Abstract
The digestibility (in vitro), toxicity and metabolic effects of rapeseed (RPH) and sunflower (SPH) protein hydrolysates have been evaluated in a murine animal model. The enzyme Alcalase® was employed to obtain a mild enzymatic hydrolysis of rapeseed and sunflower defatted seed meals (DSM) protein isolates. Both hydrolysates showed higher in vitro digestibility than the respective DSM, presumably as a consequence of the hydrolysis process that they had undergone. In vivo, RPH and SPH were well tolerated. Body and organ weights, biochemical blood parameters from treated male mice were comparable to controls. Food intake was regular in RPH and SPH animals, suggesting a good palatability of the hydrolysates. Not relevant perturbations of the principal hepatic and renal drug metabolism enzymes were observed in RPH or SPH mice. In conclusion, protein hydrolysates from sunflower and rapeseed DSM did not determine relevant toxicological effects; therefore, they could be considered as alternative protein sources and/or food ingredients.
Introduction
In the context of renewable energy, biofuel supply chains offer undeniable advantages that have led the European Union to stimulate their spread since 2003. Among these, the best known is the biodiesel chain, derived by a simple fatty acid methylation starting from renewable vegetable oils as those obtained from rapeseed, sunflower and other seeds. Nevertheless, the biodiesel penetration and diffusion in the market is still weak due to the low oil yields and insufficient valorisation of the related by-products, as glycerine and defatted seed meals (DSM), for developing industrial, agricultural and veterinary added value products.
One of the multi-applications of DSM from rapeseed and sunflower seeds is the production of protein hydrolysates to be used as an alternative source of protein for the food industry and especially for pet food. This need arises from the growing demand of products, in particular for dogs and cats, suffering from adverse food reactions (AFRs) (Kang et al. Citation2014; Willis-Mahn et al. Citation2014; Suto et al. Citation2015).
Since such disorders are mostly due to the food proteins, the treatment of these diseases involves the replacement of standard proteins with novel protein sources. In this regard, the home-prepared diet is considered the gold standard elimination diet (Scott et al. Citation2001; Biourge et al. Citation2004) but is time-consuming and requires a great deal of cooperation from the owner over a period of several weeks (Tapp et al. Citation2002). Moreover, home-made diets may also not be adequate for maintenance after diagnosis or even for diagnosis in young growing animals (Roudebush & Cowell Citation1992; Streiff et al. Citation2002).
More recently, hydrolysed veterinary diets have been proposed for the diagnosis of canine AFRs (Olivry & Bizikova Citation2010; Gaschen & Merchant Citation2011). During hydrolysis, protein sources are enzymatically broken down to polypeptides, changing and reducing the allergenic properties of the molecules (Boumans Citation2001; Cave & Guilford Citation2004). Consequently, hydrolysed proteins result less allergenic and more digestible (Sá et al. Citation2013). In this regard, in a study by Jackson et al. (Citation2003), the majority of dogs with confirmed adverse reactions to soy and corn did not show a flare-up of their skin disease when fed a soy hydrolysate and corn starch diet. Differences in the degree of hydrolysation and protein source can influence the clinical response as shown in experimentally sensitised dogs (Olson et al. Citation2000). The protein hydrolysates currently used for AFRs treatment mainly derive from animal by-products (including chicken, poultry liver, casein) and non-EU seeds as soybeans, implying an expensive economic and environmental effort. Trying to overcome this problem, EU rapeseed and sunflower DSM (derived from the biodiesel chain), were investigated for their possible application in this context, also thanks to their high-protein content (Lomascolo et al. Citation2012). In addition, the rapeseed protein isolate was considered safe by the Europeans Food Safety Authority (EFSA NDA Panel Citation2013).
In the present study, a mild enzymatic hydrolysis of DSM protein isolates, by food-grade proteases, as Alcalase® enzyme, was performed in order to obtain a product rich in small peptides, that could be more easily absorbed by the animal intestine than the original proteins, and free of non-protein anti-nutritional compounds and fibres (Parrado et al. Citation1993; Conde et al. Citation2005; Chabanon et al. Citation2007).
After rapeseed (RPH) and sunflower (SPH) protein hydrolysates production, for the first time, an investigation only focussed on their safety was conducted as a prerequisite for determining the suitability of these foodstuffs to be potentially added to pet diets. Therefore, the aim of this study was the evaluation of digestibility (in vitro), toxicity and metabolic effects (on phase I and II enzymes) of the diets containing RPH and SPH, in mice.
Materials and methods
Chemicals
For the enzymatic hydrolysis, Alcalase® (endopeptidase from Bacillus licheniformis, 2.4L) was purchased from Sigma-Aldrich (St. Louis, MO).
For the determination of the in vitro digestibility, the following chemicals were used: gastric lipase, Amano Lipase F-AP15 (Amano Enzyme Inc., Nagoya, Japan); bile salts, cholic acid–deoxycholic acid sodium salt mixture (Fluka, Milwaukee, WI); pancreatin from porcine pancreas and pepsin from porcine gastric mucosa (Sigma-Aldrich, St. Louis, MO).
For the determination of phase I and II enzymatic activities, the following chemicals were used: Nicotinamide adenine dinucleotide phosphate, oxidised and reduced form (NADP+ and NADPH), 7-ethoxyresorufin, p-nitrophenol, aminopyrine, ethoxycoumarin, pentoxyresorufin, methoxyresorufin, resorufin, ethoxycoumarin, bovin serum albumin, l-glutathione reduced, Folin–Ciocalteu reagent, 1-chloro-2,4-dinitrobenzene, 1-naphtol were purchased from Sigma Chemical Co. (St. Louis, MO); glucose 6-phosphate, glucose 6-phosphate dehydrogenase and cytochrome c were from Boehringer-Mannheim (Mannheim, Germany). All other chemicals and solvent used were of the highest available commercially purity.
Enzymatic hydrolysis
Rapeseed and sunflower DSM were produced by industrial mechanical extraction based on a hexane process, and provided by Italcol S.p.A Castelfiorentino (Florence, Italy). The two hydrolysates SPH and RPH were prepared as follows: protein isolates were obtained from DSM by alkaline extraction (pH 10.5) and isoelectric precipitation (at pH 4.3 for sunflower and pH 5.0 for rapeseed proteins) as described by Villanueva et al. (Citation1999) and Vioque et al. (Citation1999), with the difference that the sedimentation/flotation step was skipped. Hydrolysis was performed on the obtained protein isolates, suspended in distilled water (10%, w/v), adding Alcalase® (endopeptidase from Bacillus licheniformis, 2.4L), 0.2 AU g−1 (Alcalase–meal protein ratio). The process was followed by the pH-stat technique (Mettler Toledo DL 50 Graphix), for 60 min, at pH 8 and 50 °C and stopped by adjusting the pH to 6.0 with citric acid. The final hydrolysates were then recovered by centrifugation (30,000g, 30 min, 10 °C), freeze-dried, and stored at room temperature.
The composition of the two hydrolysates SPH and RPH was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 15% polyacrylamide gels in Tris-Glycine SDS running buffer (25 mM Tris-HCl pH 8.3, 192 mM Glycine, 0.1% SDS) and Coomassie blue staining technique. Hydrolysate peptide molecular weights were estimated by following the GE Healthcare protocols and by using the low molecular weight calibration kit for SDS electrophoresis (GE Healthcare, 14–94 kDa).
The SPH, RPH and protein isolates, obtained from rapeseed and sunflower DSM, were analysed by size gel filtration chromatography using an AKTA fast protein liquid chromatography (FPLC) system equipped with a Superose 12 HR 10/30 column (Amersham Biosciences) for peptide profile determination (Ugolini et al. Citation2015). The FPLC chromatograms of RPH and SPH hydrolysates were compared with the respective peptide profile of the protein isolates. Elution was monitored at 280 nm with a UV UCP-900 monitor (Amersham Biosciences, Amersham, UK). Molecular weights were determined with a calibration curve made with blue dextran (2000 kDa), catalase (240 kDa), aldolase (158 kDa) and chymotripsinogen A (25 kDa) as standards.
Free amino acids were determined by reversed phase HPLC analysis and automated precolumn derivatization with o-phtalaldehyde-3-mercaptopropionic acid (OPA) and 9-fluorenylmethylchloroformate (FMOC), as described in Ugolini et al. (Citation2015). A Hewlett-Packard Model 1100 system, coupled with a diode array detector, and a Thermo Scientific Syncronicis-C18 column (3 × 250 mm, 5 μm) was used.
In vitro digestibility
In vitro digestibility of diets containing DSM or protein hydrolysates was determined using the two-step procedure proposed by Biagi et al. (Citation2016), consisting of an incubation of the samples at 39 °C for 2 h with HCl and pepsin followed by 4 h with pancreatin; gastric lipase (10 g/L) was added to the HCl-pepsin solution and bile salts (25 g/L) were added to the pancreatin solution.
There were four replicates (flasks) for each of the following four treatments:
5 g cereal mix for dogs +4.45 g rapeseed DSM (CM + rapeseed DSM)
5 g cereal mix for dogs +4.85 g sunflower DSM (CM + sunflower DSM)
5 g cereal mix for dogs +2.43 g RPH (CM + RPH)
5 g cereal mix for dogs +1.92 g SPH (CM + SPH)
After in vitro digestion, the undigested fraction of each bottle was dried at 70 °C until a constant dry weight was obtained. Chemical composition of diets and undigested residues was determined according to AOAC standard methods (AOAC Citation2000; Method 954.01 for crude protein, Method 920.39 for ether extract, Method 942.05 for crude ash, Method 920.40 for starch) and neutral detergent fibre (NDF) was assayed accordingly to the procedure described by Van Soest et al. (Citation1991), with a heat stable amylase and expressed inclusive of residual ash. Experimental diets were used in different amounts to ensure that each flask contained the same protein amount. The cereal mix was a commercial mix of cereals and vegetables (COOP Italia, Italy). Chemical composition of the ingredients and experimental diets is reported in Table .
Table 1. Chemical composition of ingredients and experimental diets used in the in vitro digestibility trial (% of dry matter).
In vivo trial with mice
All the experimental procedures were carried out in conformity with protocols endorsed by the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Bologna (Permit Number: 10-71-2012). All efforts were made to minimise suffering. All animal studies comply with the ARRIVE guidelines.
Twenty-four male Swiss-Albino CD1 mice (Harlan Laboratories S.R.L., Udine, Italy), 5 weeks old, weighing 39.7 ± 3.4 g, were housed under controlled conditions (12 h light–dark cycle, 22 °C, 50% humidity). All animals were examined for clinical signs on receipt and none had abnormalities suggestive of ill health. After one week of acclimatisation, mice were randomly assigned to three experimental groups, 4 animals per cage: i) Control (fed a standard rodent chow, Mucedola s.r.l. Milan, Italy), ii) RPH diet (fed a diet made of standard rodent chow +10% RPH, Mucedola s.r.l.), iii) SPH diet (fed a diet made of standard rodent chow +10% of SPH, Mucedola s.r.l. Milan, Italy). Both RPH and SPH were used at 10% and provided to mice 70 and 83 g of protein per kg of diet, respectively. Food and tap water were offered ad libitum. The animals received the experimental diets for 28 consecutive days.
Body weight and food intake
Body weight was recorded at pre-test and thereafter weekly. Food intake was estimated by subtracting the amount of food left in the cages from the total amount provided every two days.
Signs and symptoms
During the study period, each mouse was visually inspected daily for the development of any physical appearance abnormalities of cutaneous apparatus, respiratory, cardiovascular and gastrointestinal systems.
Blood biochemistry parameters
Blood samples (one sample for mouse) were collected from the tail vein immediately before the sacrifice, transferred into heparinised tubes, and centrifuged for 15 min at 1500g to obtain plasma. Routinely measured biochemical parameters (glucose, creatinine, ALT, fructosamine, NEFA, total cholesterol, triglycerides, plasmatic total protein, albumin, albumin/globulins) were analysed in plasma samples by the Department of Veterinary Medical Sciences, University of Bologna. In particular, NEFA were measured using a commercial kit based on an in vitro enzymatic colorimetric method (NEFA-HR, Wako Chemicals GmbH, Neuss, Germany). The other blood parameters were assayed by colorimetric methods in automated chemistry analyser (AU 400, Olympus/Beckman Coulter, Brea CA).
Phase I and II enzymatic activities
In mice, the modulation of the main hepatic and renal enzymes of drug metabolism was assessed, in order to eventually highlight the associated implications (as drug–food interactions).
Tissue collection and preparation of subcellular fractions
Mice were fasted 16 h prior to being sacrificed. Animals were killed in accordance with approved Ministerial procedures appropriate to the species. Liver and kidney were immediately removed from each mouse, weighed and processed separately. The S9 fraction and the post-mitochondrial supernatant were prepared as recently described by Canistro et al. (Citation2016). The fractions were immediately frozen in liquid nitrogen, stored at −80 °C and used within a week for enzymatic analyses. In this short period, the activities measured in conditions of Vmax and linearity of protein contents were stable.
Phase I enzymatic activities
Aminopyrine N-demethylase (APND)
Activity was determined by the quantification of CH2O release, according to Mazel (Citation1971). The total incubation volume was 3 ml, composed of 0.5 ml of water solution of 50 mM aminopyrine and 25 mM MgCl2, 1.48 ml of 0.60 mM NADP+, 3.33 mM G6P in 50 mM Tris-HCl buffer (pH 7.4), 0.02 ml G6PDH (0.93 U/ml) and 0.125 ml of sample. After 5 minutes of incubation at 37 °C, the yellow colour developed by the reaction of the released of CH2O with the Nash reagent was read at 412 nm, and the molar absorptivity of 8,000 used for calculation (Nash Citation1953; Barillari et al. Citation2007).
p-Nitrophenol hydroxylase (p-NPH)
Activity was determined in a final volume of 2 ml: 2 mM p-nitrophenol in 50 mM Tris-HCl buffer (pH 7.4), 5 mM MgCl2, and a NADPH-generating system consisting of 0.4 mM NADP+, 30 mM isocytrate, 0.2 U of isocytrate dehydrogenase and 1.5 mg of proteins. After 10 minutes of incubation at 37 °C, the reaction was terminated by addition of 0.5 ml of 0.6 N perchloric acid. Precipitated proteins were removed by centrifugation and 1 ml of the resultant supernatant was mixed with 1 ml of 10 N NaOH. Absorbance at 546 nm was immediately recorded and 4-nitrocathecol determined (ɛ = 10.28 mM−1 cm−1) (Reinke & Mayer Citation1985; Vivarelli et al. Citation2016a).
Pentoxyresorufin O-dealkylase (PROD), ethoxyresorufin O-deethylase, (EROD) and methoxyresorufin O-demethylase (MROD)
Reaction mixture (PROD) consisted of 0.025 mM MgCl2, 200 mM pentoxyresorufin, 0.32 mg of proteins and 130 mM NADPH in 2.0 ml 0.05 M Tris-HCl buffer (pH 7.4). Resorufin formation at 37 °C was calculated by comparing the rate of increase in relative fluorescence to the fluorescence of known amounts of resorufin (excitation 563 nm, emission 586 nm) (Lubet et al. Citation1985). EROD and MROD activities were measured exactly in the same manner as described for the pentoxyresorufin assay, except that substrate concentration was 1.7 mM for ethoxyresorufin and 5 mM for methoxyresorufin (Burke et al. Citation1985; Melega et al. Citation2013).
Ethoxycoumarin O-deethylase (ECOD)
ECOD was determined by the quantification of umbelliferone formation, according to Aitio (Citation1978). Incubation mixture was consisted of 2.6 ml, composed of 1 mM ethoxycoumarin, 5mM MgCl2, NADPH-generating system (see aminopyrine assay) and 0.25 ml of sample. After 5 minutes of incubation at 37 °C, reaction was stopped by the addiction of 0.85 ml of trichloroacetic acid (TCA) 0.31 M. The pH of the mixture was brought to about 10 by adding 0.65 ml of 1.6 M NaOH-glycine buffer (pH 10.3); the amount of umbelliferone was measured fluorimetrically (excitation 390 nm; emission 440 nm) (Canistro et al. Citation2009).
Phase II enzymatic activities
Glutathione S-transferase (GST)
The incubation mixture for measuring overall GST activity contained 1 mM glutathione +1 mM 1-chloro-2,4-dinitrobenzene (CDNB) in methanol +0.025 ml of sample in a final volume of 2.5 ml 0.1 M phosphate Na+/K+ buffer (pH 6.5). The product of the reaction of the thiol group of glutathione with the electrophilic group of CDNB was read at 340 nm (ɛ = 9.6 mM−1 cm−1) (Habig et al. Citation1974; Sapone et al. Citation2016).
UDP-glucuronosyl transferase (UDP-GT)
UDP-GT was determined kinetically using 1-naphtol as substrate (final concentration, 50 mM) by the continuous fluorimetric (excitation 390 nm; emission 440 nm) monitoring of 1-naphtholglucuronide production in the presence of 1 mM uridine-5′-diphosphoglucuronic acid (Mackenzie & Hänninen Citation1980). Experiments were performed in the presence or absence of Triton X-100 (0.2%) as a detergent, in order to improve the assay sensitivity (Vivarelli et al. Citation2016b).
Protein concentration
Protein concentration was determined according to the method described in Lowry et al. (Citation1951) and revised in Bailey (Citation1967), using bovine serum albumin as standard and diluting samples 200 times to provide a suitable protein concentration.
Statistical analysis
Statistical analysis of digestibility data was performed by one-way ANOVA, with the Newman–Keuls test as the post test. Differences were considered statistically significant at p < .05. Statistical analysis of the results derived from the animal treatment was performed using Wilcoxon’s rank method as reported by Box and Hunter (Citation1978).
Results
Enzymatic hydrolysis
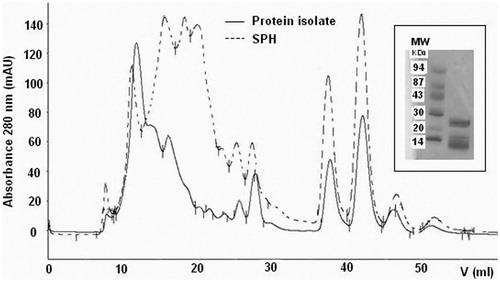
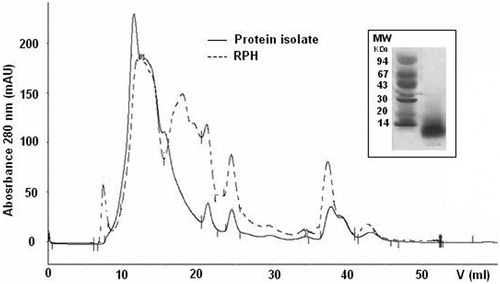
As a result of the preliminary protein isolation by the isoelectric precipitation procedure, final SPH and RPH had a high protein content of 83.2 and 70.2%, respectively (Table ) and their peptide molecular weight distribution was evaluated by gel filtration chromatography and SDS-PAGE. The SDS-PAGE analysis showed the presence of peptides with a molecular weight lower than 25 KDa and 14 KDa for SPH (Figure ) and RPH (Figure ), respectively. The gel filtration chromatography profile clearly indicated the shift towards lower molecular size protein-peptide in SPH and RPH, if compared with the protein isolates, and showed the presence of new-formed low molecular weight compounds, represented by peaks with elution volume higher than 15 ml, probably represented most by small peptides (as showed by SDS-PAGE) and low percentage of amino acids (Figures and ). The use of Alcalase alone, in fact, permitted a mild protein hydrolysis and the SPH and RPH hydrolysate content of total free amino acids determined by HPLC analysis following OPA-FMOC derivatization (Ugolini et al. Citation2015) was limited to 0.6–1.0% w/w.
In vitro digestibility
Results regarding the in vitro digestibility of diets containing DSM or protein hydrolysates are reported in Table . Compared with diets containing rapeseed and sunflower DSM, dry matter, organic matter, crude protein, ether extract and crude ash digestibility was significantly higher in diets containing the hydrolysates (p < .05). Digestibility of dry matter, organic matter and crude protein did not differ between RPH and SPH diets (p > .05).
Table 2. In vitro digestibility (%) of diets containing different sources of protein.
In vivo trial with mice
Body weight and food intake
No significant difference (p > .05) was observed in body weight gain among the different mice groups (Table ). Furthermore, compared with control, food intake was slightly numerically increased in mice eating RPH and SPH (Table ).
Table 3. Body weight and food intake in Swiss Albino male mice fed a standard chow diet (control) or a diet containing rapeseed or sunflower protein hydrolysates over 28 days period.
Signs and symptoms
No toxicological signs were recorded throughout the experiment through physical examination. During the whole study, mice were in good health and appeared to well tolerate the diets. No mortality occurred in any of the treatment groups. No abnormal behaviour was observed. No respiratory signs such as dyspnoea, cyanosis or apnoea, neither nervous signs as convulsions, piloerection or changes in motor function were shown. Gastrointestinal (i.e. diarrhoea, soft faeces) or skin (dermatitis, alopecia) toxicological signs were not observed.
Blood biochemistry parameters
Biochemical blood determinations serve as an indicator of toxicity of a test material (Schilter et al. Citation2003). As shown in Table , there were no statistically significant differences in clinical biochemical parameters among all the animal groups (p > .05). All blood biochemistry values fell into the normal physiological range (Schnell et al. Citation2002; Serfilippi et al. Citation2003).
Table 4. Blood biochemistry parameters in Swiss Albino male mice fed a standard chow diet (control) or a diet containing rapeseed or sunflower protein hydrolysates.
Terminal organ weights
There were no significant (p > .05) differences in terminal organ weights among experimental groups (data not shown).
Phase I enzymatic activities
Table reports various microsomal mixed function monooxygenases detected as single probe in the liver and kidney of mice.
Table 5. Phase I enzymatic activities in the liver and kidney of Swiss Albino male mice fed a standard chow diet (control) or a diet containing rapeseed or sunflower protein hydrolysates.
In the liver, CYP 3A1/2-supported (APND) oxidase was induced by both RPH (up to 52.7%, p < .05) and SPH (up to 18.9%, p < .05) treatments. In mice fed with RPH, the CYP2E1-linked monooxygenase (pNPH) was significantly increased (up to 18.9%, p < .01). The main up-regulations were observed for CYP2B1/2 (PROD) and CYP1A2 (MROD) -supported oxidases in animals fed with SPH (respectively, up to 24.7%, p < .01 and 44.2%, p < .01). A slight down-regulation was recorded in RPH treatment for CYP1A1-linked oxidase (EROD) (12.1% loss, p < .05), while an up-regulation was observed, for the same isoform, in the SPH group (up to 21.4%, p < .01).
In the kidney, pNPH was significantly decreased by RPH (6.3% loss, p < .05) and SPH (24.0% loss, p < .01). The main down-regulations were observed for PROD in RPH (13.6% loss, p < .01) and SPH (49.4% loss, p < .01) treatments. Significant reductions were also recorded for both CYP1A2-supported (MROD) oxidase (29.5% loss, p < .05) and CYP1A1-supported (EROD) oxidase (46.0% loss, p < .01) in mice receiving SPH.
Phase II enzymatic activities
As reported in Table , down-regulations for both RPH and SPH treatments were observed in the liver for UDP-glucuronosyl-transeferase (respectively, 19.8% loss, p < .05, and 28.0% loss, p < .01). The RPH and SPH diets did not significantly affect post oxidative enzymes in the kidney of mice.
Table 6. Phase II enzymatic activities in the liver and kidney of Swiss Albino male mice fed a standard chow diet (control) or a diet containing rapeseed or sunflower protein hydrolysates.
Discussion
The aim of this study was the evaluation of digestibility (in vitro), toxicity and metabolic effects (on phase I and II enzymes) of diets containing RPH and SPH, in mice, in order to evaluate the possibility to employ these protein hydrolysates as alternative protein sources in companion animals. Nowadays, the market of industrial food for pets, especially for those with a suspect or a confirmed diagnosis of AFRs, is currently growing. In this regard, protein hydrolysates have demonstrated to be particularly hypoallergenic, as their employment has shown to treat efficaciously food allergies even deriving from the corresponding whole protein source (Jackson et al. Citation2003).
The employment of European rapeseed and sunflower DSM (derived from the biodiesel production chain and characterised by a high protein content), easily useful and broadly available, could represent a valid alternative to this problem, reducing the costs.
The production of hydrolysates from rapeseed and sunflower DSM typically requires a first protein isolation step from the DSM that usually precedes the hydrolysis step. This procedure may decrease the protein extraction yield (Ugolini et al. Citation2015), but allows to obtain a product free of antinutritional compounds (namely clorogenic acid in sunflower DSM or fitic acid and glucosinolates in rapeseed DSM) and still rich in protein (Parrado et al. Citation1991; Parrado et al. Citation1993; Villanueva et al. Citation1999; Vioque et al. Citation1999; Chabanon et al. Citation2007). In this regard, chemical analysis confirmed a high-protein content of both RPH and SPH evaluated in the present study.
Digestibility of diets containing DSM or protein hydrolysates derived from rapeseed or sunflower were evaluated in vitro. In the present study, RPH and SPH were used as a complete replacement for the rapeseed and sunflower DSM. Compared with diets containing the hydrolysates, total dry matter digestibility was much lower in diets containing the intact DSM, presumably mainly due to their higher fibre content. In fact, fibre cannot be digested using the in vitro procedure that was performed, as it is aimed to simulate the enzymatic digestion that takes place in the stomach and small intestine of dogs. Also, the observed difference in dry matter digestibility between diets containing rapeseed and sunflower DSM may be explained by the higher fibre content of the latter. Furthermore, this nutrient may have exerted minerals binding-properties (well-known, accordingly to literature; Baye et al. Citation2017), as demonstrated by the lower crude ash digestibility in DSM-based diets. Probably, this effect contributed to influence dry matter digestibility results. Similarly, also organic matter resulted in more digestible in diets containing protein hydrolysates, probably because of the corresponding higher protein and ether extract digestibility values. Anyway, with regards to the digestibility of lipids, it can be supposed that the differences observed exerted only a limited influence on organic matter digestibility, considering the modest amounts of this nutrient in the diets evaluated. While the slightly different lipid digestibility percentages between DSM and protein hydrolysate-based diets are difficult to explain, it can be presumed that the higher protein digestibility of diets containing RPH and SPH was the consequence of the hydrolysis process. In fact, it has been shown that, in humans, the ingestion of a casein hydrolysate may result in improved protein digestion and absorption when compared with intact casein (Koopman et al. Citation2009). Similarly, nitrogen apparent digestibility was improved in Atlantic salmons fed increasing levels of fish protein hydrolysates (Refstie et al. Citation2004; Hevrøy et al. Citation2005). In another study, in vitro digestibility of casein and whey proteins was improved when they were hydrolysed to different degrees of hydrolysis (Sindayikengera & Xia Citation2006).
A range of studies is used for hazard identification and characterisation of any new material for use in food or food supplement. The mice Swiss Albino CD1 were fed for 28 consecutive days diets containing 10% of RPH or SPH with the aim of showing up both adverse toxicological effects in major vital systems and the modulation of drug metabolism enzymes.
The use of hydrolysates in the diets could reduce palatability of the feed for the presence of free amino acids and short peptides that can alter the flavour of the food making it bitterer. For this reason, food consumption and body weight were checked during the duration of the whole experiment. In contrast with the hypothesis, during the treatment, a regular food consumption was observed in treated animals compared with controls, suggesting a good palatability of the hydrolysates and the body weight gain was not affected by dietary treatment. In this regard, under various regulatory guidelines, body weight gain is an integral part of the conventional safety evaluation of a test material. Significant body weight loss is considered one of the most sensitive indicators of an animal’s deteriorating health status (Schilter et al. Citation2003). Based on these evidences, it could be stated that all animals maintained good health conditions throughout the whole study. Furthermore, concerning data on food intake, mice fed with diets containing protein hydrolysates revealed numerically slightly higher amounts of food consumed. Lacking other parameters endorsing the increased food consumption in the treated groups (such as, e.g. a strong hyperactivity), it was concluded that the observed variation may be due to the consistency of the diets containing the hydrolysates, which appeared to be more friable than the control diet, leading to higher amounts of food wasted by the animals. These data not being very reliable, statistical analysis was not applied.
The experimental diets had no significant effect on animals’ blood parameters (ALT, creatinine, glucose, fructosamine, triglycerides, NEFA, total cholesterol, total protein, albumin and albumin/globulin). The normal physiological ranges in which all the blood biochemistry values evaluated fell clearly indicate the absence of hematotoxic potential of the diets. In particular, the unchanged activity of plasmatic enzymes such as ALT (which indicates liver function), between controls and treated mice, suggests the absence of hepatic alterations attributable to diets containing protein hydrolysates. Finally, the measured parameters reveal a general metabolic profile not significantly altered in mice following RPH or SPH diet intake.
Diets containing protein hydrolysates could also set off adverse effects at enteric level, such as osmotic diarrhoea and soft faeces, as it is well known that low molecular weight compounds (such as aminoacids and small peptides) is able to increase osmolarity in the small intestine (Mahmoud Citation1994). However, during the treatment, animals did not show any gastrointestinal symptoms and food consumption was constant. In addition, there was no evidence of toxicological effects on respiratory tract, nervous system and skin in mice.
The modulation of the metabolic system was evaluated in different tissues. The observed perturbations of the oxidative and post-oxidative enzymes, although statistically significant, from a biological point of view, can be considered as not dangerous. A one-directional trend was not recorded, but a complex pattern of mild up and down-regulations that cannot affect the whole metabolism of the animal. These fluctuations, not involving the entire enzymatic patterns, do not compromise their important functions. It can be concluded that both protein hydrolysates had no important effects on drug metabolism enzymes. It is very important to evaluate the perturbation of these catalysts, because they play a predominant cellular role being implicated in endogenous and exogenous metabolism, and both their induction and/or suppression might have serious toxicological relevance.
It is well known that epigenetic mechanisms, as those linked to the positive and negative modulation of metabolising enzymes, can be associated with co-carcinogenesis, co-mutagenesis and promotion events. In fact, the increased bio-activation of ubiquitous pro-mutagens/pro-carcinogens saturating the enzymes involved in the ‘error-free repair’, can seriously pose DNA at structural damage risk. Furthermore, the up-regulation of phase I monooxygenases has been connected with the enhanced production of reactive oxygen species, a factor that may increase the incidence of several injuries (Paolini et al. Citation1994). Moreover, these enzymes not only metabolise exogenous substances, but also perform cellular and systemic functions (e.g. growth, differentiation, apoptosis, homeostasis and neuroendocrine regulation) due to their ability to recognise a wide range of endogenous substrates (steroids, corticosteroids, androgens, oestrogens, cholesterol, vitamin D, fatty acids, bile acids, thromboxanes, leukotrienes). Not least, nowadays pets are treated for various diseases (cardiovascular, cutaneous, neurological, cancers) also by multiple drugs administrations. For this reason, it is essential to know the potential modulations associated with these enzymes. In fact, the possible induction and/or inactivation of drug metabolism enzymes following the intake of xenobiotics, could affect the therapeutic efficacy and/or toxicity of co-administered drugs (pharmacokinetic drug–food interactions). As such, drug metabolism enzymes manipulation should be interpreted with care, since, in many cases, it can also contribute to increased toxicological risks. Anyway, this was not the case in the present study, as both RPH and SPH proved to be completely safe at this level.
Conclusions
This is the first report on safety assessment of RPH and SPH and the observations in this study imply that mice well tolerated the RPH and SPH diet. In the toxicity study the hydrolysates failed to induce any significant alterations in parameters, indicating safety such as food intake, growth, organ weight, blood biochemical parameters. Moreover, RPH and SPH did not compromise the hepatic and renal drug metabolising enzymes functions, even if mild fluctuations of some oxidative and post-oxidative catalysts were recorded.
In conclusion, the present findings encourage further exploration of the protein hydrolysates from sunflower and rapeseed DSM as alternative protein sources in several applications, such as food for pets suffering from adverse food reactions.
Acknowledgements
The authors thank Laura Righetti and Lorena Malaguti for cooperation in paper revision.
Disclosure statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
References
- Aitio A. 1978. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal Biochem. 85:488.
- AOAC. 2000. Official methods of analysis. 17th ed. Gaithersburg (MD): Association of Official Analytical Chemists International.
- Bailey YL. 1967. Techniques in protein chemistry. Amsterdam: Elsevier.
- Barillari J, Iori R, Broccoli M, Pozzetti L, Canistro D, Sapone A, Bonamassa B, Biagi GL, Paolini M. 2007. Glucoraphasatin and glucoraphenin, a redox pair of glucosinolates of brassicaceae, differently affect metabolizing enzymes in rats. J Agric Food Chem. 55:5505–5511.
- Baye K, Guyot JP, Mouquet-Rivier C. 2017. The unresolved role of dietary fibers on mineral absorption. Crit Rev Food Sci Nutr. 57:949–957.
- Biagi G, Cipollini I, Grandi M, Pinna C, Vecchiato CG, Zaghini G. 2016. A new in vitro method to evaluate digestibility of commercial diets for dogs. Ital J Anim Sci. 15:617–625.
- Biourge VC, Fontaine J, Vroom MW. 2004. Diagnosis of adverse reactions to food in dogs: efficacy of a soy-isolate hydrolyzate-based diet. J Nutr. 134:2062S–2064S.
- Boumans H. 2001. Physiochemical and functional properties of protein hydrolysates in nutritional products. Hill's European Symposium on Adverse Food Reactions, Symposium Proceedings, Madrid 2001, Watford (UK): Hill's Europe Publication; p. 30–33.
- Box GEP, Hunter WG. 1978. Statistics for experiments. New York (NY): Wiley.
- Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparant T, Meyer RT. 1985. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochrome P450. Biochem Pharmacol. 34:3337–3345.
- Canistro D, Bonamassa B, Pozzetti L, Sapone A, Abdel-Rahman SZ, Biagi GL, Paolini M. 2009. Alteration of xenobiotic metabolizing enzymes by resveratrol in liver and lung of CD1 mice. Food Chem Toxicol. 47:454–461.
- Canistro D, Vivarelli F, Cirillo S, Costa G, Andreotti C, Paolini M. 2016. Comparison between in toto peach (Prunus persica L. Batsch) supplementation and its polyphenolic extract on rat liver xenobiotic metabolizing enzymes. Food Chem Toxicol. 97:385–394.
- Cave NJ, Guilford WG. 2004. A method for in vitro evaluation of protein hydrolysates for potential inclusion in veterinary diets. Res Vet Sci. 77:231–238.
- Chabanon G, Chevalot I, Framboisier X, Chenu S, Marc I. 2007. Hydrolysis of rapeseed protein isolates: kinetics, characterization and functional properties of hydrolysates. Process Biochem. 42:1419–1428.
- Conde JM, Del Mar Yust Escobar M, Jimenez JJP, Rodriguez FM, Patino JMR. 2005. Effect of enzymatic treatment of extracted sunflower proteins on solubility, amino acid composition and surface activity. J Agric Food Chem. 53:8038–8045.
- EFSA NDA Panel (European Food Safety Authority Panel on Dietetic Products, Nutrition and Allergies). 2013. Scientific opinion on the safety of ‘rapeseed protein isolate’ as a novel food ingredient. EFSA J. 11:3443.
- Gaschen FP, Merchant SR. 2011. Adverse food reactions in dogs and cats. Vet Clin North Am Small Anim Pract. 41:361–379.
- Habig W, Pabst M, Jakoby W. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 249:7130–7139.
- Hevrøy EM, Espe M, Waagbø R, Sandnes K, Ruud M, Hemre GI. 2005. Nutrient utilization in Atlantic salmon (Salmo salar L.) fed increased levels of fish protein hydrolysate during a period of fast growth. Aquacult Nutr. 11:301–313.
- Jackson HA, Jackson MW, Coblentz L, Hammerberg B. 2003. Evaluation of the clinical and allergen specific serum immunoglobulin E responses to oral challenge with cornstarch, corn, soy and a soy hydrolysate diet in dogs with spontaneous food allergy. Vet Dermatol. 14:181–187.
- Kang MH, Kim HJ, Jang HJ, Park HM. 2014. Sensitization rates of causative allergens for dogs with atopic dermatitis: detection of canine allergen-specific IgE. J Vet Sci. 15:545–550.
- Koopman R, Crombach N, Gijsen AP, Walrand S, Fauquant J, Kies AK, Lemosquet S, Saris WH, Boirie Y, van Loon LJ. 2009. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 90:106–115.
- Lomascolo A, Uzan-Boukhris E, Sigoillot JC, Fine F. 2012. Rapeseed and sunflower meal: a review on biotechnology status and challenges. Appl Microbiol Biotechnol. 95:1105–1114.
- Lowry OH, Rosenbrough HJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275.
- Lubet RA, Mayer RT, Cameron JW, Nims RW, Burke MD, Wolff T, Guengerich FP. 1985. Dealkylation of pentoxyresorufin. A rapid and sensitive assay for measuring induction of cytochrome(s) P450 by phenobarbital and other xenobiotics in rat. Arch Biochem Biophys. 238:43–48.
- Mackenzie PI, Hänninen O. 1980. A sensitive kinetic assay for UDPglucuronosyltransferase using 1-naphthol as substrate. Anal Biochem. 109:362–368.
- Mahmoud MI. 1994. Physicochemical and functional properties of protein hydrolysates in nutritional products. Food Technol. 48:89–95.
- Mazel P. 1971. Experiments illustrating drug metabolism in vitro. In: LaDu BN, Mandel HG, Way EL, editors. Fundamentals of drug metabolism and drug disposition. Baltimore (MD): The Williams & Wilkins Company; p. 546–582.
- Melega S, Canistro D, Pagnotta E, Iori R, Sapone A, Paolini M. 2013. Effect of sprout extract from Tuscan black cabbage on xenobiotic-metabolizing and antioxidant enzymes in rat liver. Mutat Res. 751:45–51.
- Nash T. 1953. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 55:416–421.
- Olivry T, Bizikova P. 2010. A systematic review of the evidence of reduced allergenicity and clinical benefit of food hydrolysates in dogs with cutaneous adverse food reactions. Vet Dermatol. 21:32–41.
- Olson ME, Hardin JA, Buret AG, Gall DG, Hayek MG. 2000. Hypersensitivity reactions to dietary antigens in atopic dogs. In: Reinhart GA, Carey DP, editors. Proceedings of the IAMS Nutrition Symposium, Recent Advances in Canine and Feline Nutrition, Volume III. Wilmington (OH): Orange Frazer Press; p. 69–77.
- Paolini M, Biagi GL, Cantelli-Forti G, Bauer C. 1994. Further mechanisms of non-genotoxic carcinogenesis. Trends Pharmacol Sci. 15:322–323.
- Parrado J, Bautista J, Machado A. 1991. Production of soluble enzymatic protein hydrolysate from industrially defatted nondehulled sunflower meal. J Agric Food Chem. 39:447–450.
- Parrado J, Milla F, Hernandez-Pinzòn Bautista J, Machado A. 1993. Characterization of enzymatic sunflower protein hydrolysates. J Agric Food Chem. 41:1821–1825.
- Refstie S, Olli JJ, Standal H. 2004. Feed intake, growth, and protein utilisation by post-smolt Atlantic salmon (Salmo salar) in response to graded levels of fish protein hydrolysate in the diet. Aquaculture. 239:331–349.
- Reinke LA, Mayer MJ. 1985. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drugs Metab Disp. 13:548–552.
- Roudebush P, Cowell CS. 1992. Results of a hypoallergenic diet survey of veterinarians in North America with a nutritional evaluation of homemade diet prescriptions. Vet Dermatol. 3:23–28.
- Sá FC, Vasconcellos RS, Brunetto MA, Filho FO, Gomes MO, Carciofi AC. 2013. Enzyme use in kibble diets formulated with wheat bran for dogs: effects on processing and digestibility. J Anim Physiol Anim Nutr (Berl). 97:51–59.
- Sapone A, Canistro D, Vivarelli F, Paolini M. 2016. Perturbation of xenobiotic metabolism in Dreissena polymorpha model exposed in situ to surface water (Lake Trasimene) purified with various disinfectants. Chemosphere. 144:548–554.
- Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R. 2003. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 41:1625–1649.
- Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. 2002. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther. 13:155–161.
- Scott DW, Miller JRWH, Griffin CE, editors. 2001. Skin immune system and allergic skin diseases. Canine food hypersensitivity. In: Muller and Kirk’s small animal dermatology, 6th ed. Philadelphia (PA): W.B. Saunders Company; p. 543–666.
- Serfilippi LM, Pallman DR, Russell B. 2003. Serum clinical chemistry and hematology reference values in outbred stocks of albino mice from three commonly used vendors and two inbred strains of albino mice. Contemp Top Lab Anim Sci. 42:46–52.
- Sindayikengera S, Xia W. 2006. Nutritional evaluation of caseins and whey proteins and their hydrolysates from Protamex. J Zhejiang Univ Sci B. 7:90–98.
- Streiff EL, Zwischenberger B, Butterwick RF, Wagner E, Iben C, Bauer JE. 2002. A comparison of the nutritional adequacy of home-prepared and commercial diets for dogs. J Nutr. 132:1698S–1700S.
- Suto A, Suto Y, Onohara N, Tomizawa Y, Yamamoto-Sugawara Y, Okayama T, Masuda K. 2015. Food allergens inducing a lymphocyte-mediated immunological reaction in canine atopic-like dermatitis. J Vet Med Sci. 77:251–254.
- Tapp T, Griffin C, Rosenkrantz W, Muse R, Boord M. 2002. Comparison of a commercial limited-antigen diet versus home-prepared diets in the diagnosis of canine adverse food reaction. Vet Ther. 3:244–251.
- Ugolini L, Cinti S, Righetti L, Stefan A, Matteo R, D’Avino L, Lazzeri L. 2015. Production of an enzymatic protein hydrolyzate from defatted sunflower seed meal for potential application as a plant biostimulant. Ind Crops Prod. 75:15–23.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Villanueva A, Vioque J, Sánchez-Vioque R, Clemente A, Pedroche J, Bautista J, Millán F. 1999. Peptide characteristics of sunflower protein hydrolysates. J Am Oil Chem Soc. 76:1455–1460.
- Vioque J, Sanchez-Vioque R, Clemente A, Pedroche J, Bautista J, Millan F. 1999. Purification and partial characterization of chickpea 25 albumin. J Agric Food Chem. 47:1405–1409.
- Vivarelli F, Canistro D, Sapone A, De Nicola GR, Babot Marquillas C, Iori R, Antonazzo IC, Gentilini F, Paolini M. 2016a. Raphanus sativus cv. sango sprout juice decreases diet-induced obesity in Sprague Dawley rats and ameliorates related disorders. PLoS One. 11:e0150913.
- Vivarelli F, Canistro D, Franchi P, Sapone A, Vornoli A, Della Croce C, Longo V, Lucarini M, Paolini M. 2016b. Disruption of redox homeostasis and carcinogen metabolizing enzymes changes by administration of vitamin E to rats. Life Sci. 145:166–173.
- Willis-Mahn C, Remillard R, Tater K. 2014. ELISA testing for soy antigens in dry dog foods used in dietary elimination trials. J Am Anim Hosp Assoc. 50:383–389.