Abstract
The current study was conducted to evaluate the ability of mixed adsorbent (Na-MMT + YCW) for the preventing effect of Ochratoxin A in broiler productivity, biochemical parameters, histochemical changes and OTA residues. A total of 96 broiler chicks were grouped in three experimental and one control group (24 chicks in each group, 3 replicates each). Birds were fed basal dietary or that with Ochratoxin A and mixed adsorbent (Na-MMT + YCW) to determine the effect of this compound during mycotoxicoses. When compared with the controls, feed: gain (F/G) were significantly higher for broilers fed diets containing OTA alone (p < .05). No differences were found between the F/G of broiler chickens fed diets without OTA and those of chickens fed mixed adsorbent. The diet containing 2 mg OTA/kg without adsorbents added increased relative liver, kidney, spleen weights and decreased relative thymus, bursa weights. Alterations in the levels of serum TP, ALB, GLB, BUN and enzymatic activity of ALT, AST, ALP and GGT were observed for OTA diets, and moderate protection was provided by the sorbent. Supplementing mixed adsorbent group (Na-MMT + YCW) showed intestinal villus height and crypt depth similar to control chicks, and a significant reduction residue in livers and kidney tissue, as well as a significantly higher OTA content in the faeces compared with OTA- group were recorded. These results suggest that effects of OTA treatment were ameliorated when mixed adsorbent was used in the broiler chick diets.
Introduction
Ochratoxin A (OTA), a nephrotoxic mycotoxin mainly produced by Aspergillus ochraceus and Penicillium viridicatum, has been shown to contaminate a wide variety of cereals and feed stuffs and is extremely toxic to domestic fowls (Fakruddin et al. Citation2015; Sumbal et al. Citation2016). This mycotoxin causes detrimental effects on birds, such as growth impairment, immune depression, and paleness in broilers, which finally bring about economic losses (Garcia et al. Citation2003; Stoev Citation2010; Heussner & Bingle Citation2015; Saleemi et al. Citation2015). A particular danger is the presence of OTA residues in tissues of animals that have been given contaminated diets and which enter the human food chain. Consumption of meat and meat products from these animals is a major threat to human health (Trailovic et al. Citation2013). Long-term exposure to OTA has been implicated in Balkan Endemic Nephropathy (a human disease characterised by progressive renal fibrosis) and is associated with urinary tract tumours because of rather high OTA levels detected in food samples and in blood or urine from affected persons (Milicevic et al. Citation2011).
One of the main methods for reducing the toxic effects of mycotoxins in practice is the use of adsorbents. Recent information suggests that clay minerals, modified yeast cell wall and aluminosilicates added to mycotoxin contaminated diets reduce the bioavailability of toxins and their hazardous effects in some animal species. The major advantages of the adsorbents include low cost, safety and easy addition to animal feeds (Miazzo et al. Citation2005; Campagnollo et al. Citation2015; Bhatti et al. Citation2016). Although some literature has been devoted to the protective effects of adsorbents against OTA in poultry, there is no information available analysing the efficacy of mixed adsorbent (Na-MMT + YCW) to reduce bioavailability and diminish the toxic effects of OTA in broiler chicks through their inclusion in feed.
The purpose of this research was to obtain more information about the evaluation of adsorbents that are widely recommended in China to bind OTA toxin, under more realistic conditions.
Materials and methods
Experimental animals
All of the experiments were carried out according to the guidelines for animal experiments at the National Institute of Animal Health. A total of 96 male broiler chicks were purchased at two-weeks of age, housed in wire floor cages with continuous infra-red lighting at a temperature suitable for their age. Commercially prepared complete standard feed (Table ) suitable for the breed and age of the chicks according to the accepted standards and regulations of the country, which contained 2 mg/kg OTA with or without adsorbents supplementation, supposed to protect against the toxic effects of OTA, were available ad libitum during the whole experimental period as described in Table . The chicks were grouped in three experimental and one control group (24 chicks in each group, 3 replicates each). Birds were fed basal dietary or that with Ochratoxin A and mixed adsorbent (Na-MMT + YCW) to determine the effect of this compound during mycotoxicoses as shown in Table .
Table 1. Composition and nutrient levels of basal diets (as fed basis).
Table 2. Amount of ochratoxin A (OTA) and adsorbents in experimental feeds of chicks.
OTA production
The contaminated diet was prepared using OTA obtained by contamination of maize with Aspergillus ochraceus as described by Trailovic (Trailovic et al. Citation2013). OTA was produced using a culture of Aspergillus ochraceus. Conidiospores of A. ochraceus were cultivated on potato dextrose agar substrates, for 5 d at 27 °C. Samples (100 g) of the broken maize were taken in 500 ml glass bottles and soaked in 50 ml tap water for 1 h. After 1 h, the bottles were capped and autoclaved. Then the maize was inoculated with cultures of mould and incubated for 10 d, during which time full growth of the mould was achieved and OTA production took place. The maize with cultures of A. ochraceus was kept at the temperature of 15–25 °C during contamination. After 10 d, the maize was autoclaved and dried at 105 °C in a laboratory drying cabinet, ground and used for contamination of experimental premixes. HPLC analysis of contaminated maize revealed an OTA concentration of 230 mg/kg. This maize was used for inoculation of the broiler diet at a final OTA concentration of 2 mg/kg. The entire diet preparation with OTA was carried out with due precautionary measures and utilisation of all protective equipment.
Growth performance
Chicks were weighed individually at 30 and 40 d, and feed consumption was recorded per pen at the same time. Average daily weight gain (ADG), average daily feed intake (ADFI) and feed:gain ratio (F/G) were calculated.
Post-mortem examination
When 40-day-old, chickens per treatment were weighed and after euthanasia by cervical dislocation, the weights of liver, kidney, spleen, thymus and bursa were obtained.
Biochemical parameters
At the end of the experiment, blood samples (2 mL per bird) were collected from broiler chicks for serum biochemical determination. Within 1 h, the serum was obtained by centrifugation (2500 × g for 15 min at room temperature) and stored at −20 °C until further analysis. The concentration of serum total protein (TP), albumin (ALB), globin (GLB), blood urea nitrogen (BUN), glucose (GLU) and enzymatic activity of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) were determined with a clinical chemistry analyser (AU 5000 Olympus, Hamburg, Germany) using reagents according to the manufacturer’s instruction.
Analysis of OTA residue in organs and faeces
For this assay, 5 samples of livers, kidneys and faeces respectively from each treatment were selected. OTA in the tissues and faeces was extracted and partially purified according to the method described previously by Tavčar-Kalcher et al. (Citation2007). Briefly, the ground sample (50 g) was mixed thoroughly with 5 mL of a 20% aqueous citric acid solution and diatomaceous earth (10 g). The mixture was extracted with 100 mL of dichloromethane by shaking for 30 min. The filtered extract was dried by addition of Na2SO4 and filtered again, and an aliquot (20 mL) was evaporated to dryness. The concentrate was mixed with acetonitrile: H2O (75:25, vol/vol) and hexane and centrifuged, and 10 mL of the aqueous phase was taken and evaporated to dryness. The concentrate was mixed with methanol: water (80:20, vol/vol). The solution was applied to an Oasis cartridge (60 mg, Waters Corporation, Milford, MA) that was previously conditioned with methanol and water. OTA in the extract was detected and quantified by liquid chromatography-mass spectrometry (HPLC-MS/MS). Dried extracts were dissolved in mobile phase [acetonitrile: H2O (20:80, vol/vol)] and injected into the HPLC-MS/MS instrument. The calibration range was from 0.05 to 0.5 ng of injected OTA. The quantification limits of the analytical technique used to determine OTA residues was 0.025 ng/g.
Intestinal histological examination
Five samples with approximately 2 cm were taken from the medial region of duodenum, jejunum and ileum, respectively, and routinely processed for histology. Tissue was fixed in Bouin’s solution, dehydrated, cleared and infiltrated with paraffin wax. After embedding, cuts with 5 μm of thickness were sectioned and mounted on glass microscope slides to be stained with haematoxylin and eosin. The cuts were observed under 10 × magnification using a light microscope (Nikon E-100 equipped with a digital camera), and photographs were taken. Villus height and crypt depth were determined using the software Image J; villus height was measured from the top of the villus to the villus-crypt junction. The crypts were measured from the villus-crypt junction until the baseline. Thirty vertically oriented villi and thirty crypts were measured per sample, with a total of 120 readings per variable for each treatment.
Statistical analysis
The results are expressed as mean ± SD. The results were subjected to one-way analysis of variance (ANOVA) followed by the Student’s t test to determine the significant differences among the samples. Difference was considered to be statistically significant if p < .05.
Results
Growth performance
Growth performance data are presented in Table . Compared with the control diet, the consumption of contaminated feed resulted in a significant reduction in ADG and ADFI at day 30 and 40 (p < .05). Significantly increased ADG and ADFI during d 20–40 were observed in the two adsorbents-treated groups when compared with OTA-contaminated group (p < .05). The addition of the two adsorbents in contaminated feed significantly improved feed:gain during d 20–40 (p < .05). Significantly increased feed:gain at d 40 in OTA + Na-MMT + YCW group compared with OTA + Na-MMT group (p < .05). There was no significant difference in feed:gain between the OTA + Na-MMT + YCW group and control group at d 30 and 40 (p > .05).
Table 3. Effects of adsorbents on growth performance of broilers fed diets containing OTA at d 30 and 40.
Post-mortem examination
Data presented in Table show the effects of dietary treatments on relative organ weights (g/100 g BW). The diet containing 2 mg OTA/kg without adsorbents added increased relative liver, kidney, spleen weights and decreased relative thymus and bursa weights. However, addition of adsorbents to the OTA-contaminated diet prevented an increase in weight of liver, kidney, spleen and a decrease in weight of thymus and bursa weights. Except liver, there was no significant difference in weight of kidney, spleen, thymus and bursa between the OTA + Na-MMT + YCW group and control group (p > .05).
Table 4. Effects of adsorbents on relative organ weights organ weights of chicks fed diets containing OTA.
Biochemical parameters
The changes in the biochemical parameters for different treatments were examined, and results are shown in Table . Chickens receiving OTA had decreased serum TP, ALB, GLB, BUN concentration, while increased serum ALT, AST, ALP and GGT level. Supplementing the diet with Na-MMT plus PCM can ameliorate the action of OTA on these serum components, while this tendency was not as great when only Na-MMT was added to the OTA-contaminated diet. The serum GLU was not influenced by the use of OTA and adsorbents.
Table 5. Effects of OTA and adsorbents on serum parameters of broilers.
Analysis of OTA residue in organs and faeces
OTA levels (ng/g) in livers, kidneys and faeces of broilers fed dietary treatments are summarised in Table . After 20 d, the concentrations of OTA residues (1.79, 4.42, 8.35 ng/g, respectively) were measured in the livers, kidneys and faeces respectively of broilers given a diet containing OTA. There was a significantly lower OTA content in the livers, kidneys from the broilers whose diet contained mixed adsorbent (Na-MMT + YCW) as well as the toxin, and a significantly higher OTA content in the faeces (Table ). While in the group given diets containing Na-MMT, there was a significantly lower OTA content only in kidneys compared with OTA group. In the OTA-free treatments, OTA residues were not detected.
Table 6. Residues in the livers, kidney and faeces of broilers fed dietary treatments.
Morphological measurement of the small intestinal mucosa
Morphological measurements of the small intestinal mucosa of broilers fed dietary treatments are presented in Table . Chicks fed OTA showed decreased villus height: crypt depth ratio compared with other groups (p < .05). On the other hand, supplementing mixed adsorbent group (Na-MMT + YCW) showed intestinal villus height and crypt depth similar to control chicks, indicating that intestinal integrity was improved when adsorbents was added (Figure ). There was no significant difference between Na-MMT group and OTA-fed group.
Figure 1. Morphology of small intestinal slices in each group of chicks. A: Duodenum. B: Jejunum. C: Ileum. I: Control group. II: OTA group. III: OTA + Na-MMT group. IV: OTA + Na-MMT + YCW group.
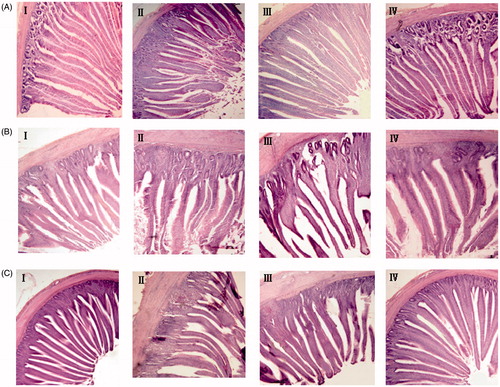
Table 7. Villus height: crypt depth ratios (μm μm−1) of duodenum, jejunum and ileum of broilers fed dietary treatments.
Discussion
Mycotoxins, particularly OTA, have been reported to produce severe economic losses and health problems in the poultry industry because of their toxicity and frequency of occurrence in feedstuffs. The current study demonstrated the toxicity of OTA in growing broiler chicks and a reduction in the ability of mixed adsorbent (Na- MMT + YCW) to decrease the toxicity of OTA.
The results obtained in this study showed that dietary OTA severely affected growth performance. The adverse effects of OTA on growth performance have been related with a decrease in protein and energy utilisation, probably as a consequence of a deterioration of the digestive and metabolic efficiency of the birds (Denli et al. Citation2009; Valtchev et al. Citation2015). The addition of the two adsorbents in contaminated feed significantly improved feed:gain during d 1–20. The presence of mixed adsorbent OTA + Na-MMT + YCW returned the growth performance parameters to normal values, showing a protective effect against mycotoxicoses.
Chronic mycotoxicoses may be diagnosed by determining serum biochemical alterations even before major clinical symptoms appear (Oguz et al. Citation2000; Hassan et al. Citation2010). In intoxicated broilers, decreases in serum TP, ALB, GLB, BUN concentrations, as well as increased serum ALT, AST, ALP and GGT level were found. Similar observations were also made by Sakthivelan and Garcı´a (Sakthivelan & Sudhakar Rao Citation2010). Huff et al. reported that the total protein and albumin were the sensitive indicator of ochratoxicosis (Huff et al. Citation1988). The mechanism by which OTA produced hypoproteinaemia and hypoalbuminaemia is due to inhibition of phenylalanyl transfer-RNA-synthetase with phenylalanine and renal leakage of albumin resulting from kidney lesions induced by OTA (Creppy et al. Citation1979; Solcan et al. Citation2015). In the present study, the serum parameters in the mixed adsorbent treatment containing OTA were close to those seen with the control diet.
Maintenance of normal microarchitecture in the small intestine is very important for proper growth and development (Cheled-Shoval et al. Citation2011; Sohail et al. Citation2012). In this study, the decreases in villus height:crypt depth ratio of small intestine in OTA-fed chicks indicate the depressing effect of OTA on small intestinal mucosa. There was an intermediate alleviation in the alterations of small intestinal mucosa by feeding mixed adsorbent in combination with OTA when compared to control values. The decreases in villus height:crypt depth ratio of small intestine in OTA-fed chicks may be related to the oxidative damage which might be one of the underlining mechanisms for mycotoxin-induced cell injury and DNA damage (Baudrimont et al. Citation1994; Shen et al. Citation1994).
Livers, kidneys and faeces of broiler chickens in group with no dietary addition of OTA (group I) were bereft of this toxin. In the group given diets containing Na-MMT, there was a significant reduction of OTA residues only in kidneys compared with OTA group. However, in the group fed mixed adsorbent (Na-MMT + YCW), a significant reduction in livers and kidney tissue, as well as a significantly higher OTA content in the faeces were recorded. The accumulation of OTA residues in tissues especially liver and kidney is probably due to enterohepatic recirculation and hepatobiliary excretion of OTA, which provoke a direct toxic effect of this toxin in these organs (Hanif et al. Citation2012). The results indicate that supplementing the diet with Na-MMT plus PCM can ameliorate the residues in liver and kidney. In addition, the increased discharge in faeces also suggests an effect of Na-MMT plus PCM to bind OTA.
Conclusions
Our study indicated that OTA in the diet at levels of 2 mg/kg resulted in reduced growth performance and an alteration of the serum biochemical, organ weight, and histological parameters of broiler chicks. The addition of mixed adsorbent (Na-MMT + YCW) to feed resulted in significant improvement related to productive and biochemical parameters in broilers. Therefore, the use of mixed adsorbent (Na-MMT + YCW) in OTA- contaminated feed is an alternative method to reduce the adverse effects of ochratoxicosis in broilers. Further studies are suggested to investigate and compare the adsorption efficiencies of adsorbents against various mycotoxins using these methods.
Acknowledgements
This work was financially supported by the programme of The-Important-of-The Importants (Zhejiang, China).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Baudrimont I, Betbeder AM, Gharbi A, Pfohl-Leszkowicz A, Dirheimer G, Creppy EE. 1994. Effect of superoxide dismutase and catalase on the nephrotoxicity induced by subchronical administration of ochratoxin A in rats. Toxicology. 89:101–111.
- Bhatti SA, Khan MZ, Saleemi MK. 2016. Aflatoxicosis and ochratoxicosis in broiler chicks and their amelioration with locally available bentonite clay. Pak Vet J. 36:68–72.
- Campagnollo FB, Franco LT, Rottinghaus GE. 2015. In vitro evaluation of the ability of beer fermentation residue containing Saccharomyces cerevisiae to bind mycotoxins. Food Res Int. 77:643–648.
- Cheled-Shoval SL, Amit-Romach E, Barbakov M, Uni Z. 2011. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult Sci. 90:2301–2310.
- Creppy EE, Lugnier AA, Fasiolo F, Heller K, Roschenthaler R, Dirheimer G. 1979. In vitro inhibition of yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem Biol Interact. 24:257–261.
- Denli M, Blandon JC, Guynot ME, Salado S, Perez JF. 2009. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B(1). Poult Sci. 88:1444–1451.
- Fakruddin M, Chowdhury A, Hossain MN. 2015. Characterization of aflatoxin producing Aspergillus flavus from food and feed samples. SpringerPlus. 4:159.
- Garcia AR, Avila E, Rosiles R, Petrone VM. 2003. Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian Dis. 47:691–699.
- Hanif NQ, Muhammad G, Muhammad K, Tahira I, Raja GK. 2012. Reduction of ochratoxin A in broiler serum and tissues by Trichosporon mycotoxinivorans. Res Vet Sci. 93:795–797.
- Hassan AM, Kenawy AM, Abbas WT, Abdel-Wahhab MA. 2010. Prevention of cytogenetic, histochemical and biochemical alterations in Oreochromis niloticus by dietary supplement of sorbent materials. Ecotoxicol Environ Safe. 73:1890–1895.
- Heussner AH, Bingle LEH. 2015. Comparative ochratoxin toxicity: a review of the available data. Toxins. 7:4253–4282.
- Huff WE, Kubena LF, Harvey RB. 1988. Progression of ochratoxicosis in broiler chickens. Poult Sci. 67:1139–1146.
- Miazzo R, Peralta MF, Magnoli C, Salvano M, Ferrero S, Chiacchiera SM, Carvalho EC, Rosa CA, Dalcero A. 2005. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult Sci. 84:1–8.
- Milicevic D, Jovanovic M, Matekalo-Sverak V, Radicevic T, Petrovic MM, Lilic S. 2011. A survey of spontaneous occurrence of ochratoxin A residues in chicken tissues and concurrence with histopathological changes in liver and kidneys. J Environ Sci Health. 29:159–175.
- Oguz H, Kececi T, Birdane YO, Onder F, Kurtoglu V. 2000. Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Res Vet Sci. 69:89–93.
- Sakthivelan SM, Sudhakar Rao GV. 2010. Effect of ochratoxin a on body weight, feed intake and feed conversion in broiler chicken. Vet Med Int. 2010:590432.
- Saleemi MK, Khan MZ, Khan A. 2015. Embryotoxic and histopathological investigations of in-ovo inoculation of aflatoxigenic fungal extracts in chicken embryos. Pak Vet J. 35:403–408.
- Shen HM, Shi CY, Lee HP, Ong CN. 1994. Aflatoxin B1-induced lipid peroxidation in rat liver. Toxicol Appl Pharmacol. 127:145–150.
- Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, Shabbir MZ, Rehman H. 2012. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 91:2235–2240.
- Solcan C, Bocaneti F, Fantanariu M. 2015. Kidney myelolipoma and amyloidosis associated with lung osseous metaplasia in broiler chicken. Pak Vet J. 35:123–126.
- Stoev SD. 2010. Studies on carcinogenic and toxic effects of ochratoxin A in chicks. Toxins (Basel). 2:649–664.
- Sumbal GA, Shar ZH, Sherazi STH. 2016. Decontamination of poultry feed from ochratoxin A by UV and sunlight radiations. J Sci Food Agr. 96:2668–2673.
- Tavčar-Kalcher G, Vrtač K, Pestevšek U, Vengušt A. Validation of the procedure for the determination of aflatoxin B1 in animal liver using immunoaffinity columns and liquid chromatography with postcolumn derivatisation and fluorescence detection. Food Control. 18:333–337.
- Trailovic JN, Stefanovic S, Trailovic SM. 2013. In vitro and in vivo protective effects of three mycotoxin adsorbents against ochratoxin A in broiler chickens. Br Poult Sci. 54:515–523.
- Valtchev I, Koynarski T, Sotirov L. 2015. Effect of aflatoxin B1 on Moulard Duck's natural immunity. Pak Vet J. 35:67–70.
