Abstract
The study examined meat quality and carcase haemorrhage in goats subjected to different methods of pre-slaughter electrical stunning or slaughtered without stunning. Thirty-two Boer crossbred bucks were randomly assigned to low-frequency head-only (LFHO; 1 A for 3 s at a frequency of 50 Hz), low-frequency head-to-back (LFHB: 1 A for 3 s at a frequency of 50 Hz) or high-frequency head-to-back (HFHB; 1 A for 3 s at a frequency of 850 Hz) pre-slaughter electrical stunning or slaughter without stunning (SWS). All the 32 animals were bled to drain excess blood from the carcase. The slaughter was performed by a licenced slaughter man by severing carotid artery, jugular vein, trachea and oesophagus. At 12 h post-mortem, LFHO, LFHB and HFHB had lower (p < .05) glycogen and higher lactate and glycolytic potential values than SWS. A faster (p < .05) rate of pH decline was found in LFHO, LFHB and HFHB compared to SWS. No physicochemical parameters except cooking loss differed between treatments. Cooking loss was higher (p < .05) in LFHO, LFHB and HFHB compared to SWS at 7 and 14 d post-mortem. Incidences of carcase haemorrhages in electrically stunned goats were higher than SWS. Nonetheless, HFHB had lower (p < .05) haemorrhages than LFHB and LFHO. Electrical stunning prior slaughter increased carcase haemorrhages and cooking loss but did not affect other meat quality traits in goats.
Introduction
Historically, it is well known that humans have been slaughtering animals for meat consumption. However, little attention was paid to the stress, pain and suffering that animal may feel during the slaughter and exsanguination. About a hundred and fifty years ago, the Western world found it ethically right to reduce the potential pain and distress that animals undergo during slaughter and have devised a variety of methods collectively known as ‘stunning’ to accomplish this purpose (Zivotofsky & Strous Citation2012). The main principle of these systems is to render animals unconscious and insensible to pain for durations long enough for death to occur (Linares & Vergara Citation2012). According to the EU Council Directive (EU Citation1993) and Council Regulation ((EC) No 1099/2009) (EC Citation2009) on the protection of animals, at the time of slaughter it is stated that animals shall be stunned to make them unconsciousness and insensibility before bleeding; however, in respect for human rights and freedom of worship, some exceptions have been given to the ritual slaughter. Currently, permitted methods for stunning which are classified as being humane (i.e. painless) in the United States, Europe and other foreign countries are mechanical, electrical and gas stunning (Lambooij et al. Citation2012). Electrical stunning which is achieved by the passage of a sufficient amount of current through the central nervous system (McNeal et al. Citation2003) is the most common stunning application (Nakyinsige et al. Citation2013). It works by inducing brain dysfunction and unconsciousness either temporarily in which case the animal dies as a result of bleeding, as is the case with head-only electrical stunning or with subsequent killing by cardiac arrest in head-to-back electrical stunning (Farouk Citation2013; Llonch et al. Citation2015).
During the tonic and clonic stages, head-only electrical stunning method which is accepted by halal market prompts an increase in blood pressure (Kirton et al. Citation1978) and when it is accompanied by intense skeletal muscle activity can lead to both forms of carcase haemorrhages (blood splash and speckle) in the carcase (Vergara et al. Citation2005; Gregory Citation2007) and reduces carcase quality (Farouk et al. Citation2014). In low-frequency head-to-back electrical stunning technique (50 Hz), the current passed through the spine and restrained the function of the spinal nerve and decreased clonic convulsions (Gilbert et al. Citation1984). This minimises the increase in blood pressure and intensity of muscle contraction, thereby limiting the occurrence and severity of haemorrhages (Petersen et al. Citation1986). However, the current passing through the heart leads to ventricular fibrillation, stoppage of the heart and death of the animal (Wotton & Gregory Citation1986) and therefore the stun is not acceptable to the halal market. Additionally, the poor bleeding efficiency associated with head-to-back stunned animals is considered to be a major quality defect that negatively affect meat colour, cause undesirable discolouration and decrease the shelf life of meat. Since conventional methods of electrical stunning are associated with adverse effects on carcase and meat quality (Farouk et al. Citation2014), the application of high frequencies to stun animals prior to slaughter was introduced. The modified system at higher frequency can be applied from head-to-back. The major benefit of a high-frequency head-to-body component is that the technique avoids or reduces cardiac arrest and muscle activity associated with convulsions and the incidence of carcase haemorrhages and blood spots (Farouk et al. Citation2014). However, there is hardly any comprehensive scientific study proving or justifying this claim. The hypothesis of this study was that goats stunned using the high-frequency head-to-back would enhance meat quality and reduce carcase damage. Thus, this study aimed at comparing the effects of different methods of pre-slaughter electrical stunning [low-frequency head-only (LFHO) electrical stunning, low-frequency head-to-back (LFHB) electrical stunning and high frequency head-to-back (HFHB) electrical stunning] with slaughter without stunning (SWS) on carcase and meat quality in goats.
Materials and methods
Animal welfare
The research protocol was approved by the institutional animal care and use committee (IACUC) of the Universiti Putra Malaysia (approval no: R052/2015).
Animals, stunning and slaughter procedure
Thirty-two Boer crossbred bucks (8–10 months old; average body weight of 29.9 ± 1.6 kg) reared under similar management system, were purchased from a commercial goat farm. The goats were randomly allotted into four groups of eight animals each and subjected to either slaughter without stunning (SWS), low-frequency head-only (LFHO), low-frequency head-to-back (LFHB) and high-frequency head-to-back (HFHB) pre-slaughter electrical stunning. The slaughtering protocol was carried out at the Research Abattoir of Animal Science Department, Faculty of Agriculture, Universiti Putra Malaysia. Prior to stun and slaughter procedures, all animals were subjected to minimal anaesthesia model which was conducted using the technique of Gibson et al. (Citation2009). The current study used minimal anaesthesia as a model on animals for research purpose which is not an allowed practice for animals slaughtered for human consumption. The minimal anaesthesia required to ensure that a fixed amount of electrical current is delivered to the precise anatomical locations as intended. This is crucial as mis-stunning incidences is fairly common among conscious animals (Zivotofsky & Strous Citation2012). Mis-stunning not only causes unintended tissue damage and welfare issues, but could result in higher number of animals required for the trial. Our prior studies (Sabow et al. Citation2015a, Citation2015b) have also shown that there were no significant differences in terms of bleeding efficiency, physicochemical characteristics and shelf life between fully conscious and minimally anaesthetised goats. The model was performed by rapid injection through the cephalic vein using 5 mg/kg propofol and maintained with halothane in 100% oxygen (Sabow et al. Citation2016c). The goats of the SWS groups were slaughtered without stunning following the traditional halal slaughtering method as described in the Malaysian Standard MS1500:2009 (Department of Standards Malaysia Citation2009). The halal slaughter was carried out by a certified slaughter man using an exquisitely sharp knife. Regenstein (Citation2012) and Rosen (Citation2004) reported that the use of an extremely sharp knife produces minimal behavioural reactions in animals to the neck cut and as a result, there was little or no vocalisation and movements observed during and post slaughter of the animal. The neck cut severed skin, muscle, oesophagus, trachea, carotid arteries, jugular veins and major nerves without decapitating the head during the process. The other three groups of goats were electrically stunned using electrical stunning transformer type CS-1 system manufactured by Karl Schermer (Ettlingen, Baden-Württemberg, Germany). The electrical stunning procedure was described in detail elsewhere (Sabow et al. Citation2016b). Briefly, the stunning system was connected with double-electrode scissor-type dry stunning tongs (Z3, Karl Schermer, Ettlingen, Germany) for LFHO and triple-electrode tongs (2A Handset, Kentmaster, Hartlebury, United Kingdom) for LFHB and HFHB electrical stunning, respectively. In the LFHO electrical stunning method, the head electrodes were placed between the eyes and the base of the ears on both sides of the head. In the LFHB and HFHB electrical stunning methods, the head electrodes were placed behind the ears on both sides of the head while the third electrode was placed over the spine of the animal in the region of the last thoracic and the first lumbar vertebrae. In LFHO and LFHB, a constant electrical current of 1.0 A at 50 Hz for 3.0 s were used based on the requirements of Regulation EC (Citation2009) and the recommended guideline parameters for goats stated by MUIHA2310 (Citation2012). For HFHB, the stunner output was pre-selected to deliver a 1.0 A constant electrical current for 3.0 s at 850 Hz. Based on the impedance of the goat’s tissues, voltage was automatically adjusted in all electrical stunning applications. The voltage to which goats were subjected did not differ significantly between the LFHO, LFHB and HFHB stunning techniques (203.50 ± 8.74, 216.13 ± 8.32 and 219.25 ± 5.47, respectively). The average stun-to-stick interval was 7.81 ± 0.31 s, with no significant differences (p > .05) between stunning systems. Thereafter, all the electrically stunned goats were slaughtered by severing the throat, carotid arteries and jugular veins.
Muscle sampling
After evisceration and carcase dressing, about 20 g muscle cuts from the left Semitendinosus (ST) muscle were sampled, put in a plastic bag, vacuum packaged, stored in a chiller (4 °C) and assigned for drip loss determination. The left Longissimus lumborum (LL) muscle between the 6th and 8th lumbar vertebra was detached and separated into two parts, snap frozen in liquid nitrogen and stored at −80 °C. The first part was used for the determination of pH, lactate acid and glycogen concentration on d 0 post-mortem. The second part was assigned for the evaluation of myofibrillar fragmentation index at d 0. Samples from the LL muscle between the 9th and 10th lumbar vertebra were collected and assigned for the analysis of colour, cooking loss and shear force at d 0. Subsequent sampling was carried out at specific post-mortem periods on carcases hung in 4 °C. Muscle samples from the left LL between the 11th and 12th lumbar vertebra were divided into four parts, stored at −80 °C and assigned for the analysis of glycogen, lactate and pH at 12 h, 1, 3 and 7 d post-mortem. The right LL muscle was divided into two parts: from the 6th to 10th lumbar vertebra for physical meat quality traits and from 11th to 12th lumbar vertebra for myofibrillar fragmentation index and sarcomere length. Muscle samples from both portions were trimmed free of fat and connective tissue and subjected to 3 specific periods; that is, 24 h and 7 and 14 d post-mortem storage.
Muscle lactate concentration
The muscle lactate concentration was determined using EnzyChrom™ Lactate Assay Kit # ECLC-100 (BioVision, Milpitas, CA) following the manufacturer’s procedure.
Muscle glycogen content
At the death of an animal, the supply of oxygen to the muscles stops because of the failure of the blood circulatory system. This scenario necessitates anaerobic glycolysis in which ATP is generated from glycogen (Henckel et al. Citation2002; Mačanga et al. Citation2011). Thus, the amount of muscle glycogen at slaughter plays vital role in post-mortem energy metabolism. Total muscle glycogen concentration was measured using colorimetric assay EnzyChrom™ Glycogen Assay kit # E2GN-100 (Bio Assays, Belford, MA) as outlined in the instruction manual. Glycolytic potential, the estimate of the amount of glycogen present in the muscle at the time of slaughter, has been considered a good predictor of ultimate pH (Bertol et al. Citation2005). Glycolytic potential is an important parameter for determining the extent of post-mortem muscle glycolysis (Xu et al. Citation2011). Evaluation of muscle glycolytic potential was calculated using the equation (2 × muscle glycogen content + muscle lactate concentration) proposed by Zhang et al. (Citation2009).
Muscle pH
The pH of the LL muscle was measured using a portable pH metre (Mettler Toledo, Switzerland). About 1 g of each sample was crushed and homogenised (Wiggen Hauser® D-500, Berlin, Germany) for 30 s in 20 ml ice cold deionised water in the presence of 5 mM sodium iodoacetate (Merck Schuchardt OHG, Hohenbrunn, Germany) to inhibit further glycolysis (specifically glyceraldehyde 3-phosphate dehydrogenase) and the production of lactic acid (AMSA Citation2012). The pH electrode was used for the determination of the pH of the resultant homogenates. Muscle pH change after 12 and 24 h post-mortem was calculated using the equation described by Onenc and Kaya (Citation2004):
Colour values
Meat colour values were determined using Color Flex spectrophotometer (Hunter Lab, Reston, VA) based on the International Commission on Illumination (CIE) Lab-values (also known as L*, a*, b*) with D56 illuminant and 10° standard observer, tristimulus values (X,Y,Z) and reflectance at specific wavelength (400–700) nm to express the meat colour data. Prior to use, the colorimeter was calibrated against black and white tiles. The samples of the LL muscle of about 10 mm of thickness were subjected to blooming for 30 min. The bloomed sample was placed facing base of the colorimeter cup. Triplicate readings for each sample were recorded for L* (lightness), a* (redness) and b* (yellowness) values and then averaged (Hunt Citation1980). Ratios of hue angle [tan-1 (b*/a*)] and saturation index or chroma √(a2 + b2) were calculated (MacDougall Citation1982).
Water holding capacity
The water holding capacity (WHC) of the meat samples was evaluated by measuring cooking and drip losses following the procedure of Honikel (Citation1998). Approximately 20 g of fresh meat samples from the left side of ST muscle were collected, weighed individually and recorded as initial weight (W1) for drip loss. After weighing, samples were put in polyethylene bags then vacuum packaged and stored in a 4 °C chiller for 14 d. After the completion of designated aging period, samples were removed from the polyethylene bags, blotted gently to dry, reweighed and recorded as W2. The percentage drip loss was calculated using the following formula:
For the evaluation of the cooking loss, samples (∼50 g) of LL muscles were weighed (W1), placed in polyethylene bags and vacuum packed. The samples were cooked in a pre-heated water bath set at 80 °C for 10 min once the internal temperature of the samples reached 78 °C. After removal of the cooked samples from the water bath and subsequent cooling at room temperature, the samples were blotted gently dry and reweighed (W2). The percentage of cooking loss was estimated using the following formula:
Shear force
The meat samples used for the determination of cooking loss were prepared and evaluated for texture using a texture analyser (Stable Micro System, Surrey, UK). Calibration of the device was done at 5 kg for weight and the speed of the blade and distance for height were set at 10 mm/s. Samples were prepared according to the method of Sazili et al. (Citation2005). Triplicate blocks of 1 cm (height) × 1 cm (width) × 2 cm (length) were cut from each sample and the average shear force of the blocks was used for the analysis.
Myofibril fragmentation index
The myofibril fragmentation index (MFI) was determined in line with the method of Hopkins et al. (Citation2000). Approximately 2.5 g of pulverised LL muscle were homogenised with 30 ml 20 mM ice-cold potassium phosphate buffer (pH 7.0) containing 100 mM KCl, 1 mM EDTA and 1 mM MgCl2 (Wiggen Hauser, Germany) for 60 s. The homogenates were then centrifuged at 1000g for 15 min at 2 °C. The supernatant obtained was discarded while the pellets were re-suspended in 25 ml phosphate buffer and repeated centrifugation step was ensured. Similarly, the pellets were suspended in 15 ml of buffer and vortexed after the resulted supernatant was discarded. In order to remove the remaining connective tissues, the myofibril suspensions were filtered into 50 ml conical centrifuge tubes using 1.0 mm polyethylene strainers and then rinsed with additional 15 ml of buffer. The concentration of total protein for the final suspension was assessed (Bradford Citation1976) using Bio-Rad Protein Assay Kit II 500-0002 following the manufacturer’s procedure. Standards were prepared using Bovine Serum Albumin and absorbance was measured at 595 nm using a spectrophotometer. The myofibril suspension was diluted with potassium phosphate buffer to a final protein concentration of 0.5 ± 0.05 mg/ml and the absorbance of the diluted myofibril suspensions was measured at 540 nm with a spectronic®20 GENESYSTM spectrophotometer (Spectronic Instruments, USA). Triplicate absorbance readings were averaged and multiplied by 150 to obtain the index values for myofibrillar fragmentation (Nakyinsige et al. Citation2014).
Sarcomere length
Sarcomere length was measured from the LL muscles based on the method of Kandeepan et al. (Citation2009). Two grams sample of muscle tissues were placed in 30 ml of fixative solution (5 mM EDTA, 0.039 M boric acid and 0.1 M KCl in 2.5% glutaraldehyde) and incubated for 2 h at room temperature. The muscle samples were then transferred to 30 ml of storage solution (5 mM EDTA, 0.039 M boric acid and 0.025 M KCl in 2.5% glutaraldehyde) and incubated for 24 h at 4 °C chiller. After incubation, the muscle samples were homogenised in 14 ml of 0.25 M sucrose solution for 15 s with a homogeniser (T18 digital ULTRA-TURRAX® – IKA, Germany) set at ∼6000 rpm speed. A single drop of the resulted homogenate was placed on a microscope glass slide and covered using a glass cover slip and then examined with an image analyser microscope (Olympus, BX. 51. TF, Tokyo, Japan) as described by Sabow et al. (Citation2015a). Ten myofibrils (each myofibril contains at least ten sarcomeres) were selected to calculate the average length of the sarcomere.
Haemorrhages and bone fracture evaluation
Carcase evaluation was carried out following the method of Velarde et al. (Citation2003). The carcases were scored according to the location and size of the haemorrhage. To assess the incidences of speckles (petechial haemorrhages) and blood splash (ecchymosis), tissues of the shoulders, loins and legs were used. The fiery pinpoint bleeding located in the fat or connective tissue layers over muscle was classified as petechial haemorrhages or speckles. The area of blood that is greater than 10 mm, darker in colour and located in the muscles tissues were classified as ecchymosis whereas the incidence of wider blood splashes on shoulders, back, and legs were classified as haematomas. The incidence and severity of speckles on the carcase was estimated visually, using a five-point grading scale (0 score, no speckle, to a 5 score which indicates large fiery red areas) developed by the New Zealand Meat Industry Research Institute (Kirton et al. Citation1981). On the scale, score 0 indicates the absence of splash, scores 1 and 2 indicate the incidence of one or very few mainly small haemorrhages and, at the other extreme, scores 3 and 4 show a very badly splashed carcase while score 5 indicates large fiery red areas from which many pinprick-like areas merge. In order to assess the incidence of blood splash and bone fractures in the carcases, they were dissected into shoulder (cut between the fifth and sixth thoracic vertebrae), loin (cut at the last lumbar vertebrae) and leg primals and weighed. All bones were removed from the shoulder, loin and leg primals and assessed for bone fractures and any muscle tissue affected by ecchymosis was removed, weighed and recorded (Channon et al. Citation2002). The incidences of blood splash were expressed as a percentage of shoulder, loin and leg weight.
To link the occurrence of haemorrhages to the degree of skeletal muscle contractions during the clonic phase of electrical stunning, the intensity of muscular activities was individually scored in each animal as described by Llonch et al. (Citation2015). The score 2 was considered severe when muscular convulsion included movements from the body, head and extremities, while score 1 was considered moderate when the movements were from either the extremities or the head.
Data analysis
The experiment followed a completely randomised design. Data obtained for the carcase quality were subjected to the generalised linear model (GLM) procedure of Statistical Analysis System (SAS) package Version 9.2 software (SAS Citation2007) in which the parameters were fitted as dependent variables while slaughter methods were fitted as fixed effect. Data obtained for meat quality traits were analysed using the GLM procedure of SAS in which stunning methods, post-mortem storage time and interaction between slaughter methods and post-mortem storage time were fitted as fixed effects in a repeated measure analysis of variance. Means were separated by Duncan’s multiple range test at a significance level of .05.
Results and discussion
Muscle glycogen and lactate concentration
Table shows the effect of different slaughter methods on the glycogen concentration of the LL muscle from goats. At 0, 1, 3 and 7 d post-mortem, glycogen content did not differ (p > .05) between the slaughter methods. However, at 12 h post-mortem, the LL muscle from goats subjected to SWS treatment had higher (p < .05) glycogen content compared to those subjected to LFHO, LFHB and HFHB treatments. This finding is in tandem with those of Nakyinsige et al. (Citation2014) who reported that the LL muscles from un-stunned rabbits presented higher glycogen than those from the CO2 stunned rabbits. In contrast, Onenc and Kaya (Citation2004) found that electrical stunning minimised glycogen depletion prior to slaughter of cattle. The low glycogen concentration in LFHO, LFHB and HFHB groups compared to the SWS group could be attributed to the way in which the slaughter method was achieved. Regardless of the slaughter method, muscle glycogen concentration declined significantly (p < .05) with increase in post-mortem period. This observation could be attributed to post-mortem anaerobic glycolysis which necessitates the conversion of glycogen to lactic acid.
Table 1. Differences in the glycogen and lactate content and glycolytic potential of Longissimus lumborum muscle during post-mortem aging periods in goats subjected to slaughter without stunning and slaughter following different methods of electrical stunning (n = 8).
As glycogen is broken down so lactic acid accumulates. The inability of the blood system to remove the lactic acid is responsible for the post-mortem acidification of muscle. The accumulation of lactate is an important indicator of rate of pH decline and ultimate pH of muscle (Choe et al. Citation2008). The results of lactate concentration in the LL muscle obtained from goats subjected to different slaughter methods are presented in Table . Slaughter method did not affect (p > .05) lactate concentration at 0, 1, 3 and 7 d post-mortem. Nonetheless, at 12 h post-mortem, the LL muscle from goats subjected to LFHO, LFHB and HFHB had higher lactate than those from the SWS group. These results coincide with those of glycogen concentration. As found in the current findings, increased lactate concentration was observed in turkeys (Sante et al. Citation2000) and broilers (Xu et al. Citation2011) subjected to high-frequency electrical stunning compared to low frequency. In geese and ducks, Fernandez et al. (Citation2010) also observed an increment in lactate concentrations at 30 min post-mortem after head-only electrical stunning as compared to the gas stunning. Regardless of slaughtering technique, concentration of lactate increased (p < .05) over storage. This could be attributed to the breakdown of glycogen to form lactate and the inability of the blood systems to remove the formed lactate (Kylä-Puhju et al. Citation2004).
At 12 h, the reduced glycogen and developed lactate content in electrically stunned goats could be due to the observed muscular convulsions and kicking which caused an increased usage of adenosine triphosphate (ATP) by the muscle tissues. The increase in the rate of glycogen metabolism has been previously attributed to the effect of kicking during electric stunning (Devine et al. Citation1984). When glycogen reserves are low at the time of slaughter, a small amount of lactic acid is formed during early rigour development. Animals subjected to electrical stunning which show response to stimuli by body movements is typical indicator of physiological stress (Linares et al. Citation2007; Lambooij et al. Citation2012). Farouk et al. (Citation2014) and Nakyinsige et al. (Citation2013) reported that most stunning methods increased the intensity of muscular convulsions at the beginning of the current flow. Nonetheless, these muscular activities may not necessarily signify that the welfare of an animal has been compromised, which could be explained the similarity of glycogen and lactate levels at 24 h among slaughter method groups.
Muscle glycolytic potential
The results for lactate concentration in LL muscle from goats are presented in Table . At 0, 1, 3 and 7 d post-mortem, muscle glycolytic potential was not significantly different among the treatments. Similarly, Fernandez et al. (Citation2010) found no effect of electrical stunning techniques (water bath and head-only) on muscle glycolytic potential in ducks or geese. Also, Xu et al. (Citation2011) reported no significant differences in glycolytic potential values between broiler chickens subjected to high-frequency (400 and 1000 Hz) electrical stunning and those subjected to low frequency (160 Hz). At 12 h post-mortem, the LFHO, LFHB and HFHB muscles had higher (p < .05) glycolytic potential compared to the SWS muscles. This finding can be attributed to the higher rate of post-mortem glycolysis in electrically stunned goats. The glycolytic potential increased over storage.
Muscle pH values
The ultimate pH is an important factor which influences the physical and chemical traits of meat (Mačanga et al. Citation2011; Mortimer et al. Citation2014). Table shows the results of the pH of the LL muscle from goats. Slaughtering methods had no effect (p > .05) on the pre-rigour pH (pH0) and pH at 1, 3 and 7 d post-mortem. At 12 h post-mortem, a lower (p < .05) muscle pH was observed in goats subjected to LFHO, LFHB and HFHB. This might be due to the high rate of post-mortem glycolysis. The similarity in ultimate pH across slaughter methods is consistent with the results of Onenc and Kaya (Citation2004) in cattle and Velarde et al. (Citation2003) and Vergara et al. (Citation2005) in lambs who reported similar ultimate pH in non-stunned and electrical stunned animals.
Table 2. Differences in pH and pH decline of Longissimus lumborum muscle during post-mortem aging periods in goats subjected to slaughter without stunning and slaughter following different methods of electrical stunning (n = 8).
At 12 h post-mortem, the rate of muscle pH decline (pH0 – pH12h) was faster (p < .05) in electrical stunned goats compared to SWS goats. Electrical stunning methods resulted in a slightly lower drop in pH compared with SWS at 24 h. This finding contrasts those of Onenc and Kaya (Citation2004) but agrees with the findings of Büyükünal and Nazli (Citation2007) who reported that electrical stunned animals have a higher rate of pH decline compared to the un-stunned ones. The rapid decline in muscle pH of the electrical stunned goats after 12 h post-mortem could be due to the passing of current flow through animals causing an increase in muscular activities which hastens post-mortem glycolysis (Petersen & Blackmore Citation1982; Troeger & Woltersdorf Citation1990). Likewise, Nowak et al. (Citation2007) showed that a fast decline in pH is a result of the lactate yielded during muscle spasm. Further evidence was provided by the high level of lactate found in the plasma of lambs that were electrically stunned (Bórnez et al. Citation2009). Irrespective of slaughter method, the pH values reduced significantly (p < .05) over storage. The decline in pH is due to post-mortem glycolysis (Scheffler et al. Citation2013). Usually, when animals are slaughtered and bled, they suffer hypoxia, the muscle metabolism shifts to anaerobic glycolysis. For this reason, glycogen reserves are exhausted since they are metabolised to lactic acid bringing about a reduction in pH.
Muscle colour values
The first assessment of quality of red meat by consumers is based on its colour. Meat colour is linked to both perception and real values (Holman et al. Citation2015). The influence of the slaughter methods on colour coordinates (L*, a* and b*) of LL muscle in goats is shown in Table . Slaughter method had no effect on muscle colour coordinates. This finding is in agreement with those of Vergara and Gallego (Citation2000), Velarde et al. (Citation2003) in lambs and Onenc and Kaya (Citation2004) in cattle who reported a similarity between un-stunned and electrical stunned animals for L*, a*and b* values. Irrespective of slaughter method, values of lightness increased significantly, while the values of redness and yellowness decreased (p < .05) with increasing post-mortem aging. Santé-Lhoutellier et al. (Citation2008) reported that the oxidation of myoglobin during aging time prompted a significant reduction in colour characteristics, particularly a* values. The chroma (C*) and hue (H°) were not affected by slaughter methods. Similarly, Onenc and Kaya (Citation2004) reported that electrically stunned cattle had similar C* and H° values as those slaughtered without electrical stunning.
Table 3. Differences in colour coordinates of Longissimus lumborum muscle during post-mortem aging periods in goats subjected to slaughter without stunning and slaughter following different methods of electrical stunning (n = 8).
Water-holding capacity
Water-holding capacity (WHC) is defined as the ability of meat to preserve an essential quantity of both inherent and supplemented water which are quality attributes for the industry and also for the consumers (Prevolnik et al. Citation2010; Modzelewska-Kapituła et al. Citation2015). The results of drip and cooking losses in LL muscle obtained from goats subjected to different slaughtering methods are presented in Table . Slaughter methods had no effect (p < .05) on drip loss at 1, 3 and 7 d post-mortem. The similarity in drip loss could perhaps be due to the similarity in ultimate pH across the slaughter methods. Nakyinsige et al. (Citation2014) reported that WHC is affected by muscle pH and temperature decline during post-mortem. The present findings are in agreement with those of Onenc and Kaya (Citation2004) who observed that cattle subjected to pre-slaughter electrical stunning had similar WHC as those slaughtered without stunning. Similarly, Agbeniga et al. (Citation2013) reported that drip loss in beef cattle was not affected by captive bolt stunning and kosher method of slaughtering. In addition, Vergara and Gallego (Citation2000) and Vergara et al. (Citation2005) found no significant differences in drip loss between electrically stunned lambs and those not stunned. At d 14 post-mortem, the LL muscle from goats subjected to LFHO, LFHB and HFHB had higher (p < .05) drip loss compared with those from SWS goats. This observation could be attributed the rapid pH decline in electrically stunned goats compared to the SWS goats. Schäfer et al. (Citation2002) and Huff-Lonergan and Lonergan (Citation2005) reported that the rapid decline in muscle pH increases drip loss. Moreover, Linares et al. (Citation2007) reported that increase in catecholamines as a result of stress associated with electrical stunning technique has a significant influence on proteolysis since it reduces space of muscle fibres and consequently decreases water-holding capacity. Irrespective of treatment groups, drip loss (p < .05) increased over storage. This observation may be due to the degradation of myofibrillar proteins and collagens in the course of aging which reduces the ability of the myofibrillar proteins to hold water (Pearce et al. Citation2011; Sabow et al. Citation2016a).
Table 4. Differences in drip loss, cooking loss and shear force values of Longissimus lumborum muscle during post-mortem aging periods in goats subjected to slaughter without stunning and slaughter following different methods of electrical stunning (n = 8).
Slaughter methods had a significant (p < .05) impact on cooking loss on 7 and 14 d post-mortem. The cooking loss for SWS was lower (p < .05) than that of LFHO, LFHB and HFHB on 7 and 14 d post-mortem. It has been demonstrated that the rate of decline in pH of muscles plays an important role in cooking loss of meat (Lyon & Buhr Citation1999). As observed in the current study, Linares et al. (Citation2007) found a higher cooking loss in the electrically stunned lambs compared to those slaughtered without stunning. Onenc and Kaya (Citation2004) also reported that pre-slaughter electrical stunning increased cooking loss in beef at 7 and 14 d post-mortem. According to Gregory (Citation2008), most workers posited that quality of meat from animals slaughtered without stunning is comparable to those stunned. Conversely, there is a growing body of evidence that meat obtained from non-stunned animals is better in terms of cooking loss (Onenc & Kaya Citation2004; Linares et al. Citation2007; Agbeniga et al. Citation2013; Nakyinsige et al. Citation2014). Regardless of the slaughtering technique, cooking loss reduced (p < .05) with increase in post-mortem periods. Similarly, Sabow et al. (Citation2015a) and Kannan et al. (Citation2006) found that cooking loss of LL muscle decreased over storage. The decrease in cooking loss over storage could be attributed to the structural breakdown of myofibrilar proteins caused by the blockage of the channels of skeletal muscle via which water is lost causing the formation of a ‘sponge effect’ which holds the water and inhibits it from getting lost (Huff-Lonergan & Lonergan Citation2005; Farouk Citation2012).
Shear force values
Tenderness is one of the most significant component of meat quality that influences consumers’ eating satisfaction (Hildrum et al. Citation2009) and it is inversely related to shear force. The shear force results are shown in Table . Slaughter method had no effect on shear force values throughout storage. In line with the present findings, Büyükünal and Nazli (Citation2007) reported that shear force values of meat from lambs subjected to head-only and head-to-back electrical stunning and those slaughtered without stunning were similar. Linares et al. (Citation2007) also reported that shear force values were not significantly different between electrically stunned and non-stunned lambs. The values of shear force reduced (p < .05) with aging. This could be due to the degradation of myofibrillar proteins (Li et al. Citation2012) by endogenous proteases during aging (Sazili et al. Citation2013).
Myofibril fragmentation index (MFI) and sarcomere length
Evaluating the degree of fragmentation in myofibrils is a good indicator of the degradation of the structure of the myofibrillar proteins during post-mortem aging (Kandeepan et al. Citation2009). Also, the fragmentation index of myofibrils is a reliable indicator of the degree of muscle proteolysis which is reflected in the breakdown of structural proteins, especially the breaking down of the I-band of the sarcomere and rupture of myofibril linkages (Li et al. Citation2012). Table shows the result for myofibrillar fragmentation index. On d 1, meat samples from SWS group exhibited lower (p < .05) MFI values than those from LFHO, LFHB and HFHB groups. This explains why meat samples from the SWS had a higher numerical shear force compared with meat samples from other treatments. Hou et al. (Citation2014) and Marino et al. (Citation2013) found a strong negative relationship between MFI and shear force. The high MFI in electrically stunned goat could be attributed to the faster pH decline caused by electrical stunning which might be responsible for the stimulation of muscle proteolysis. Sierra et al. (Citation2012) posited that proteolytic changes in both myofibrillar and sarcoplasmic portions occur earlier in muscles having rapid reduction in pH. Slaughter method had no effect (p > .05) on MFI values on 7 and 14 d post-mortem. This observation contrasts that of Nakyinsige et al. (Citation2014) who observed that meat obtained from gas stunned rabbits showed significantly lower MFI than un-stunned rabbits. However, Rees et al. (Citation2003) found that stunning technique (head-to-heart electrical stunning and carbon dioxide stunning) did not influence MFI values post rigour. Sabow et al. (Citation2015a) reported that MFI values in goats subjected to minimal anaesthesia prior slaughter was not different from those slaughtered without anaesthesia. Irrespective of slaughter method, the MFI values increased (p < .05) with increase in post-mortem aging period. The increment in MFI over storage could be due to the degradation of the muscle myofibrillar proteins into segments at, or near, the Z-disk during post-mortem storage. This observation is in agreement with the results of Hou et al. (Citation2014) and Hopkins et al. (Citation2004) who showed that aging increased the MFI of the muscle in cattle and sheep, respectively.
Table 5. Differences in myofibril fragmentation index (MFI) and sarcomere length of Longissimus lumborum muscle during post-mortem aging periods in goats subjected to slaughter without stunning and slaughter following different methods of electrical stunning (n = 8).
Sarcomere length is a useful indicator of meat tenderness (Li et al. Citation2012). Weaver et al. (Citation2008) reported that muscle fibres longer in lengths of sarcomere are more susceptible to post-mortem proteolysis. As shown in Table , the changes in sarcomere length in goat muscles obtained from different methods of slaughtering were not significantly different (p > .05). This suggest that all methods of slaughter reduce overlapping of actin and myosin at the same rate. This finding contradicts those of Kim et al. (Citation2013), but it is in line with those of Sabow et al. (Citation2015a) who found that the sarcomere length values in goats were not influenced by slaughter methods. Regardless of slaughter method, sarcomere length increased (p < .05) over storage.
Carcass quality
Ecchymosis (blood splash) and petechiae (speckles) are forms of haemorrhages that occur in livestock (Farouk et al. Citation2014). The former is spots of blood in muscles while the latter is petechial haemorrhages in fat or connective tissues overlying the muscle. They range in size from microscopic to several centimetres, which can affect the appearance and acceptability of the carcase (Leet et al. Citation1997). In Figures and , details of the comparison of the incidences of petechiae and ecchymosis in shoulder, loin and leg between different slaughter methods are presented. Goats slaughtered without stunning (SWS) had significantly lower (p < .05) incidence of petechiae and ecchymosis in the loin, shoulder and leg primal cuts than those from LFHO, LFHB and HFHB. No incidence of haematomas and broken bones was observed in all slaughter groups. After electric stunning, the incidence of haemorrhages in the loin, shoulder and leg increased which may be because of direct stimulation by high-energy input through the tonic and clonic phase in muscles. Similarly, Daly (Citation2005) and Gregory (Citation1998) reported that the passage of electrical current through animal tissue presents some inevitable quality consequences with the formation of haemorrhages within primal hindquarter cuts that may result from the simultaneous maximal contraction of normally opposing muscles groups. Also, Kirton et al. (Citation1981) observed no petechiae, ecchymosis/blood splash, haematomas and bone fractures in carcase and organs obtained from the un-stunned lambs compared to those from head-only and head-to-back electrical stunning groups. Velarde et al. (Citation2003) reported that haemorrhages, petechiae, ecchymosis, haematomas and bone fracture incidence in lambs subjected to head-only electrical stunning were absent. However, the authors observed higher occurrence of petechiae in the hearts of head-only electrically stunned lambs compared to non-stunned lambs. In a recent review, Farouk et al. (Citation2014) outlined the influence of stunning techniques on the severity of blood splash is in the order: no stun < percussion < captive bolt < head-to-back electrical stun < head-only electrical stun which is in tandem with the current findings.
Figure 1. Effect of slaughter without stunning and slaughter following different methods of electrical stunning on the scores of petechial haemorrhages (speckles) in goat (A) shoulder, (B) loin and (C) leg primal. SWS: slaughter without stunning; LFHO: low-frequency head-only, electrical stunning; LFHB: low-frequency head-to-back electrical stunning; HFHB: high-frequency head-to-back electrical stunning. Values with different letters are significantly different at p < .05. *0 score indicates the absence of splash; 1 and 2 score indicates the incidence of one or very few mainly small haemorrhages; 3 and 4 score shows a very badly splashed carcase and score 5 indicates large fiery red areas from which many pinprick-like areas merge (Kirton et al. Citation1981).
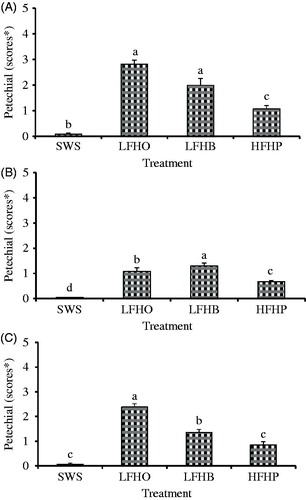
Figure 2. Effect of slaughter without stunning and slaughter following different methods of electrical stunning on the percentage of ecchymosis-affected (blood splash) meat trimmed from the (A) shoulder, (B) loin and (C) leg primal in goats. SWS: slaughter without stunning; LFHO: low-frequency head-only, electrical stunning; LFHB: low-frequency head-to-back electrical stunning, HFHB: high-frequency head-to-back electrical stunning. Values with different letters are significantly different at p < .05.
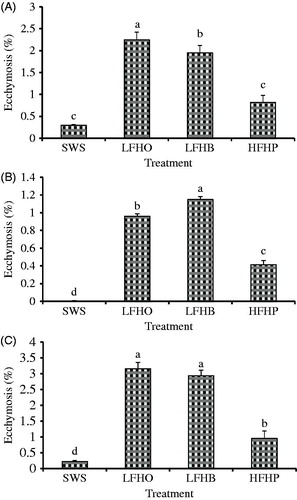
Carcasses from HFBH had lower petechiae and ecchymosis (p < .05) than those from LFHO and LFHB. The current findings show that the use of low-frequency electrical currents to stun animals trigger a higher intensity of muscle contraction through the tonic phase and a rise in blood pressure with rupture of blood vessels showing the presence of haemorrhages in the muscle tissues of the three cuts studied. According to Gregory (Citation1998), the use of low-frequency electric current (50 Hz) to stun red meat animals pre-slaughter stimulates the peripheral motor neuron, muscle fibres and motor areas in the brain which results in muscle contraction as well as capillary rupture (blood splash/haemorrhage) due to the nature of muscle contractile forces. However, high-frequency currents flow close to the surface of a conductor, hence may produce less severe muscular convulsions at the beginning of the current flow (Gregory et al. Citation1991) which were 1.63 ± 0.18, 0.48 ± 0.11 and 0.76 ± 0.13 for LFHO, LFHB and HFHB, respectively, in this study. This finding is in agreement with that of Monk (Citation1990) who reported that the level of carcase downgrading in pigs can be reduced by increasing the frequency from 50 to 1500 Hz when using head-to-back electrical stunning. Similarly, Wilkins and Wotton (Citation2002) reported that carcases from turkeys subjected to high-frequency electrical stunning had significantly lower haemorrhages than those from low-frequency electrical stunning. A number of studies relating to carcase characteristics and meat quality assessment of poultry subjected to electrical water-bath stunning at high frequencies were reviewed by Prinz (Citation2009). The author concluded that the incidences of bone breakage and haemorrhage in breast muscles in stunned broilers with high frequency of 1500 Hz were significantly lower compared to 50 Hz.
Conclusions
The present findings indicate that except for cooking loss, most chemical and physical properties of LL muscle from goats slaughtered without stunning are comparable to those subjected to electrical stunning prior to slaughter. Although carcases from electrical stunned groups had higher incidence of petechiae and ecchymosis than those slaughter without stunning, the use of high frequency head-to-back electrical stunning reduced the occurrence of petechiae and ecchymosis in the shoulder and loin and leg primals compared to low-frequency head-only or head-to-back electrical stunning. Further studies to examine the efficacies of higher electrical frequencies and the development of new electrical stunning techniques which would fulfil welfare of animals and carcase and meat quality requirements are suggested.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Agbeniga B, Webb E, O’Neill H. 2013. Influence of Kosher (Shechita) and conventional slaughter techniques on shear force, drip and cooking loss of beef. S Arf J Anim Sci. 43:98–102.
- AMSA. 2012. AMSA meat color measurement guidelines. Illinois, USA: American Meat Science Association.
- Bertol T, Ellis M, Ritter M, McKeith F. 2005. Effect of feed withdrawal and handling intensity on longissimus muscle glycolytic potential and blood measurements in slaughter weight pigs. J Anim Sci. 83:1536–1542.
- Bórnez R, Linares M, Vergara H. 2009. Systems stunning with CO(2) gas on Manchego light lambs: physiologic responses and stunning effectiveness. Meat Sci. 82:133–138.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Büyükünal S, Nazli B. 2007. Effect of different electrical stunning methods on meat quality of marmara Kivircik breed lamb in Turkey Republic. Vet Gla. 61:155–162.
- Channon HA, Payne AM, Warner RD. 2002. Comparison of CO(2) stunning with manual electrical stunning (50 Hz) of pigs on carcass and meat quality. Meat Sci. 60:63–68.
- Choe J, Choi Y, Lee S, Shin H, Ryu Y, Hong K, Kim B. 2008. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. 80:355–362.
- Daly C. 2005. The use of alternative electrical frequencies for stunning of livestock before religious slaughter. Anim Welfare Ritual Slaugh. 4:77–84.
- Department of Standards Malaysia. 2009. MS1500:2009 (1st revision) Halal food-production, preparation, handling and storage-general guideline; p. 1–13.
- Devine C, Ellery S, Wade L, Chrystall B. 1984. Differential effects of electrical stunning on the early post-mortem glycolysis in sheep. Meat Sci. 11:301–309.
- EC. 2009. European Community Council Regulations (EC) No 1099/2009 on the protection of animals at the time of killing. Off J Eur Union L. 303:1–30.
- EU. 1993. Directive 93/119/EU on the protection of animals at the time of slaughter or killing. Off J Eur Commun. 340:21–34.
- Farouk M. 2012. New Zealand meat industry must optimise halal market opportunities. New Zealand Food Technology. 47:9.
- Farouk M. 2013. Advances in the industrial production of halal and kosher red meat. Meat Sci. 95:805–820.
- Farouk M, Al-Mazeedi H, Sabow A, Bekhit A, Adeyemi K, Sazili A, Ghani A. 2014. Halal and Kosher slaughter methods and meat quality: a review. Meat Sci. 98:505–519.
- Fernandez X, Lahirigoyen E, Auvergne A, Molette C, Bouillier-Oudot M. 2010. The effects of stunning methods on product qualities in force-fed ducks and geese. 1. Carcass downgrading and meat quality. Animal. 4:128–138.
- Gibson T, Johnson C, Murrell J, Hulls C, Mitchinson S, Stafford K, Johnstone A, Mellor D. 2009. Electroencephalo-graphic responses of halothane-anaesthetised calves to slaughter by ventral-neck incision without prior stunning. NZ Vet J. 57:77–83.
- Gilbert K, Devine C, Hand R, Ellery S. 1984. Electrical stunning and stillness of lambs. Meat Sci. 11:45–58.
- Gregory N. 1998. Animal welfare and meat science. Chapter 4. UK, Wallingford: CABI publishing; p. 64–92.
- Gregory N. 2007. Animal welfare and meat production, 2nd ed. Wallingford, UK: CABI Publishing; p. 213–226.
- Gregory N. 2008. Animal welfare at markets and during transport and slaughter. Meat Sci. 80:2–11.
- Gregory N, Wilkins L, Wotton S. 1991. Effect of electrical stunning frequency on ventricular fibrillation, downgrading and broken bones in broilers, hens and quails. Br Vet J. 147:71–77.
- Henckel P, Karlsson A, Jensen MT, Oksbjerg N, Petersen JS. 2002. Metabolic conditions in Porcine longissimus muscle immediately pre-slaughter and its influence on peri- and post mortem energy metabolism. Meat Sci. 62:145–155.
- Hildrum KI, Rødbottez R, Høy M, Berg J, Narum B, Wold JP. 2009. Classification of different bovine muscles according to sensory characteristics and Warner Bratzler shear force. Meat Sci. 83:302–307.
- Holman BWB, Ponnampalam EN, van de Ven RJ, Kerr MG, Hopkins DL. 2015. Lamb meat colour values (HunterLab CIE and reflectance) are influenced by aperture size (5 mm v. 25 mm). Meat Sci. 100:202–208.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:447–457.
- Hopkins D, Littlefield P, Thompson J. 2000. A research note on factors affecting the determination of myofibrillar fragmentation. Meat Sci. 56:19–22.
- Hopkins D, Martin L, Gilmour A. 2004. The impact of homogenizer type and speed on the determination of myofibrillar fragmentation. Meat Sci. 67:705–710.
- Hou X, Liang R, Mao Y, Zhang Y, Niu L, Wang R, Liu C, Liu Y, Luo X. 2014. Effect of suspension method and aging time on meat quality of Chinese fattened cattle M. Longissimus dorsi. Meat Sci. 96:640–645.
- Huff-Lonergan E, Lonergan SM. 2005. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 71:194–204.
- Hunt MC. 1980. Meat color measurements. Meat conference of the American Meat Science Association. Lafayette, Indiana, USA: Purdue University; p. 41–46.
- Kandeepan G, Anjaneyulu A, Kondaiah N, Mendiratta S, Lakshmanan V. 2009. Effect of age and gender on the processing characteristics of buffalo meat. Meat Sci. 83:10–14.
- Kannan G, Gadiyaram K, Galipalli S, Carmichael A, Kouakou B, Pringle T, McMillin K, Gelaye S. 2006. Meat quality in goats as influenced by dietary protein and energy levels, and postmortem aging. Small Rumin Res. 61:45–52.
- Kim GD, Lee HS, Jung EY, Lim HJ, Seo HW, Lee YH, Jang SH, Baek SB, Joo ST, Yang HS. 2013. The effects of CO2 gas stunning on meat quality of cattle compared with captive bolt stunning. Livest Sci. 157:312–316.
- Kirton A, Bishop W, Mullord M, Frazerhurst L. 1978. Relationships between time of stunning and time of throat cutting and their effect on blood pressure and blood splash in lambs. Meat Sci. 2:199–206.
- Kirton A, Frazerhurst L, Bishop W, Winn G. 1981. A comparison of the effects of electrical, captive bolt or percussion stunning on the incidence of blood splash in lambs. Meat Sci. 5:407–411.
- Kylä-Puhju M, Ruusunen M, Kivikari R, Puolanne E. 2004. The buffering capacity of porcine muscles. Meat Sci. 67:587–593.
- Lambooij E, van der Werf J, Reimert H, Hindle V. 2012. Restraining and neck cutting or stunning and neck cutting of veal calves. Meat Sci. 91:22–28.
- Leet N, Devine C, Gavey A. 1997. The histology of blood splash in lamb. Meat Sci. 1:229–234.
- Li K, Zhang Y, Mao Y, Cornforth D, Dong P, Wang R, Zhu H, Luo X. 2012. Effect of very fast chilling and aging time on ultra-structure and meat quality characteristics of Chinese Yellow cattle M. Longissimus lumborum. Meat Sci. 92:795–804.
- Linares M, Bórnez R, Vergara H. 2007. Effect of different stunning systems on meat quality of light lamb. Meat Sci. 76:675–681.
- Linares M, Vergara H. 2012. Effect of gas stunning and modified-atmosphere packaging on the quality of meat from Spanish Manchego light lamb. Small Rumin Res. 108:87–94.
- Llonch P, Rodríguez P, Casal N, Carreras R, Muñoz I, Dalmau A, Velarde A. 2015. Electrical stunning effectiveness with current levels lower than 1 A in lambs and kid goats. Res Vet Sci. 98:154–161.
- Lyon C, Buhr R. 1999. Biochemical basis of meat texture. In: RI Richardson, GC Mead, editor. Poultry meat science. New York, NY: CAB Int; p. 99–126.
- Mačanga J, Koréneková B, Nagy J, Marcinčák S, Popelka P, Kožárová I, Korének M. 2011. Post-mortem changes in the concentration of lactic acid, phosphates and pH in the muscles of wild rabbits (Oryctolagus cuniculus) according to the perimortal situation. Meat Sci. 88:701–704.
- MacDougall D. 1982. Changes in the colour and opacity of meat. Food Chem. 9:75–88.
- Marino R, Albenzio M, della Malva A, Santillo A, Loizzo P, Sevi A. 2013. Proteolytic pattern of myofibrillar protein and meat tenderness as affected by breed and aging time. Meat Sci. 95:281–287.
- McNeal W, Fletcher D, Buhr R. 2003. Effects of stunning and decapitation on broiler activity during bleeding, blood loss, carcass, and breast meat quality. Poult Sci. 82:163–168.
- Modzelewska-Kapituła M, Kwiatkowska A, Jankowska B, Dąbrowska E. 2015. Water holding capacity and collagen profile of bovine m. infraspinatus during postmortem ageing. Meat Sci. 100:209–216.
- Monk J. 1990. Electrical stunning in pigs (high frequency) [M.Sc. dissertation]. UK: University of Bristol.
- Mortimer S, van der Werf J, Jacob R, Hopkins D, Pannier L, Pearce K, Gardner G, Warner R, Geesink G, Hocking Edwards J. 2014. Genetic parameters for meat quality traits of Australian lamb meat. Meat Sci. 96:1016–1024.
- MUIHAS2310. 2012. Guidelines of halal assurance system criteria on slaughter-houses. Majelis Ulama Indonesia. LPPOM MUI. Available from: http://www.mui.or.id/
- Nakyinsige K, Che Man Y, Aghwan Z, Zulkifli I, Goh Y, Abu Bakar F, Al-Kahtani H, Sazili A. 2013. Stunning and animal welfare from Islamic and scientific perspectives. Meat Sci. 95:352–361.
- Nakyinsige K, Sazili A, Zulkifli I, Goh Y, Fatimah A, Sabow A. 2014. Influence of gas stunning and halal slaughter (no stunning) on rabbits welfare indicators and meat quality. Meat Sci. 98:701–708.
- Nowak B, Mueffling T, Hartung J. 2007. Effect of different carbon dioxide concentrations and exposure times in stunning of slaughter pigs: impact on animal welfare and meat quality. Meat Sci. 75:290–298.
- Onenc A, Kaya A. 2004. The effects of electrical stunning and percussive captive bolt stunning on meat quality of cattle processed by Turkish slaughter procedures. Meat Sci. 66:809–815.
- Pearce KL, Rosenvold K, Andersen HJ, Hopkins DL. 2011. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes-A review. Meat Sci. 89:111–124.
- Petersen G, Blackmore D. 1982. The effect of different slaughter methods on the post mortem glycolysis of muscle in lambs. N Z Vet J. 30:195–198.
- Petersen G, Carr D, Davies A, Pickett B. 1986. The effect of different methods of electrical stunning of lambs on blood pressure and muscular activity. Meat Sci. 16:1–15.
- Prevolnik M, Čandek-Potokar M, Škorjanc D. 2010. Predicting pork water-holding capacity with NIR spectroscopy in relation to different reference methods. J Food Eng. 98:347–352.
- Prinz S. 2009. Electrical stunning of broiler chickens. World Poultry Science Association, Proceedings of the 19th European Symposium on Quality of Poultry Meat, 13th European Symposium on the Quality of Eggs and Egg Products, Turku, Finland, 21-25 June 2009. World's Poultry Science Association (WPSA); p. 1–8.
- Rees MP, Trout GR, Warner RD. 2003. The influence of the rate of pH decline on the rate of ageing for pork. I: interaction with method of suspension. Meat Sci. 65:791–804.
- Regenstein JM. 2012. The Politics of Religious Slaughter-How Science Can be Misused, 65th Annual Reciprocal Meat Conference at North Dakota State University in Fargo, ND.
- Rosen S. 2004. Physiological insights into Shechita. Vet Rec. 154:759–765.
- Sabow A, Sazili A, Aghwan Z, Zulkifli I, Goh Y, Ab Kadir M, Nakyinsige K, Kaka U, Adeyemi K. 2016a. Changes of microbial spoilage, lipid-protein oxidation and physicochemical properties during postmortem refrigerated storage of goat meat. Anim Sci J. 87:816–826.
- Sabow AB, Zulkifli I, Goh YM, Ab Kadir MZA, Kaka U, Imlan JC, Abubakar AA, Adeyemi KD, Sazili AQ. 2016b. Bleeding efficiency, microbiological quality and oxidative stability of meat from goats subjected to slaughter without stunning in comparison with different methods of pre-slaughter electrical stunning. PLoS One. 11:e0152661. Available from: http://dx.doi.org/10.1371/journal.pone.0152661
- Sabow A, Goh Y, Zulkifli I, Sazili A, Kaka U, Ab Kadi M, Ebrahimi M, Nakyinsige K, Adeyemi K. 2016c. Blood parameters and electroencephalographic responses of goats to slaughter without stunning. Meat Sci. 121:148–155.
- Sabow A, Sazili A, Zulkifli I, Goh Y, Ab Kadir M, Adeyemi K. 2015a. Physico-chemical characteristics of longissimus lumborum muscle in goats subjected to halal slaughter and anesthesia (halothane) pre-slaughter. Anim Sci J. 86:981–991.
- Sabow A, Sazili A, Zulkifli I, Goh Y, Ab Kadir MA, Abdulla N, Nakyinsige K, Kaka U, Adeyemi K. 2015b. A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Sci. 104:78–84.
- Santé-Lhoutellier V, Engel E, Gatellier P. 2008. Assessment of the influence of diet on lamb meat oxidation. Food Chem. 109:573–579.
- Sante V, Le Pottier G, Astruc T, Mouchoniere M, Fernandez X. 2000. Effect of stunning current frequency on carcass downgrading and meat quality of turkey. Poult Sci. 79:1208–1214.
- SAS. 2007. User’s Guide 9. 2nd ed. Cary, (NC): SAS Inst. Inc.
- Sazili A, Norbaiyah B, Zulkifli I, Goh Y, Lotfi M, Small A. 2013. Quality assessment of longissimus and semitendinosus muscles from beef cattle subjected to non-penetrative and penetrative percussive stunning methods. Asian-australas J Anim Sci. 26:723.
- Sazili A, Parr T, Sensky P, Jones S, Bardsley R, Buttery P. 2005. The relationship between slow and fast myosin heavy chain content, calpastatin and meat tenderness in different ovine skeletal muscles. Meat Sci. 69:17–25.
- Schäfer A, Rosenvold K, Purslow PP, Andersen HJ, Henckel P. 2002. Physiological and structural events post mortem of importance for drip loss in pork. Meat Sci. 61:355–366.
- Scheffler TL, Scheffler JM, Kasten SC, Sosnicki AA, Gerrard DE. 2013. High glycolytic potential does not predict low ultimate pH in pork. Meat Sci. 95:85–91.
- Sierra V, Fernández-Suárez V, Castro P, Osoro K, Vega-Naredo I, García-Macía M, Rodríguez-Colunga P, Coto-Montes A, Oliván M. 2012. Identification of biomarkers of meat tenderisation and its use for early classification of Asturian beef into fast and late tenderising meat. J Sci Food Agric. 92:2727–2740.
- Troeger K, Woltersdorf W. 1990. Electrical stunning and meat quality in the pig. Fleischwirtschaft. 70:901–904.
- Velarde A, Gispert M, Diestre A, Manteca X. 2003. Effect of electrical stunning on meat and carcass quality in lambs. Meat Sci. 63:35–38.
- Vergara H, Gallego L. 2000. Effect of electrical stunning on meat quality of lamb. Meat Sci. 56:345–349.
- Vergara H, Linares M, Berruga M, Gallego L. 2005. Meat quality in suckling lambs: effect of pre-slaughter handling. Meat Sci. 69:473–478.
- Weaver A, Bowker B, Gerrard D. 2008. Sarcomere length influences postmortem proteolysis of excised bovine semitendinosus muscle. J Anim Sci. 86:1925–1932.
- Wilkins L, Wotton S. 2002. Effect of frequency of the stunning current waveform on carcase and meat quality of turkeys processed in a commercial plant in the UK. Br Poult Sci. 43:231–237.
- Wotton S, Gregory N. 1986. Pig slaughtering procedures: time to loss of brain responsiveness after exsanguination of cardiac arrest. Res Vet Sci. 40:148–151.
- Xu L, Zhang L, Yue H, Wu S, Zhang H, Ji F, Qi G. 2011. Effect of electrical stunning current and frequency on meat quality, plasma parameters, and glycolytic potential in broilers. Poult Sci. 90:1823–1830.
- Zhang L, Yue H, Zhang H, Xu L, Wu S, Yan H, Gong Y, Qi G. 2009. Transport stress in broilers: I. Blood metabolism, glycolytic potential, and meat quality. Poult Sci. 88:2033–2041.
- Zivotofsky AZ, Strous RD. 2012. A perspective on the electrical stunning of animals: Are there lessons to be learned from human electro-convulsive therapy (ECT)? Meat Sci. 90:956–961.
