; Facchin et al. Citation1988). In rabbits, as in the other livestock species, the AI benefits consist of a better reproductive management, such as the control of genetic improvement and variability, the planning of parturitions and weaning and the decrease of the infectious disease spreads (Morrell Citation1995; Castellini Citation1996). Since in rabbit industry a single ejaculated can be diluted to prepare almost fifty AI doses (Lavara et al. Citation2005), this technique is increased in most of the intensive rabbits farming in many European countries, as Italy, France, Spain and Hungary (Roca et al. Citation2000). A good spermatozoa progressive motility is a main male factor for the successful reproduction, so an easy but efficient evaluation of fresh or chilled semen is an important tool to avoid economic losses. For these reasons, in most of the AI centre the sperm motility is mainly assessed subjectively by visual scoring (Lavara et al. Citation2005). Currently, rabbit insemination is mostly performed with fresh diluted semen collected and conditioned on site or with cooled semen provided by a semen production farm, generally stored for no longer than 18 h (Morrell Citation1995; Roca et al. Citation2000; Brun et al. Citation2002; Nagy et al. Citation2002; Daniel & Renard Citation2010). The availability of semen that can be stored for longer periods would allow an extension of the interval between semen collection and insemination of the females (Di Iorio et al. Citation2014). Thus, enhancing AI performance in case of farms without male located far away from semen collection centres (Gogol, Citation2013). Previous researches showed that the fertilizing ability of rabbit spermatozoa was preserved for up to 48 h using chilled storage techniques (Roca et al. Citation2000; López-Gatius et al. Citation2005; Rosato & Iaffaldano Citation2011), thus enabling the transportation of superior genetics between nearby farms. Moreover, extending the interval of liquid semen storage beyond 48 h or freezing would facilitate the widespread transport of rabbit semen to more distant locations and this remains one of the major goals in the meat rabbit industry (Roca et al. Citation2000). Indeed, semen preservation is a prominent limiting factor for extensive commercial application programmes in rabbits (Curry Citation2000). The cryopreservation techniques for rabbits semen remain suboptimal and the use of frozen-thawed rabbit spermatozoa generally result in lower fertility rates (Mocé & Vicente Citation2009; Mocé et al. Citation2010; Rosato & Iaffaldano Citation2011). Only recently, a few reports have shown satisfactory results with frozen semen, nevertheless, the freezing technique is too expensive (Iaffaldano et al. Citation2012; Rosato & Iaffaldano Citation2013). For these reasons, freezing is not preferred while the preservation of fresh extended semen at temperature of 15–17 °C, to preserve vitality, is common in the rabbit industry. The temperature at which perturbation of the rabbit sperm cell occurs has not been established, the studies comparing various temperatures concluded that 15 °C is more appropriate than 5 °C to store chilled rabbit semen (Castellini Citation1996). The aims of the present study were to evaluate the in vitro total and progressive motility of rabbit semen during storage at 17 °C for 108 h and the reproductive efficiency (pregnancy rate, number of kits born alive) in rabbit AI of a new innovative semen extender (Formula®) in comparison with one (Lepus®) commonly used in four farms without bucks.
Materials and methods
Animals
In the present study, 70 adult bucks of different hybrid genetic lines, as Grimand, Ila, Hical, 6 months old and weighed 6–7 kg were considered. The semen donors were from the same rabbit semen reproductive centre located in the central part of Italy. A total of 2792 does belonging to four farms for meat production were enrolled in the study to evaluate the reproductive efficiency of the two extenders. The 2792 females were sexually mature hybrid rabbits, 130 days old and weighed about 4 kg. The doe’s farms followed the ‘all full-all empty’ method, with cycles of 40 days. In both kind of farms, the animals were housed in individual cages and pregnant females were kept in a separate area, with controlled light (16 h light/8 h dark photoperiod). Room temperature ranged from 16 to 28 °C. All the animals received a commercial diet and water provided ad libitum.
Preparation of the extender for rabbit semen
The Formula® (Medinova® Reggio Emilia, Italia) extender batch was prepared at a laboratory scale in an orbital mixer for 1 h at 32 rpm by mixing potassium chloride, gentamicin sulphate, sodium bicarbonate, disodium EDTA, sodium citrate hydrate, sucrose and invertase. The obtained mixture was divided into 51 g shares. The amount of sucrose and gentamicin were such to obtain a final concentration of1.28 g/L and 40 g/L, respectively while the invertase one (Maxinvert® 200000-DSM) was greater than 5000 SU/L (equivalent to >1640 U/L). On each morning of the days of semen collection (8 times), the share was diluted in 1 L of purified water in order to prepare 1 L of semen extender by means of a magnetic stirrer. The Lepus® (Medinova© Reggio Emilia, Italia) extender was also prepared by dissolving the powder in 1000 mL of purified water. The composition of Lepus® is undisclosed because of commercial interest. The extenders were maintained at 35 °C during the transport to the breeding centre until the semen collection.
Semen collection, processing and assessment of spermatozoa viability
All the bucks were of proved fertility and normally employed in the commercial production of semen doses for AI. Semen was collected three times a week in the afternoon using a warmed (40–45 °C) and lubricated artificial vagina (homemade by a technician) from male rabbits, housed in a farm dedicated only to the bucks far away from the female. After collection, any gel plug was removed with a disposable cellulose filter, sperm evaluation was performed to assess the initial seminal quality and only white opalescent ejaculates were used for the dilution. Sperm motility and morphological evaluations were performed on each fresh pooled sample to assess the seminal quality. Sperm motility was assessed at 37 °C using an optic microscope by the same trained technician. For each samples four fields were analysed and a minimum of 100 sperms were evaluated. Sperm concentration was evaluated using Burker’s chamber and in parallel a calibration curve by a photometer (Jenway LTD 60-51®, Bibby Scientific Equipment Division, Staffordshire, UK) was made. To be diluted the ejaculates shown at least 90% of forward progressive motility. The spermatozoa numbers of each doses was adjusted to 6 × 106 in 0.5 mL of both extenders (Lepus® and Formula®), stored at 17 °C, to be transported and used 24 h later at the farm. A minimum of dilution ratio of 1:10 (volume: volume) was utilised for semen doses preparation. For every semen pool (8) an aliquot for each extender was stored at 17 °C. The total and the progressive motility were checked at 0, 4, 12, 18, 24, 36, 48, 60, 72, 84, 96 and 108 h of storage, every 12 h until motility decreased below 40%. Visual scoring with a light microscope for both the extenders checked the motility parameters. Briefly, a drop (10 μL) of each diluted pooled semen was examined on warmed (37 °C) microscope slide overlaid with a coverslip and observed for sperm motility by phase contrast microscopy at 200× magnification in at least four fields on the slide taking the average of these readings to obtain the final estimate motility. On each of the fresh heterospermic-pooled sample, semen morphology was assessed using eosin–nigrosin staining following standard procedures (Shipley Citation1999) and Osmotic Resistance Test (ORT) was performed according to the Rosato and Iaffaldano (Citation2011) technique.
Synchronisation of oestrus, induction of ovulation, insemination and pregnancy diagnosis
Each insemination consisted of 6 million sperms in 0.5 mL and the following was performed in the morning after collecting the semen. For oestrus synchronisation a PMSG injection (20UI, Folligon®, Intervet, Italy) was performed before IA; while a gonadotropin releasing hormone (GnRH) analogue (e.g. buserelin acetate) 0.8 μg (Receptal® Hoechst, UK) administered by subcutaneous injection was performed to induce ovulation at the time of AI. A total of 2792 receptive females (identified by red vulvar lips) were inseminated, using a lubricated A.I. glass catheter (IMV Technologies®, France) with disposable AI plastic sheath (IMV, Technologies, France). Pregnancy diagnosis was performed by palpation 12 days after insemination, and confirmed at 21 days if the first diagnosis was negative. The pregnancy rate, the number of females that gave birth and the number of rabbit born and born alive per female were considered and recorded.
Statistical analysis
The data on spermatozoa motility (total motility and progressive motility at different storage time) were analysed by ANOVA (SAS Citation2008), using the type of extender (2 levels), time (12 levels: from 0 to 108 h) and interaction as fixed effects. Data from pregnancy rate and newborn rabbits were analysed by ANOVA (SAS Citation2008), using a linear model with type of extender (2 levels), herd (4 levels) and parity of female (3 levels: 1st, 2nd and >2nd parity) as fixed effects. The data relating to percentage of pregnancy have been previously processed to guarantee the normality.
Results
The mean sperm concentration of fresh pooled semen collected during the trials was 655 ± 71 × 106/mL, the mean osmotic resistance value was 93.74 ± 2.54%, while the percentage of morphological abnormal spermatozoa were 3.7 ± 1.3%. No significant differences were observed about the morphology and the membrane integrity (ORT) between the semen pools during the experiment (data not tabulated). Total sperm motility did not differ between the two extenders immediately after dilution. The overall total and progressive motility were 77.23% and 72.85% vs 62.50% and 55.53% for Formula® and Lepus®, respectively. The values during storage were significantly higher for the Formula® extender (p < .01). The Formula® maintained total motility at more than 70% for at least 72 h compared with 24 h of storage for the other extender (Figure ). The progressive motility was maintained over 70% for at least 60 h vs 18 h of storage for the other extender (Figure ), with significant differences for both parameters from 12 h of storage (p < .05). At 108 h the total and progressive motility declined at 56% and 45% for Formula®, while for Lepus® reached 30% and 20%, respectively already at 84 h of storage. The results regarding the AI trial are reported in Table . A total number of 2792 AIs were performed, 1267 of which were performed using the semen diluted by the extender object of this patent application (Formula®) and 1525 were carried out using the other extender (Lepus®). As shown in Table , the use of the rabbit semen diluted with the new extender reported a higher pregnancy rate (p < .05), compared to the results obtained using the traditional extender. Furthermore, with the new extender the number of rabbits born is on average 9.25 per litter, while using the traditional extender the average is 8.83, therefore the use of the new extender resulted in a significant increase of the number of rabbit born (p < .05). Regarding the mean of the newborn alive (9.08 using Formula vs 8.51 with Lepus) the difference between the two extenders was also significant (p < .05).
Figure 1. Effects of the two extenders (Formula®, Lepus®) on total motility of rabbit semen (least square means) × time interaction. a,bDifferent superscripts within the same time of storage indicate a significant difference (p < .05).
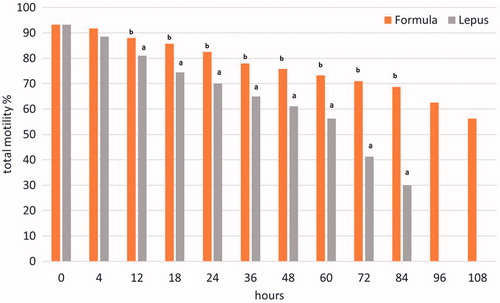
Figure 2. Effects of the two extenders (Formula®, Lepus®) on progressive motility of rabbit semen (least square means) × time interaction. a,bDifferent superscripts within the same time of storage indicate a significant difference (p < .05).
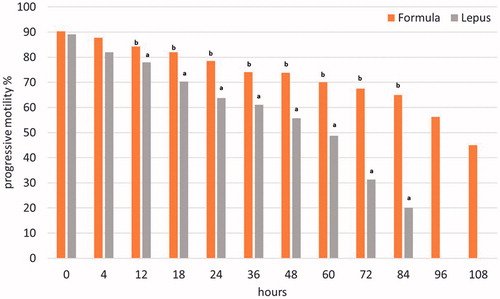
Table 1. Reproductive performances of rabbit does after insemination with semen diluted with Formula© and Lepus© stored at 17 °C for 24 h.
Discussion
The first objective of this study was to evaluate the total and progressive motility in rabbit spermatozoa diluted with Formula® in comparison to Lepus® extender, during storage at 17 °C. The results clearly show that when rabbit spermatozoa suspended in the first extender maintained a forward progressive motility of almost 70% till 72 h of storage at 17 °C. This successful result may be attributed to the metabolic activity of the spermatozoa, which use fructose as the major source of energy (El-Tarabany et al. Citation2015). In the present invention, the polysaccharide and/or oligosaccharide work as energy source precursors. Sperms cells metabolism is able to exploit various energy sources with different efficiencies, mainly simple sugars such as fructose (Bresciani et al. Citation2013), glucose mannose, sorbitol, ribose, galactose as well as non-monosaccharide energy source such as lactate, pyruvate, citrate, glycerol, glycerol-3-phosphate and short-chain fatty acids (Jones & Chantrill Citation1989; Jones & Bubb Citation2000). There is no indication that spermatozoa may use complex sugars, or that spermatozoa have membrane transporters suitable to internalise such molecules or the hydrolytic enzyme needed to obtain energy. For this reason, disaccharides (sucrose or trehalose) are present in extenders formulations for semen freezing mainly as non-permeating cryoprotective agents (Yildiz et al. Citation2000). The hydrolysis of sucrose by invertase is known: in 1928 Nelson et al. have prepared aqueous solutions of sucrose and invertase, and measured the rate of hydrolysis of sucrose. In spite of the wide use of the invertase enzymes in food industry(Nelson & Schubert, Citation1928), no disclosure exists wherein the saccharolytic enzymes are somehow associated with the preparation of formulations for animal semen extenders (Bettini et al. International Publication Number WO 2015/193265 A1). The use of sucrose associated to invertase is the innovative concept of our new extender formulation for cooled semen storage. The Authors belief is that the use of this metabolic way to provide energy exploiting the ‘time-reaction’ between invertase and sucrose to provide glucose and fructose in the medium allow the improvement to the efficiency of the extender during storage at 17 °C. The common purpose of the semen extender is to allow the increase of a single ejaculate, get more semen doses, as well as to improve spermatozoa vitality during storage. The effectiveness is based on the capacity to supply energy source needed to sustain the metabolic activity of sperm cells, to control pH and osmotic pressure of the medium and to inhibit the microbial growth. Since the true fertilising potential of an ejaculate can only be determined after using the semen to artificially inseminated females (Lavara et al. Citation2005), our reproductive performances results demonstrated the higher efficacy of the Formula® extender for rabbit AI programmes. Moreover, as reported from other studies, there is a positive correlation between sperm motility and the number of newborns delivered at birth (Ruiz-Sánchez et al., Citation2006). In our study, semen doses were used after 24 h of storage for the AI trial, because the four commercial farms enrolled do not accept change in the timing of semen preservation to avoid any risk of economic losses. Nevertheless, the use of Formula® extender allowed an improvement of the number of newborns and this result could be related to the higher spermatozoa motility. Further investigations are needed to evaluate the reproductive efficiency after 48 and 72 h of storage. Nowadays, the scientific research in rabbit reproduction meat industry is lacking both in diagnostic imaging evaluation (Aksoy et al. Citation2009; Volta et al.Citation 2014) as in the development of new innovative formulations to improve the shelf life of doses for AI and the reproduction efficiency. Recently, Gogol et al. (Citation2014) reported the effect of the GnRH analogue on the quality of rabbit spermatozoa stored at 17 °C for 3 days. The conclusions were that the extender supplementation with GnRH did not negatively affect the motility parameters of rabbit spermatozoa during storage but did not improve the reproductive efficiency. While Di Iorio et al. (Citation2014) made a comparison between three different extenders (Cortalap®, Lepus®, Merk III®) to assess the in vitro preservability of rabbit spermatozoa stored for 72 h at 5 °C. They demonstrated that storage of rabbit semen at 5 °C is not recommended for AI use, even if the extender Cortalap® that showed the best in vitro results has been used. As reported by Lavara et al. (Citation2005) in a study on a total of 2765 AI in rabbit a significant correlation exists between kindiling rate and the percentage of total motile cells in the semen doses. In our study, we evaluated the use of an innovative source of energy (sucrose) associated to hydrolytic enzyme for the rabbit spermatozoa metabolic needs during liquid storage at 15–17 °C (Bettini et al. International Publication Number WO 2015/193265 A1). Formula® semen extender allows a better condition for the sperm doses viability for a longer time useful for distance transport. Further investigations should be undertaken to identify the bacterial species and antibiotic susceptibility of bacterial contamination of rabbit semen as already reported in the boar semen, since this contamination could affect the quality and viability of rabbit semen storage (Bresciani et al. Citation2014; Bryła & Trzcińska, Citation2015; Sepúlveda et al., Citation2014, Citation2016).
Conclusion
In field, rabbit AI is usually carried out with fresh diluted semen within 6–12 h of collection (Roca et al. Citation2000), Formula® extender, over the good reproduction efficiency results obtained, allowed a good storage with an in vitro motility till 72 h of almost 70% of progressive motility. In conclusion, the results reported in this study allow us to confirm that Formula® is recommended for rabbit semen AI programmes and it could allow better doses management prolonging the shelf-life of each dose.
References
- Aksoy M, Erdem H, Hatipo?lu F, Lehimcio?lu NC, Akman O, Özkan K. 2009. Ultrasonographic examination of the scrotal content in the rabbit. Reprod Dom Anim. 44:156–160.
- Bettini R, Parmigiani E, Bresciani C, Bianchera A. 2015. International patent application PCT/EP2015/063394. International Publication Number WO 2015/193265 A1.
- Bresciani C, Morini G, Bettini R, Bigliardi E, Di Ianni F, Cabassi CS, Sabbioni A, Parmigiani E. 2013. Reproductive efficiency of a new modified boar semen extender for liquid storage. Livest Sci. 157:384–388.
- Bresciani C, Cabassi CS, Morini G, Taddei S, Bettini R, Bigliardi E, Di Ianni F, Sabbioni A, Parmigiani E. 2014. Boar semen bacterial contamination in Italy and antibiotic efficacy in a modified extender. Ital J Anim Sci. 13:83–87.
- Brun JM, Theau-Clément M, Bolet G. 2002. The relationship between rabbit semen characteristics and reproductive performance after artificial insemination. Anim Reprod Sci. 70:139–149.
- Bryła M, Trzcińska M. 2015. Quality and fertilizing capacity of boar spermatozoa during liquid storage in extender supplemented with different antibiotics. Anim Reprod Sci. 163:157–163.
- Castellini C. 1996. Recent advances in rabbit artificial insemination. World Rabbit Sci. 2:9–12.
- Curry MR. 2000. Cryopreservation of semen from domestic livestock. Rev Reprod. 5:46–52.
- Daniel N, Renard JP. 2010. Artificial insemination in rabbits. Cold Spring Harb Protoc. 2010. doi:10.1101pdb.prot.5358.
- Di Iorio M, Marchisi A, Rocco M, Chrenek P, Iaffaldano N. 2014. Comparison of different extenders on the preservability of rabbit semen stored at 5 °C for 72 hours. Ital J Anim Sci. 13:710–714.
- El-Tarabany MS, El-Bayomi K, Abdelhamid T. 2015. Semen characteristics of purebred and crossbred male rabbits. PLoS One. 10:e0128435.
- Facchin E, Zanirato MG, Gualterio L, Valentini A. 1988. AI in rabbit breeding. Note 1: AI service for meat rabbit breeding. In: Proceedings of 4th World Rabbit Congress, Budapest, Hungary; 2: 121–130.
- Gogol P. 2013. Motility parameters and intracellular ATP content of rabbit spermatozoa stored for 3 days at 15 degrees C. Folia Biol (Krakow). 61:87–91.
- Gogol P, Trzcińska M, Bryla M. 2014. Motility, mitochondrial membrane potential and ATP content of rabbit spermatozoa stored in extender supplemented with GnRH analogue [des-Gly10, D-Ala6]-LH-RH ethylamide. Polish J Vet Sci. 17:571–575.
- Iaffaldano N, Di Iorio M, Rosato MP. 2012. The cryoprotectant used, its concentration, and the equilibration time are critical for the successful cryopreservation of rabbit sperm: dimethylacetamide versus dimethylsulphoxide. Theriogenology. 78:1381–1389.
- Jones AR, Chantrill LA. 1989. Glycolytic enzyme activity is essential for domestic cat (Felis catus) and cheetah (Acinonyx jubatus) sperm motility and viability in a sugar-free medium. Reprod Fertil Dev. 1:357–367.
- Jones AR, Bubb WA. 2000. Substrates for endogenous metabolism by mature boar spermatozoa. J Reprod Fertil. 119:129–135.
- Lavara R, Mocè E, Lavara F, Viudes De Castro MP, Vicente JS. 2005. Do parameters of seminal quality correlate with the results of on farm inseminations in rabbits? Theriogenology. 64:1130–1141.
- López-Gatius F, Sances G, Sancho M, Yániz J, Santolaria P, Gutiérrez R, Núñez M, Núñez J, Soler C. 2005. Effect of solid storage at 15 degrees C on the subsequent motility and fertility of rabbit semen. Theriogenology. 64:252–260.
- Mocé E, Lavara R, Vicente JS. 2010. Effect of cooling rate to 5°C straw size and farm on fertilising ability of cryopreserved rabbit sperm. Reprod Domest Anim. 45:1–7.
- Mocé E, Vicente JS. 2009. Rabbit sperm cryopreservation: a review. Anim Reprod Sci. 110:1–24.
- Morrell JM. 1995. Artificial insemination in rabbits. Br Vet J. 151:477–488.
- Nagy S, Sinkovics G, Kovács A. 2002. Viability and acrosome integrity of rabbit spermatozoa processed in a gelatin-supplemented extender. Anim Reprod Sci. 70:283–286.
- Nelson JM, Schubert MP. 1928. Water concentration and the rate of hydrolysis of sucrose by invertase. J Am Chem Soc. 50:2188–2193.
- Roca J, Martınez S, Vázquez JM, Lucas X, Parrilla I, Martınez EA. 2000. Viability and fertility of rabbit spermatozoa diluted in Tris-buffer extenders and stored at 15 degrees C. Anim Reprod Sci. 64:103–112.
- Rosato MP, Iaffaldano N. 2011. Effect of chilling temperature on the long-term survival of rabbit spermatozoa held either in a Tris-based or a jellified extender. Reprod Domest Anim. 46:301–308.
- Rosato MP, Iaffaldano N. 2013. Cryopreservation of rabbit semen: comparing the effects of different cryoprotectans, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectans on sperm survival and fertility after standard vapour freezing and vitrification. Theriogenology. 79:508–516.
- Ruiz-Sánchez AL, O’Donoghue R, Novak S, Dyck MK, Cosgrove JR, Dixon WT, Foxcroft GR. 2006. The predictive value of routine semen evaluation and IVF technology for determining relative boar fertility. Theriogenology. 66:736–748.
- SAS. 2008. SAS/STAT® User’s Guide Version 9.2. Cary (NC): SAS Institute Inc.
- Sepúlveda L, Bussalleu E, Yeste M, Bonet S. 2014. Effects of different concentrations of Pseudomonas aeruginosa on boar sperm quality. Anim Reprod Sci. 150:96–106.
- Sepúlveda L, Bussalleu E, Yeste M, Bonet S. 2016. Effect of Pseudomonas aeruginosa on sperm capacitation and protein phosphorylation of boar spermatozoa. Theriogenology. 85:1421–1431.
- Shipley C. 1999. Breeding soundness examination in the boar. Swine Health Prod. 7:117–120.
- Sinkovicks G, Medgyes I, Paljak J. 1983. Some results of artificial insemination in rabbits. J Appl Rabbit Res. 6:43–48.
- Volta A, Manfredi S, Vignoli M, Russo M, England GCW, Rossi F, Bigliardi E, Di Ianni F, Parmigiani E, Bresciani C, Parmigiani E. 2014. Use of contrast-enhanced ultrasonography in chronic pathologic canine testes. Reprod Dom Anim. 49:202–209.
- Yildiz C, Kaya A, Aksoy M, Tekeli T. 2000. Influence of sugar supplementation of the extender on motility, viability and acrosomal integrity of dog spermatozoa during freezing. Theriogenology. 54:579–585.
