Abstract
The pathogenicity of Enterotoxigenic Escherichia coli (ETEC) expressing F4ac (K88) fimbrial adhesin and its adhesion to the pig jejunum surface is genetically controlled by the presence of a specific receptor glycoprotein on brush borders of the epithelium. We firstly screened the degree of inhibition obtained with ten galactose-recognising lectins – respectively from Abrus precatorius (APA-I); Coregonus maraena; Euphorbia characias; Crotalaria juncea; Sambucus ebulus (SEL1d); Sambucus nigra (SNAV); Coregonus peled; Momordica charantia (MCL); Trychosanthes kirilowii; Adenia racemosa –, by an ELISA test based on intestinal brush border sensitive for E. coli F4ac adhesion and ETEC F4ac fimbrial antigen. Three lectins significantly inhibited the fimbrial adhesion and the highest value was obtained by Crotalaria juncea lectin (p < .01). The results were then confirmed by an in vitro inhibition test of ETEC adhesion to villi isolated from ETEC susceptible pigs. The favourable results obtained with the supplementation of two d-galactose lectins against the ETEC adhesion to intestinal villi stimulate in vivo testing of the addition of these lectins in the piglet feeding
Introduction
The enterotoxigenic Escherichia coli (ETEC) is the main cause of post-weaning diarrhoea in piglet. The ETEC types more frequently isolated in Italy possess F4 or F18 fimbriae that mediate the adhesion of the microbe to the pig jejunum. The adhesion of F4 depends on the genetically controlled presence of a specific receptor glycoprotein on the brush borders of the pig epithelium (Sellwood & Kearns Citation1979). This adhesion is the initial step in the colonisation of the small intestine and the development of the enteric infection (Van den Broeck et al. Citation1999). Finding natural products that when added in the diet could counteract the adhesion of ETEC to the intestinal receptor, could contribute to better health and performance of young pigs.
Three antigenic variants of this adhesin are recognised as F4ab, F4ac and F4ad, with F4ac being the principal variant associated with piglet diarrhoea (Westerman et al. Citation1988). The fimbrial adhesins present lectin domains; the interaction between intestinal receptors and F4ac fimbriae is strong and stable (Verdonck et al. Citation2004), and inhibits the ability of peristaltic action of the intestine to remove pathogens. Putative receptors for F4 have been identified on porcine epithelial cells. These include intestinal mucin-type glycoproteins (IMTGP), which bind F4ab and F4ac (Francis et al. Citation1998). Two glycoproteins from brush borders of porcine intestinal cells were identified as specific receptors for F4ac. Grange et al. (Citation1998) determined that β-linked galactose was an essential component in recognition of IMTGP receptors by F4ac adhesions. The important role for galactose residue in the F4 receptor structure is still recognised (Moonens et al. Citation2015).
Two different strategies can be suggested to potentially control ETEC infections: agents which are either (i) bactericidal/bacteriostatic and act directly against the bacteria or (ii) which block the attachment of E. coli to their intestinal receptor. The opportunities and the problems related to the strategies for using isolated natural anti-adhesion molecules were reviewed by Ofek et al. (Citation2003). Lectins of vegetable origin could be used to protect piglets against E. coli invasion by blocking E. coli F4ac adhesion to the intestine. For this goal, lectins that recognise sugar motifs based on galactose are the best candidates.
We set up both an ELISA test to screen a panel of galactose recognising lectins, for their ability to inhibit the adhesion of F4 fimbriae, and an in vitro test on jejunum villi to assess if the same lectins were also able to inhibit the intestinal receptors for E. coli F4ac.
Materials and methods
Preparation of brush borders
Brush borders were isolated from the jejunum of ten two-week-old piglets. Each intestinal piece was tied of at one end then reverted over a glass rod and filled with PBS at 4 °C and tied at other end. This was done to produce ‘sausage’ which had the mucosal surface on the outside. It was then placed in a bottle containing PBS at 4 °C and agitated gently. The PBS was discarded and replaced with fresh solution, until the solution remained clear. This was then replaced with hypotonic EDTA buffer at 4 °C, and shaken in a cold room for 30 min. The suspension of dislodged cells was left to stand for 1 hour at room temperature to lyse the cells. Lysed cell membrane and brush borders were centrifuged (1100 g, 10 min, 4 °C) and the pellet re-suspended in PBS. Washing process was repeated several times, until the supernatant was clear. The pellet was then re-suspended in PBS buffer and centrifuged at 150 g for 10 min. The supernatant was collected and the pellet was discarded. This was repeated three times. The pooled supernatant was filtered and centrifuged at 550 g for 10 min. The pellet containing brush borders was re-suspended in PBS and the brush borders were counted on a haemocytometer and the suspension adjusted so that it contained 1 × 107 brush borders per ml.
Bacterial preparation
ETEC F4ac O149 was grown in Tryptone Soy Broth (Oxoid, Basingstoke, England). After overnight incubation at 37 °C, the bacterial cells were harvested by centrifugation at 3000 g and re-suspended in PBS (pH 7.0) to obtain a concentration of approximately 1 × 1010, subsequently confirmed by densitometry and serial dilution followed by viable plate counts on Triptone Soy Agar (Oxoid).
Preparation of fimbriae from E. coli F4ac
The fimbriae from F4ac positive ETEC were purified with the method of Van den Broeck et al. (Citation1999). The purity of F4ac solution was assessed by SDS-PAGE on 12% polyacrilamide gel: the major subunit of F4ac was clearly visible, among other minor proteins. The gel was stained and the relative band intensity was determined. F4ac represented 78% of the isolated protein. The F4 fimbriae were also identified in Western blot with a F4ac-specific MAb.
Lectin purification
The agglutinin from the seeds of Abrus precatorius (APA-1) was purified by lactamyl-Sepharose affinity chromatography followed by gel filtration and DEAE-Sephacel column chromatography and eluted with a sodium acetate gradient (Hegde et al. Citation1991).
The lectins from Momordica charantia (MCL) (Barbieri et al. Citation1980). Euphorbia characias (Barbieri et al. Citation1983), Sambucus ebulus (SEL1d) (Rojo et al. Citation2003), Trychosanthes kirilowii (Falasco et al. Citation1989) and Adenia racemosa (Pelosi et al. Citation2005) were purified by acid-treated CL Sepharose 6B eluted with 0.1 M galactose. Crotalaria juncea lectin was purified by affinity chromatography by coupling galactose to Sepharose 6B activated with divinyl sulphone (Ersson Citation1977). Sambucus nigra agglutinin V (SNAV) was purified by affinity chromatography as described in (Van Damme et al. Citation1996). The lectin from Coregonus lavaretus maraena was purified by affinity chromatography on O-alpha-d-galactosyl polyacrylamide gel (Krajhanzl et al. Citation1978). Coregonus peled lectin was purified by affinity chromatography on asialofetuin-Sepharose 4B as described by Jung et al. (Citation2003). Molecular weight and purity, reported in Table , were determined by SDS-PAGE. The initial concentration of each lectin was 1g/l.
Table 1. Origin of the lectins tested in the ELISA inhibition test (References are given in brackets).
ELISA test
The ability of the lectins to inhibit the E. coli F4 adhesion on the brush border membranes from ETEC susceptible pigs, was assayed by ELISA. Each step of this analysis was separated by washing the plate six times with PBS–Tween 20. The wells of a 96-well microtiter plate (NUNC, Life Technologies, Milan, Italy) were coated with intestinal brush border (concentration 1:800). After incubation at 37 °C for 3 h and at 4 °C overnight, PBS and chicken serum 2% (vol/vol) (Sigma-Aldrich, Milan, Italy) was added for 1 h at room temperature to block any non-specific binding. Lectin was added at the initial concentration of 0.5g/L and serial dilutions in PBS buffer were made. In the last column of the plate no lectin was added as a positive control. The plate was incubated at 37 °C for 2 h, then F4ac l antigen was added at a dilution of 1:800 and the plate was incubated for 1 h. The wells were treated with rabbit anti-K88 (VLA, Addlestone, UK). After incubation at 37 °C for 1h, the wells were treated with a monoclonal anti-rabbit IgG conjugated to horseradish peroxidase (Sigma-Aldrich Milan, Italy) and incubated again for 1 h. After addition of o-phenylenediamine dihydrochloride) substrate (Sigma-Aldrich, Milan, Italy) and incubation for 10 min, the reaction was stopped with HCl 3N and the OD 492 nm were read on a microplate reader (Sunrise Microplate Reader, TECAN, Milan, Italy). For each lectin, we performed a total of three or four replicates in two different batches.
In vitro inhibition assay of ETEC adhesion to the villi
The villi used for the test were from sets previously obtained by pigs that were confirmed to be strongly susceptible for the binding of ETEC to villi, utilising the method of Van den Broeck et al. (Citation1999), previously adopted also in several other researches by the authors. The same basic procedure was also used here for testing the lectins for the inhibition of adhesion, as adapted by Trevisi et al. (Citation2012). ETEC adhesion was determined by phase-contrast microscopy (1000 ×). Per each lectin, the villi from each 3 or 4 donor pigs were used; per each pig, 20 well-shaped villi were captured by digital camera in 32–40 fields and the number of bacteria was counted in each field. The bacterial adhesion per 250 μm length of villous border was calculated. Villi used in subsequent assays were finally washed in buffer, recovered by filtration and divided into subsets: control and treatments. For the control, villi were suspended in PBS with 1% d-mannose (Sigma-Aldrich, Milan, Italy), while for the treatments each villi subset was incubated in medium containing one of the three different Lectins or F4 fimbriae, both used at the same concentration of 0.25 g/l. After incubation, villi were washed again and suspended in the same buffer like the control. All the villi suspensions were then incubated for 1 h at room temperature with ETEC (4 × 109 bacteria/ml PBS), and observed by phase-contrast microscopy to determine bacterial adhesion.
Statistical methods
For the ELISA test, per each lectin, optical density values obtained per each dilution were fitted with a linear-quadratic model using the PROC REG of SAS and the observed values were plotted against the predicted. The lowest concentrations that showed inhibition of binding when compared to controls were also calculated by Student's t-test.
Count numbers of adhering bacteria obtained by the addition of lectins or fimbriae in the in vitro inhibition assay of ETEC adhesion to the villi were compared with control values by Student's t-test.
Results
Inhibition test by ELISA method
Three galactose-recognising lectins inhibited fimbrial F4 adhesion on pig intestinal brush border receptors, as indicated by the fitting of the dose response to each one by a linear-quadratic model (Table ): the plot of observed versus predicted values is presented in Figure . The results of the other lectins tested were all negative and are not shown. Control OD values obtained were always high and very stable, and indicative of adhesion in the absence of the lectins.
Figure 1. Plot of the curvilinear inhibitory effect of Lec1 (A), Lec4 (B) and Lec7 (C) against the adhesion of F4ac fimbriae on pig intestinal brush borders by ELISA test. Observed values: (o). Lower OD values indicate increasing inhibition. Table displays the names and the characteristics of the lectins.
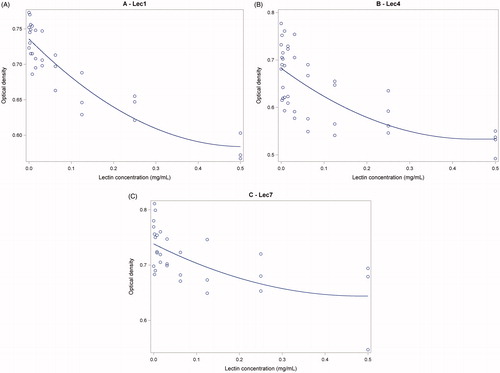
Table 2. Parameters of the equations showing the curvilinear effect of the doses of each lectin on the adhesion of F4ac fimbriae on pig intestinal brush borders by ELISA test, where x0 is intercept, x1 is the concentration of each lectin and x2 = x1 . x1.
The best fitting (correct R2 = .807) was obtained with Lec1, as compared with Lec4, that was the second, and Lec7. The lowest concentrations that showed inhibition of binding when compared to controls (p < .05) were: 0.00195 mg/l; 0.0312 mg/l; 0.250 mg/l, respectively for Lec4, Lec1 and Lec7.
In vitro inhibition assay of ETEC adhesion to villi
After the incubation with lectins, the villous were still integer, as appreciated by visualisation at contrast microscope. The lectins that reduced the number of ETEC adhering on treated villous (Table ) were: Lec4, −49.4%; p < .01; Lec1, −33.8; p < .05; Lec2, −12.3; not significant. In a second assay (test 2), both Lec4 and F4 reduced the number of ETEC adhering on treated villous, respectively by 70.7 and 62.0% (p < .01) vs. control.
Table 3. Inhibitory effect of ETEC adherence to villi by different lectins and by F4.
Discussion
The galactose-binding property of the highly purified lectin from Abrus precatorius was firstly observed by Olsnes et al. (Citation1974). For the lectin from Crotalaria juncea it was early demonstrated by Ersson (Citation1977) that the hemagglutination activity is strongly inhibited by d-galactose and d-galactosamine. Our results confirm the observation that a lectin of Euonymus europeaus which specifically recognises the Gal alpha (1-3)Gal sequence, blocked the binding of F4 fimbriae to mucus proteins (Willemsen & De Graaf Citation1992). Our data support the evidences that terminal β-Galactose is involved in the binding of F4 (Payne et al. Citation1993) and the link of this sugar to N-acetylhexosamines enhances F4 binding (Grange et al. Citation2002).
Not all the tested lectins efficiently blocked the adhesion of F4 to receptive intestinal brush borders. Given that all the tested lectins are classified by their ability to bind galactose, we suggest that with the lectins which were not effective, there must be some steric impediments to the link. Interestingly, Jacalin, another Galactose binding lectin, has one of the highest scores of affinity for porcine ileal epithelial border (based on lectin histochemistry), yet two other galactose lectins (from Arachis hypogea and Erythrina cristagalli) had very low-affinity scores (George et al. Citation2007), suggestive that lectins with the same sugar specificity can bind differently on the brush border. Steric hindrance upon binding to moieties close to F4 receptors can explain also the case of Lec7, were an apparent lack of dose dependency was seen. Finally, it could be hypothesised that the different degree of purity could have affected the response of each lectin. Nevertheless all the lectins used had a high degree of purity (Table ) and in any case there was no association between the degree of purity and the performance, as indicated by the absence of significant inhibition in the ELISA test with the very pure Lec6.
Lec4 was as efficient as the isolated F4 fimbriae, in the in vitro inhibition of adhesion of ETEC to susceptible villi. This apparently indicates that Lec4 reached the maximum block that was possible with the method that we used. Indeed, the inhibition of ETEC adhesion was not total also with F4. This could be explained by the fact that the concentration that we used in vitro was not sufficient or by the fact that with this method some of the adhering molecules are mechanically washed with the treatment before the addition with the ETEC suspension.
In conclusion, galactose-binding lectins extracted from Abrus precatorius and Crotalaria juncea efficiently compete with F4, and with Escherichia coli F4, for the adhesion to receptive porcine intestinal villi. The seeds of Abrus precatorius contain different toxins (abrins), but the two adhesins isolated from this species (APA-I e APA-II) differ for the properties and the molecular weight (Hegde et al. Citation1991). A 200–2000-fold higher concentration of APA-I is required to induce a protein synthesis inhibition similar to abrin (Bagaria et al. Citation2006). Concerning Crotalaria juncea, an alcoholic extract of the seed, presumably containing alkaloids, caused degeneration of different tissues of rat after oral supply (Prakash et al. Citation1995). More data are necessary to evaluate the impact of those lectins on overall gut health; particularly toxicity and immunogenicity should be considered if a practical in vivo application of the two lectins is envisaged.
Conclusion
The availability of two different methods that validate to each other, could permit the evaluation of other products potentially able to compete with the enterotoxigenic E. coli F4, and that ultimately could reduce its infection. The present research stimulates further studies to be conducted in vivo to assess the practical relevance of blocking galactose in intestinal receptors of pigs susceptible to enterotoxigenic E. coli F4. The assessment should be extended, considering also the effect of the Crotalaria juncea lectin on gut health and function.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bagaria A, Surendranath K, Ramagopal UA, Ramakumar S, Karande AA. 2006. Structure-function analysis and insights into the reduced toxicity of Abrus precatorius agglutinin I in relation to abrin. J Biol Chem. 281:34465–34474.
- Barbieri L, Falasca A, Franceschi C, Licastro F, Rossi CA, Stirpe F. 1980. Inhibition of protein synthesis in vitro by proteins from the seeds of Momordica charantia (bitter pear melon). Biochem J. 186:443–452.
- Barbieri L, Falasco A, Franceschi C, Licastro F, Rossi CA, Stirpe F. 1983. Purification and properties of two lectins from the latex of the euphorbiaceous plants Hura crepitans L. (sand-box tree) and Euphorbia characias L. (Mediterranean spurge). Biochem J. 215:433–439.
- Ersson B. 1977. A phytohemagglutinin from Sunn hemp seeds (Crotalaria juncea). II. Purification by a high capacity biospecific affinity adsorbent and its physicochemical properties. Biochim Biophys Acta. 494:51–60.
- Falasco AI, Abbondanza A, Barbieri L, Bolognesi A, Rossi CA, Stirpe F. 1989. Purification and partial characterization of a lectin from the seeds of Trichosanthes kirilowii Maximowicz. FEBS Lett. 246:159–162.
- Francis DH, Grange PA, Zeman DH, Baker DR, Erickson AK. 1998. Expression of mucin-type glycoprotein K88 receptors strongly correlates with piglet susceptibility to K88(+) enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect Immun. 66:4050–4055.
- George S, Oh Y, Lindblom S, Vilain S, Rosa AJ, Francis DH, Brözel VS, Kaushik RS. 2007. Lectin binding profile of the small intestine of five-week-old pigs in response to the use of chlortetracycline as a growth promotant and under gnotobiotic conditions. J Anim Sci. 85:1640–1650.
- Grange PA, Erickson AK, Anderson TJ, Francis DH. 1998. Characterization of the carbohydrate moiety of intestinal mucin-type sialoglycoprotein receptors for the K88ac Fimbrial Adhesin of Escherichia coli. Infect Immun. 66:1613–1621.
- Grange PA, Mouricout MA, Levery SB, Francis DH, Erickson AK. 2002. Evaluation of receptor binding specificity of Escherichia coli K88 (F4) fimbrial adhesin variants using porcine serum transferrin and glycosphingolipids as model receptors. Infect Immun. 70:2336–2343.
- Hegde R, Maiti TK, Podder SK. 1991. Purification and characterization of three toxins and two agglutinins from Abrus precatorius seed by using lactamyl-Sepharose affinity chromatography. Anal Biochem. 194:101–109.
- Jung WK, Park PJ, Kim SK. 2003. Purification and characterization of a new lectin from the hard roe of skipjack tuna, Katsuwonus pelamis. Int J Biochem Cell Biol. 35:255–265.
- Krajhanzl A, Horejsí V, Kocourek J. 1978. Studies on lectins. XLI. Isolation and characterization of a blood group B specific lectin from the roe of the powan (Coregonus lavaretus maraena). Biochim Biophys Acta. 532:209–214.
- Moonens K, Van den Broeck I, De Kerpel M, Deboeck F, Raymaekers H, Remaut H, De Greve H. 2015. Structural and functional insight into the carbohydrate receptor binding of F4 fimbriae-producing enterotoxigenic Escherichia coli. J Biol Chem. 290:8409–8419.
- Murayama K, Taka H, Kaga N, Fujimura T, Mineki R, Scindo N, Morita M, Hosono M, Nitta K. 1997. The structure of Silurus asotus (catfish) roe lectin (SAL): identification of a noncovalent trimer by mass spectrometry and analytical ultracentrifugation. Anal Biochem. 247:319–326.
- Ofek I, Hasty DL, Sharon N. 2003. Anti-adhesion therapy of bacterial diseases: prospects and problems. FEMS Immunol Med Microbiol. 38:181–191.
- Olsnes S, Saltvedt E, Pihl A. 1974. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 249:803–810.
- Payne D, O'Reilly M, Williamson D. 1993. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to beta 1-linked galactosyl residues in glycosphingolipids. Infect Immun. 61:3673–3677.
- Pelosi E, Lubelli C, Polito L, Barbieri L, Bolognesi A, Stirpe F. 2005. Ribosome-inactivating proteins and other lectins from Adenia (Passifloraceae). Toxicon. 46:658–663.
- Prakash AO, Dehadrai S, Jonathan S. 1995. Toxicological studies on the ethanolic extract of Crotalaria juncea seeds in rats. J Ethnopharmacol. 45:167–176.
- Rojo MA, Citores L, Arias FG, Ferreras JM, Jimenez P, Girbés T. 2003. cDNA molecular cloning and seasonal accumulation of an ebulin l-related dimeric lectin of dwarf elder (Sambucus ebulus L) leaves. Int J Biochem Cell Biol. 35:1061–1065.
- Sellwood R, Kearns MJ. 1979. Inherited resistance to Escherichia coli diarrhoea in pigs: the genetics and nature of the intestinal receptor. In: Janowitz HD, Sachar DB, editors. Proceedings of international colloquium on frontiers of knowledge in the diarrhoeal diseases. Upper Montclair, NJ: Projects in Health. Inc. p. 113–122.
- Trevisi P, Priori D, Gandolfi G, Colombo M, Goossens T, Bosi P. 2012. In vitro test on the ability of a yeast cell wall based product to inhibit the Escherichia coli F4ac adhesion on the brush border of porcine intestinal villi. J Anim Sci. 90(suppl.4):275–277.
- Van Damme EJ, Barre A, Rougé P, Van Leuven F, Peumans WJ. 1996. Characterization and molecular cloning of Sambucus nigra agglutinin V (nigrin b), a GalNAc-specific type-2 ribosome-inactivating protein from the bark of elderberry (Sambucus nigra). Eur J Biochem. 237:505–513.
- Van den Broeck W, Cox E, Goddeeris BM. 1999. Receptor-dependent immune responses in pigs after oral immunization with F4 fimbriae. Infect Immun. 67:520–526.
- Verdonck F, Cox E, Vancaeneghem S, Goddeeris BM. 2004. The interaction of F4 fimbriae with porcine enterocytes as analysed by surface plasmon resonance. FEMS Immunol Med Microbiol. 41:243–248.
- Wei CH, Koh C, Pfuderer P, Einstein JR. 1975. Purification, properties, and crystallographic data for a principal nontoxic lectin from seeds of Abrus precatorius. J Biol Chem. 250:4790–4795.
- Westerman RB, Mills KW, Phillips RM, Fortner GW, Greenwood JM. 1988. Predominance of the ac variant in K88-positive Escherichia coli isolates from swine. J Clin Microbiol. 26:149–150.
- Willemsen PT, de Graaf FK. 1992. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 12:367–375.
