Abstract
Several studies reveal that coenzyme Q (CoQ) and vitamin E (Vit. E) act against oxidative deterioration, and that CoQ restores the active and antioxidant form of Vit. E. These two antioxidants, acting against lipid peroxidation, seem to be able to improve motility parameters of spermatozoa. The objective of this study is to evaluate the addition of CoQ and Vit. E to semen extender for equine spermatozoa in order to evaluate possible effects on semen motility. First, immediately after collection, semen samples were diluted with 1mM of CoQ and 1mM of CoQ plus 1mM of Vit. E and prepared for frozen storage in liquid nitrogen. After thawing (37 °C/30 s), samples were maintained at 37 °C and subjected to analysis after 0, 2 and 4 h for motility parameters with CASA (Computer-Assisted Sperm Analysis) method. In a second experiment, after the collection, semen samples were diluted with 1mM of CoQ, in presence or absence of seminal plasma where Vit. E is normally present, and prepared for cooled storage at 4 °C. The effects on motility parameters were determined with CASA at 0, 24, 31 and 48 h after collection. During the analysis, samples were kept at 4 °C. The CASA variables were examined with a mixed linear model. No improvement (p > .05) in motility parameters results from the addition of CoQ and Vit. E in frozen or cooled-stored equine semen when compared to control group.
Introduction
In 1943, John MacLeod associated male infertility to oxidative stress (OS); he related the loss of sperm motility to the increased oxygen tension in human semen (Griveau & Le Lannou Citation1997). The OS occurs during normal physiological processes at cellular level and it is associated to the disruption of the physiological equilibrium between production and elimination of reactive oxygen species (ROS), which can lead to sperm damages and male infertility (Aitken & Roman Citation2008; Bansal & Bilaspuri Citation2010).
In the ejaculate, sources of ROS could come not only from spermatozoa mitochondria, but also from leucocytes, such as neutrophils and macrophages (Bansal & Bilaspuri Citation2010). Oxidative sperm damages include DNA fragmentation, altered mobility and reduced capacity of the spermatic membrane to fuse with oocytes (Aitken & Roman Citation2008). The susceptibility of spermatozoa to OS damage is due to (i) large amounts of polyunsaturated fatty acids (PUFAs) in phospholipid membrane; (ii) a limited anti-oxidising capacities of the cell; and (iii) the great ability of spermatozoa to generate ROS, although indispensable, at physiological levels, for adequate sperm functionality (Sharma & Agarwal Citation1996; Agarwal et al. Citation2006; Bansal & Bilaspuri Citation2010).
The testicles are naturally equipped with large amount of antioxidants, which can be traced back both inside the spermatozoa and in the seminal plasma (Sharma & Agarwal Citation1996; Agarwal et al. Citation2006). The removal of the plasma in equine semen is still contradictory. In fact, its presence causes a reduction of sperm motility due to substances contained (Carver & Ball Citation2002). However, some of its components, like proteins, ROS inhibitors or antioxidants, seem to help in maintaining a good motility and the integrity of the acrosome, protecting spermatozoa from cold shock damage and helping the fertilisation process (Crockett et al. Citation2001; Pérez-Pé et al. Citation2001; Troedsson et al. Citation2005; Aitken & Roman Citation2008; Ball Citation2008).
Antioxidants have been successfully used to treat male infertility and to improve motility and membrane integrity in cryopreservation process in man, bull, ram, goat, boar and dog (Bansal & Bilaspuri Citation2010). Among substances with recognised antioxidants effect, coenzyme Q (CoQ) has been identified as a natural lipophilic organic compound preventing OS on spermatozoa; it is present in the mitochondria of spermatozoa and in seminal plasma and its addition to semen has given good results in preventing male infertility (Balercia et al. Citation2004). The CoQ occurs in three redox states: ubichinone, ubisemichinone and ubichinol, which allow it to act both as antioxidant and as pro oxidant. As antioxidant, CoQ interferes with both the onset and propagation of lipid peroxidation, and indirectly by regenerating the antioxidant form of vitamin E (Vit. E) (Bentinger et al. Citation2007).
The Vit. E is a powerful lipophilic antioxidant, it exists in different isoforms, four tocopherols and four tocotrienols, and the more abundant form found in mammalian tissue is α-tocopherol (Aitken & Roman Citation2008). The α-tocopherol is absolutely vital for the maintenance of mammalian spermatogenesis (Almeida & Ball Citation2005). Mammalian cells do not synthesise this chain breaking antioxidant, and only membrane tocopherol is consumed by OS creating a relatively stable radical tocopheroxyl (Almeida & Ball Citation2005). Previous studies have demonstrated that the administration of Vit. E improves sperm survival, membrane integrity and reduces the loss of motility after 48 h of storage (Bansal & Bilaspuri Citation2010).
The ROS production and the consequent spermatozoa damages increase during the processing and conservation of semen, leading to a significant decrease in fertility (Ball Citation2008; Bansal & Bilaspuri Citation2010). Semen motility is the most sensitive indicator of OS in equine semen; indeed, ROS cause a reduction of ATP production and damages to the axoneme (Baumber et al. Citation2000).
The purpose of the present study is to determine if the addition of exogenous antioxidants, CoQ and Vit. E, influenced the motility parameters of equine frozen or cooled semen. We decided to use the concentration of 1mM of each antioxidant according to results previously obtained in boar semen (Pindaru et al. Citation2015). In the first part, we evaluate the effectiveness of the addition of CoQ and CoQ plus Vit. E in frozen semen; in the second part, we assess if the CoQ, in the presence or absence of antioxidants contained in seminal plasma, improves sperm functions in cooled semen.
Materials and methods
Semen collection
Semen was obtained by means of a Missouri model artificial vagina on a phantom and collected through a 150 × 60 mm cellulose filter (Minitübe, Germany) to eliminate the gel fraction. Each animal provided semen samples three times at 48 h intervals, to obtain three replicate per stallion in each experiment. Descriptive statistics of semen collected from each stallion are reported on Table .
Table 1. Descriptive statistics obtained after semen collection from CASA analysis for each stallion (indicated with letters) used in part 1 and part 2 of the experiment.
Reagents
Unless otherwise indicated, all reagents used in the experiment were obtained from Sigma–Aldrich Company (St. Louis, MO). We used: (±)-α-Tocopherol, DL-all-rac-α-Tocopherol as Vit. E and coenzyme Q10 ≥98% (HPLC) as CoQ.
Experimental design
Effects of addition of CoQ and Vit.E in frozen semen
Five Standardbred stallions were used (age range 5–16 years), stabled in boxes measuring 5 × 5 m and fed twice a day with meadow hay and a mixture of cereals (33% barley flakes; 33% oats; and 34% corn). The collected semen was divided into two aliquots, diluted 1:1 with a commercial extender Equipro (Equipro, Minitüb, Germany) and centrifuged at 1400 g/min for 14 min at 4 °C. Once the seminal plasma had been eliminated, the sperm pellets were diluted to a final concentration of 100 × 106 sperm/ml with Equipro (untreated control, CTR group), or with Equipro added with CoQ (at a concentration of 1mM; Q group), or with Equipro added with CoQ plus Vit. E (at a concentration of 1 mM for each substance; Q + E group). After dilution, in all three groups 4% egg yolk and 3% glycerol were added, and the extended semen was equilibrated for 60 min at 4 °C to allow the glycerol to penetrate. Straws were tagged and filled with suitable pipettes, loading with 0.5 ml of semen, each. Straws were then sealed by ultrasound and frozen in an automatic Ice Cube model 1800 (SY-LAB), with a freezing curve based on a temperature reduction of 5 °C/min from +4 to −15 °C (freezing point) and then by a reduction of 20 °C/min from −15 to −140 °C. Straws were then stored in liquid nitrogen. Analyses were carried out in all treatment group in the pre-freezing phase (i.e. at the beginning of equilibration phase), and after freezing at 0 (H0), 2 (H2), and 4 h (H4) from thawing, by mixing for each treatment group and replicates straws belonging to the same batch. Before post freezing analysis, the semen was thawed over a water bath at 37 °C for 30 s and maintained in Eppendorf tube in water bath at 37° C up to the last analysis carried out following thawing.
Effects of addition of CoQ in cooled semen
Four Standardbred stallions (age range 9–15 years) and four Holstein stallions (age range 8–19 years) were used. Animals were housed in 6 × 5 m stables and fed with alfalfa hay and a mixture of cereals (30% flaked barley, 30% oats, 10% corn) containing also 30% of pellet based on wheat bran and soybean meal. In each day of collection, each semen sample was divided into three aliquots as follows: the first contained 0.5 ml of semen added with 2.5 ml of Equipro plus CoQ at a final concentration of 1 mM (plasma and CoQ, P + Q group); the spermatozoa of the second and third group, once the seminal plasma had been eliminated by centrifugation at 1600 g/min for 8 min, were re-suspended with 2.5 ml of Equipro (untreated control, CTR group) or with 2.5 ml of Equipro plus 1 mM CoQ (CTR + Q group). All experimental groups had a final dilution of semen and extender of 1:5 and a final concentration of 100 × 106 sperm/ml. Each final aliquot was assessed four times: immediately after dilution (H0), at 24 (H24), 31 (H31) and 48 h from collection (H48). After the first assessment, the semen was maintained at 4 °C until the last analysis was carried out.
Motility analysis
Motility analysis comprised assessment of sperm motility with the CASA method (Computer-Assisted Sperm Analysis; Hamilton-Thorne Biosciences; IVOS, Animal Version 12.3D Bild 002). At each assessment, 15 μl of semen was placed on a pre-warmed Cell-Vu slide (Millennium Science Inc., New York, NY) with two counting chambers. Ten fields per sample were chosen manually at 40×. The settings for the CASA analysis, drawn from previous studies (Jasko et al. Citation1990; Mantovani et al. Citation2002) were as follows: frame acquired, 20 (min no. 15); acquisition rate 50 Hz; min. contrast, 45; min cell size, 4; threshold straightness, 80; medium velocity (VEL) cut off, 25 μm/s; low VEL cut off, 24.9 μm/s; non motile head size, 13; non motile head intensity, 25; static size limits, 0.53–3.40; static intensity limits, 0.26–3.36; static elongation limits, 12–80. A preliminary measure of repeatability within sample was assessed and, analyses started when motility parameters differed ≤3% between subsequent measurements. The parameters examined were: percentages of motile (% MOT) and progressively motile spermatozoa (% PMS), average path velocity (VAP; μm/s), average curvilinear speed (VCL; μm/s), average straight velocity (VSL; μm/s), average straightness (STR = VSL/VAP), average linearity (LIN = VSL/VCL), and average lateral head movements (ALH; μm).
Statistical analysis
In both part of the study, a similar statistical analysis was carried out by means of a mixed linear model for repeated measures applying the mixed procedure of SAS (SAS Institute 2009. SAS/STAT 9.2 User’s Guide, Second Edition. SAS Inst. Inc., Cary, NC). The two separated analysis accounted for the fixed effects of the group (or treatment; CTR, Q and Q + E; P + Q, CTR and CTR + Q for first and second part, respectively), the time at which the CASA variables were obtained (0, 2 and 4 h after thawing for first part; 0, 24, 31 and 48 h after collection for the second part, respectively), and the group (or treatment) × time interaction. Due to the differences in the number of time series analysed, the different nature of the measurement (i.e. after thawing or after collection and cooling), and distances from time 0, a unique model considering the experiment effect was not possible. In Addition, considering the longitudinal nature of CASA analysis (i.e. time series) and the aim of evaluating a time and group (or treatment) × time effect, the main plot (group or treatment effect) was tested on its specific error term represented by the stallion × repeat within the group random effect. Comparison between least square means belonging to different groups, times or interaction group × treatment were analysed with the Bonferroni correction method. In addition to the CASA variables analysed in both parts of the study, for the first part the differences between pre-freezing and post-thawing (at hours) CASA measures were also analysed by applying the same model and comparisons previously described.
Results
Effects of addition of CoQ and Vit.E in frozen semen
In the first part of the study, no improvement (p > .05) was found for the addition of CoQ and CoQ plus Vit. E compared to the CTR, which represent the usual extender commonly applied today for the transport of equine frozen semen (Table ). In Figures and , are presented the decay curves from 0H to 4H after thawing for the total motility and the progressive motile spermatozoa. There are no differences among CTR and Q or Q + E extender even at 2 or 4 h from thawing. As regards to the quality of sperm movement, the addition of CoQ and Vit. E did not influence velocity parameters of semen after thawing (Table ). Indeed, the VAP, VSL and VCL value for the three extenders were not different. However, comparing the differences between CASA variables measure before freezing and at 0H after thawing, some variables resulted significantly influenced by the extender used. Particularly, CoQ produced an improvement of +4.4% in STR and +3.5% in LIN (Figure ). Like for other parameters of sperm movement, Q + E showed lower performances as compared to the addition of CoQ alone, restricting the efficiency to +0.8% in STR and +2.3% in LIN (Figure ). Comparing CASA data pre-freezing and after 2H from thawing, the addition of CoQ or CoQ plus Vit. E caused a reduction of motility compared to CTR of 2.9% and 5.7%, for Q and Q + E, respectively (data not shown). However, these antioxidants increase PMS of 6.3% in Q and of 2.5% in Q + E, compared to CTR after 2 h from thawing, respectively (data not shown). At 4H after thawing, the differences in motility as compare to pre-freezing value resulted close to zero, indicating again the absence of any effect due to the addition of CoQ or CoQ plus Vit. E in the motility of semen.
Figure 1. Decay curve for the motility (%MOT) on frozen-thawed spermatozoa suspended with standard extender (CTR) or with extender added with coenzyme Q (Q) or coenzyme Q plus vitamin E (Q + E). Values are least square means ± SE.
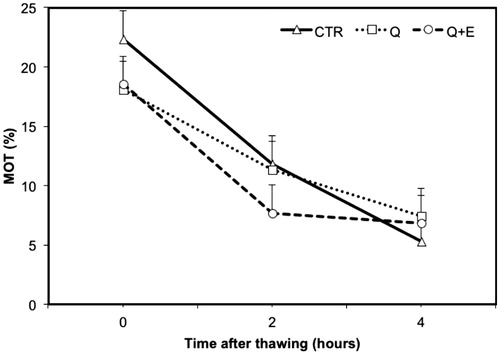
Figure 2. Decay curve of the progressively motility (%PMS) on frozen-thawed spermatozoa suspended with standard extender (CTR) or with extender added with coenzyme Q (Q) or coenzyme Q plus vitamin E (Q + E). Values are least square means ± SE.
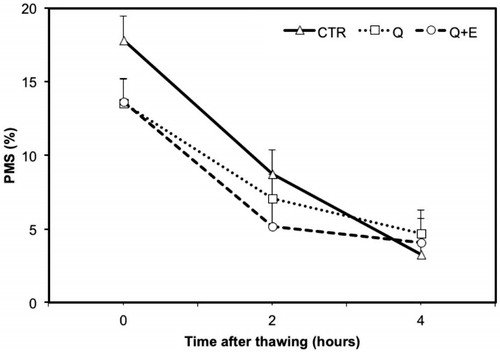
Figure 3. Variation on straightness (STR) and average linearity (LIN) measured before freezing and after thawing on frozen semen suspended with standard extender (CTR) or with extender added with coenzyme Q (Q) or coenzyme Q plus vitamin E (Q + E). Values are least square means ± SE. Bars containing values with different superscript (a or b) differ for p < .05.
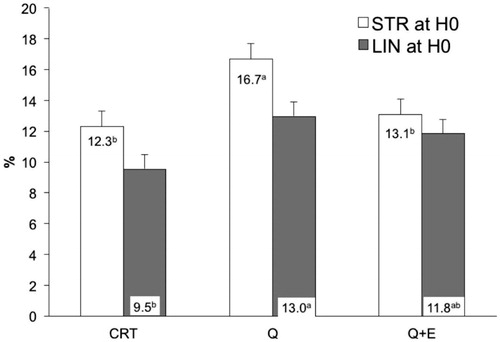
Table 2. Motility parameters of frozen-thawed semen diluted with standard extender (CTR) or with extender added with coenzyme Q (Q) or coenzyme Q plus vitamin E (Q + E).
Effects of addition of CoQ in cooled semen
Data reported in Table indicates that no improvement (p > .05) was attributable to the addition of CoQ in cooled equine semen, both in the presence or in the absence of seminal plasma, as regard % MOT, % PMS and ALH (μm). However, comparing the differences between CASA variables measure at 48 h after collection, sperm motility resulted influenced by the presence of seminal plasma (p = .0001). Particularly, seminal plasma reduced of about 24% the % MOT compared to CTR after 48 h from semen collection (Figure ).
Figure 4. Decay curve for the motility (% MOT) on cooled-stored semen diluted with standard extender (CTR) or with extender added with coenzyme Q in presence (P + Q) or absence (CTR + Q) of seminal plasma. Values are least square means ± SE. Different letters (a or b) indicate differences between least square means at p < .05.
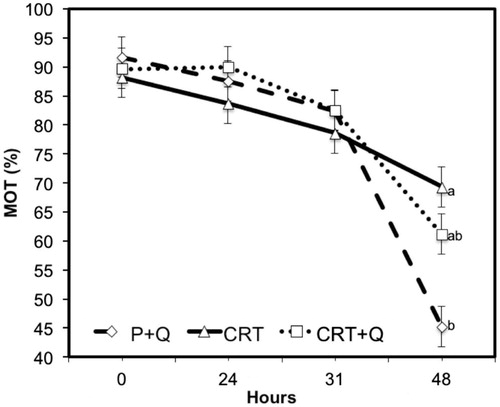
Table 3. Motility parameters of cooled-stored semen diluted with standard extender (CTR) or with extender added with coenzyme Q in presence (P + Q) or absence (CTR + Q) of seminal plasma.
Comparing the differences between CASA variables measured at subsequent time from collection, no significant changes were noticed between CTR and CTR + Q, indicating the absence of any effect due to the addition of CoQ in the quality of treated semen. However, comparing P + Q with CTR or CTR + Q, the first group performed always better than the other two groups, with the only exception for the % MOT variable that was similar among groups at collection (0H) and after 24 or 31 h. P + Q group showed lower motility that CTR after 48 h of refrigeration (Figure ). Multiple comparison test with the Bonferroni correction method shows improvements (p < .05) in the quality of semen, especially for VAP (μm/s) and VSL (μm/s) at all time from collection (Figures and ). In addition, differences were observed also for LIN after 31 and 48 h from collection comparing CTR + Q and CTR, respectively (Figure ). STR and VCL (μm/s) were improved in P + Q, compared to CTR (p < .001), at 48 h from collection and at the time of collection, respectively (data not shown).
Figure 5. Decay curve for the average path velocity (VAP; μm/s) on cooled-stored semen diluted with standard extender (CTR) or with extender added with coenzyme Q in presence (P + Q) or absence (CTR + Q) of seminal plasma. Values are least square means ± SE. Different letters (a or b) indicate differences between least square means at p < .05.
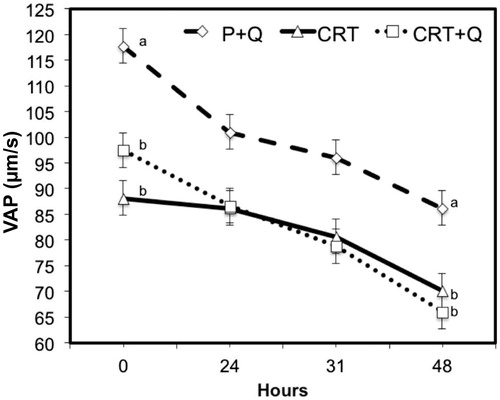
Figure 6. Decay curve for the average straight velocity (VSL; μm/s) on cooled-stored semen diluted with standard extender (CTR) or with extender added with coenzyme Q in presence (P + Q) or absence (CTR + Q) of seminal plasma. Values are least square means ± SE. Different letters (a or b) indicate differences between least square means at p < .05.
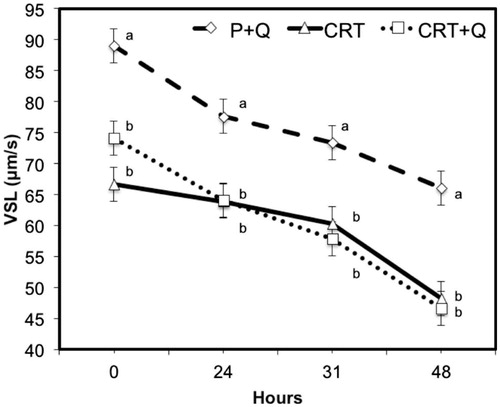
Figure 7. Decay curve for the average linearity (LIN = VSL/VCL) on cooled-stored semen diluted with standard extender (CTR) or with extender added with coenzyme Q in presence (P + Q) or absence (CTR + Q) of seminal plasma. Values are least square means ± SE. Different letters (a or b) indicate differences between least square means at p < .05.
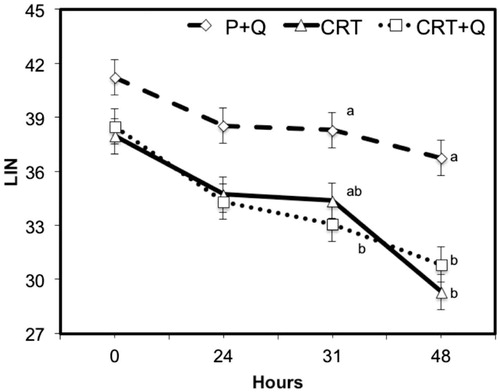
Discussion
The current study demonstrated that there is no improvement in motility parameters in the addition of CoQ (1mM) or CoQ (1mM) plus Vit. E (1mM) in equine frozen semen and in the addition of CoQ in equine cooled semen, with or without seminal plasma. The improvements shown by P + Q in the velocity parameters (Table ) can be due only to the seminal plasma activity (Rigby et al. Citation2001).
After the first experiment in frozen semen, we hypothesised that the lack of improvements that we expected from the addition of CoQ was due to the limited contact time between spermatozoa and CoQ, our balancing time pre-freezing was 60 min. The decay curves (Figures and ) showed a lower reduction of motility parameters refers to the increase of contact time. Therefore, although not supportable as a therapy in frozen semen, the addition of CoQ looked like it could be used in cooled and shipped semen that is generally used between 24 and 36 h post collection. However, increasing the contact time trying the addition of CoQ in cooled semen, no improvement has occurred.
A recent study shows that serum concentration of CoQ10 in horse increases with oral supplementation (Sinatra et al. Citation2013). This way of administration seems to give statistically significant improvements in human species for motility parameters in semen and a positive correlation was found between treatment duration with CoQ10 and sperm count, motility and morphology (Balercia et al. Citation2004); these improvements seem to be due by a more active spermatogenesis by the seminiferous tubules and to an alteration of the reproductive hormone profiles (Safarinejad Citation2009). Indeed, testes have a very low level of oxygen tension and this is further reduced by the activity of mitochondria and germ cells involved in the spermatogenesis (Aitken & Roman Citation2008). In the testes, OS is capable of destroying the steroidogenic capacity of Leydig cells and the ability to differentiate normal sperm from the germinal epithelium (Aitken & Roman Citation2008). Looking at the results in human (Safarinejad Citation2009), it is possible that the main activity of CoQ is to protect cells and tissues involved in spermatogenesis, resulting in an increase in the number of spermatozoa. This hypothesis is also supported by the fact that in elderly subjects the amount of antioxidants in the tissues is reduced (Navas et al. Citation2007), and this could be a cause of low sperm counts typical of old age subjects.
The addition of CoQ in the ejaculate does not seem to perform particular antioxidant action; however, in the uterus CoQ and ubiquinol may be involved in the mechanism of inhibition of post-coital endometritis. The effect of reduction of post-coital endometritis performed by equine seminal plasma has already been demonstrated (Troedsson et al. Citation2001), and OS has been proposed as a potential factor involved in the pathogenesis of the endometritis in mares (Yaralioglu-Gurgoze et al. Citation2005). Some authors demonstrated that intramuscular treatments with antioxidants increase the recovery rate in chronic endometritis in cows (Segupta & Nandi Citation2013). Its effect in the uterus may explain its presence in seminal plasma, as it does not seem to give any improvement directly in the spermatozoa.
The results of the direct addition of CoQ in equine semen were unpredictable, given the poor literature available in equine species; on the other hand, surprising was the lack of improvement of the addition of Vit. E to CoQ. Indeed, the Vit. E gave discordant results in the improvement of motility parameters (Almeida & Ball Citation2005; Thuwanut et al. Citation2008; Silva et al. Citation2013). Several studies conducted in equine and other species report ameliorative effects in the use of α-tocopherol (Vasconcelos Franco et al. Citation2013). However, other studies have shown that lipid peroxidation does not increase substantially during stallion semen storage (Kankofer et al. Citation2005) or that stallion sperms seem resistant to induction of membrane peroxidation (Neild et al. Citation2005). In our study, the expected effect of reducing the ROS damage to the spermatozoa, improving their motility parameters, was based on the recognised ability of CoQ to regenerate the active form of α-tocopherol (Bentinger et al. Citation2007). Indeed, equine seminal plasma is a powerful source of ROS scavengers, which function is to protect ejaculated sperm through defence enzymes and small molecular antioxidants such as α-tocopherol (Vasconcelos Franco et al. Citation2013). However, the additive effect that derived from the synergy of these two substances did not occur, probably for the inability of the kind of Vit. E used, to enter in the cell. The interaction between Vit. E and biological membranes depend on both the nature of the chains of the fatty acids of membrane phospholipids, both by the length of the side chains of the tocopherol. The penetration of the α-tocopherol in a phospholipid monolayer is strictly dependent on the amount and the kind of PUFAs. It is also reported that the optimum interaction between tocopherol and phospholipids membranes occurs when the length of their hydrophobic portions is almost the same (Maggio et al. Citation1977). Tocopherol succinate was superior to α-tocopherol in reducing oxidation because it’s more water soluble, and therefore it's more suitable to reach subcellular membranes within the mitochondria (Zhang et al. Citation2001; Almeida & Ball Citation2005); this fact has demonstrated the importance of the integration of Vit. E in membrane to carry out its antioxidant and cytoprotective action. Given the considerable variability in lipid composition between different individuals and different ejaculates, even in the same stallion (Aurich Citation2005), and knowing the importance of the integration of Vit. E in the membrane to prevent OS (Maggio et al. Citation1977), the failure in the entrance of the Vit. E in the membrane of the spermatozoa could be the basis of lack of the expected effect. In equine spermatozoa, the addition of 120 μM Trolox, a water-soluble Vit. E analogue, to extender in the post-thaw moment improved total and progressive motility, after 120 min of incubation (Silva et al. Citation2009). In 2013, the same authors achieved improvements in the parameters of motility adding Trolox in pre-freezing extender (Silva et al. Citation2013). These results demonstrated that Vit. E protects sperm cells during cryopreservation. Comparing our results with these it is possible that our kind of Vit. E was not able to enter the spermatozoa membrane or the lack of effect can be due to membrane damage induced by cryoconservation; indeed, the effect of antioxidants may vary not only depending on concentration also depending on the species, medium or temperature.
In a recent study in human, the addition of Vit. E in the semen extender results in increase in post-thaw motility in subjects ≥40 years compared with younger patients (Taylor et al. Citation2009). We compared the obtained results by dividing the stallions into two groups, subjects ≥10 years or subjects <10 years, however, no improvement was found from the addition of CoQ and/or Vit. E correlated to the age of the subjects. We suppose that the limited number of subjects and the great variability between semen of different stallions were the main cause of the absence of results.
Our results are in contrast with those obtain by Yousefian et al. (Citation2014) and Nogueira et al. (Citation2015). Our difference value measured at 48 h (Figure ) between CRT and CRT + Q for motility can be explained with a possible negative effect due to the high amount of antioxidant used. However, these studies differ for the extenders used (Equipro and Kenney, respectively), the breed of stallions (Standardbred and Holstein compared with Caspian, respectively) and the number of stallions (5 and 8 stallions in our study, 3 and 10 subjects in the other abovementioned studies).
Conclusions
The addition of antioxidants, as Vit. E and CoQ, directly in the semen at the concentration of 1mM, does not improved motility parameters in frozen or cooled equine semen; on the contrary, it may compromise semen quality over time. Our results, together with data reported by other authors, suggest a species-specific effect probably depending not only on the type of antioxidant added, but also on its concentration. In accordance to other studies, it is possible that the ameliorative effects of CoQ could be due to his effects in testicles tissue, improving spermatogenesis, and not directly in the spermatozoa, underline the importance to improve its presence in feed and not in the semen extender. However, some studies have also suggested that lipid peroxidation is not substantial during semen conservation and that stallion semen seem resistant to the induction of membrane peroxidation. Therefore, further research is required in order to clarify the exogenous antioxidant effect in stallion semen and/or to select the best concentration of antioxidants, or combination of antioxidants, the way of administration and the effects that they can produce at spermatogenesis level. Additionally, results from this study should be further improved by increasing the number of stallions in order to obtain more exhaustive results.
Acknowledgements
Preliminary results were presented as an Abstract in Reproduction in Domestic Animals, Supplement: 5, n°47 pag. 82; August 2012.
Disclosure statement
The authors deny any financial interest or benefit arising from the direct applications of their research. No competing interests have been declared.
Funding
This work was supported by Research funded by the University of Padova-Ministry of the University and of Scientific and Technologic Research (MURST, Italy) - Ex 60%.
References
- Agarwal A, Gupta S, Sikka S. 2006. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 18:325–332.
- Aitken RJ, Roman SD. 2008. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 1:15–24.
- Almeida J, Ball BA. 2005. Effect of alpha-tocopherol and tocopherol succinate on lipid peroxidation in equine spermatozoa . Anim Reprod Sci. 87:321–337.
- Aurich C. 2005. Factors affecting the plasma membrane function of cooled-stored stallion spermatozoa. Anim Reprod Sci. 89:65–75.
- Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, Littarru G. 2004. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril Jan. 81:93–98.
- Ball BA. 2008. Oxidative stress, osmotic stress and apoptosis: impacts on sperm function and preservation in the horse. Anim Reprod Sci. 107:257–267.
- Bansal AK, Bilaspuri GS. 2010. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int. 7 p.
- Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. 2000. The effect of reactive oxigen species on equine sperm motility, viability, acrosomal integrity, mithocondrial membrane potential, and membrane lipid peroxidation. J Androl. 21:895–902.
- Bentinger M, Brismar K, Dallner G. 2007. The antioxidant role of coenzyme Q. Mitochondrion. 7 Suppl:S41–S50.
- Carver DA, Ball BA. 2002. Lipase activity in stallion seminal plasma and the effect of lipase on stallion spermatozoa during storage at 5 degrees C. Theriogenology. 58:1587–1595.
- Crockett EC, Graham JK, Bruemmer JE, Squires EL. 2001. Effect of cooling of equine spermatozoa before freezing on post-thaw motility: preliminary results. Theriogenology. 55:793–803.
- Griveau JF, Le Lannou D. 1997. Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 20:61–69.
- Jasko DJ, Lein DH, Foote RH. 1990. A comparison of two computer-automated semen analysis instruments for the evaluation of sperm motion characteristics in the stallion. J Androl. 11:453–459.
- Kankofer M, Kolm G, Aurich J, Aurich C. 2005. Activity of glutathione peroxidase, superoxide dismutase and catalase and lipid peroxidation intensity in stallion semen during storage at 5 degrees C. Theriogenology. 63:1354–1365.
- Maggio B, Diplock AT, Lucy JA. 1977. Interactions of tocopherols and ubiquinones with monolayers of phospholipids. Biochem J. 161:111–121.
- Mantovani R, Rota A, Falomo ME, Bailoni L, Vincenti L. 2002. Comparison between glycerol and ethylene glycol for the cryopreservation of equine spermatozoa: semen quality assessment with standard analyses and with the hypoosmotic swelling test. Reprod Nutr Dev. 42:217–226.
- Navas P, Villalba JM, De Cabo R. 2007. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 7 Suppl:S34–S40.
- Neild DM, Brouwers JF, Colenbrander B, Agüero A, Gadella BM. 2005. Lipid peroxide formation in relation to membrane stability of fresh and frozen thawed stallion spermatozoa. Mol Reprod Dev. 72:230–238.
- Nogueira BG, Sampaio BFB, Souza MIL, Costa e Silva EV, Zúccari CE. 2015. Coenzyme and α?tocopherol prevent the lipid peroxidation of cooled equine semen. Reprod Dom Anim. 50:1003–1010.
- Pérez-Pé R, Cebriàn-Pérez JA, Muino-Blanco T. 2001. Semen plasma proteins prevent cold-shock membrane damage to ram spermatozoa. Theriogenology. 56:425–434.
- Pindaru L, Cenariu M, Pall E, Groza IS. 2015. Effects of Coenzyme on sperm viability during storage of boar semen at 17 °C. Scientific Works. Series C. Veterinary Medicine. Vol. LXI:32?36. Available from: http://veterinarymedicinejournal.usamv.ro/pdf/2015/issue_2/Art5.pdf.
- Rigby SL, Brinsko SP, Cochran M, Blanchard TL, Love CC, Varner DD. 2001. Advances in cooled semen technologies: seminal plasma and semen extender. Anim Reprod Sci. 68:171–180.
- Safarinejad MR. 2009. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 182:237–248.
- Segupta D, Nandi PR. 2013. Effect of oestradiol, vitamin A, E and selenium treatment with varying sexual rest period on recovery rate in cross-bred cows with chronic endometritis. Vet World. 6:106–108.
- Sharma KR, Agarwal A. 1996. Role of reactive oxygen species in male infertility. Urology. 48:835–850.
- Silva KMG, Moraes TAP, Silva ECB, Gamboa SC, Guerra MMP. 2009. Effect of trolox and pentoxifylline on motility and integrity of, acrossome and DNA of equine spermatozoa after thawing. Arq Bras Med Vet Zootec. 61:42–49.
- Silva SV, Soares AT, Batista AM, Almeida FC, Nunes JF, Peixoto CA, Guerra MMP. 2013. Vitamin E (Trolox) addition to Tris-egg yolk extender preserves ram spermatozoon structure and kinematics after cryopreservation. Anim Reprod Sci. 137:37–44.
- Sinatra ST, Chopra RK, Jankowitz S, Horohov DW, Bhagavan HN. 2013. Coenzyme Q10 in equine serum: response to supplementation. J Equine Vet Sci. 33:71–73.
- Taylor K, Roberts P, Sanders K, Burton P. 2009. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod Biomed Online. 18:184–189.
- Thuwanut P, Chatdarong K, Techakumphu M, Axnèr E. 2008. The effect of antioxidants on motility, viability, acrosome integrity and DNA integrity of frozen-thawed epididymal cat spermatozoa. Theriogenology. 70:233–240.
- Troedsson MHT, Desvousges A, Alghamdi AS, Dahms B, Dow CA, Hayna J, Valesco R, Collahan PT, Macpherson ML, Pozor M, et al. 2005. Components in seminal plasma regulating sperm transport and elimination. Anim Reprod Sci. 89:171–186.
- Troedsson MHT, Loset K, Alghamdi AM, Dahms B, Crabo BG. 2001. Interaction between equine semen and the endometrium: the inflammatory response to semen. Anim Reprod Sci. 68:273–278.
- Vasconcelos Franco JS, Chaveiro A, Góis A, Da Silva FM. 2013. Effects of α-tocopherol and ascorbic acid on equine semen quality after cryopreservation. J Equine Vet Sci. 33:787–793.
- Yaralioglu-Gurgoze S, Cetin H, Cen O, Yilmaz S, Atli MO. 2005. Changes in malondialdehyde concentrations and glutathione peroxidase activity in purebred Arabian mares with endometritis. Vet J. 170:135–137.
- Yousefian I, Zare-Shahneh A, Zhandi M. 2014. The Effect of coenzyme Q10 and α-tocopherol in skim milk-based extender for preservation of caspian stallion semen in cool condition. J Equine Vet Sci. 34:949–954.
- Zhang JG, Nicholls-Grzemski FA, Tirmenstein MA, Fariss MW. 2001. Vitamin E succinate protects hepatocytes against the toxic effect of reactive oxygen species generated at mitochondrial complexes I and III by alkylating agents. Chem Biol Interac. 138:267–284.
