Abstract
The objective of this study was to evaluate the effects of diets rich in omega-3 and -6 fatty acids (FA) during the transition period and early lactation on size and number of follicles, oocyte and embryo quality, and blood metabolites of Holstein cows. Forty-two cows were assigned to the diets: control (CON, with no fat sources and dietary ether extract content of 28.8 and 24.8 g/kg during the pre- and post-partum periods, respectively); whole flaxseed (WF, diet rich in omega-3 FA), 60 and 80 g/kg of WF during the pre and post-partum periods, respectively; whole raw soybeans (WS, diet rich in omega-6 FA), 120 and 160 g/kg of WS during the pre and post-partum periods; and calcium salts of unsaturated fatty acids (CSFA, diet rich in omega-6 FA), 24 and 32 g/kg of CSFA during the pre and post-partum periods. Diets were supplied from 35 d of the expected calving date until 84 days in milk (DIM). The ovaries of cows were examined from 14 to 84 DIM. Ovum pick-ups were performed on days 35 ± 7 and 65 ± 7 of lactation. Diets rich in omega-3 and -6 FA had greater follicle number than the CON. No differences were detected among diets rich in omega-3 and omega-6 FA on number and size of follicles and corpus luteum area. Cows had similar oocyte quality regardless of diet supplied. Although FA supplementation had no effect on embryo quality (number of cleaved and viable embryos), diets rich in omega-6 FA had lower viable embryos compared with WF. Supplementation with diets rich in omega-3 and -6 FA resulted in greater blood cholesterol concentration in early lactation cows. Fat supplementation increased the number of small and total follicles of early lactating cows. However, diets rich in omega-3 or -6 FA had no effect on oocyte quality. Cows fed WS during the transition period had the least viable embryos, and consequently cows fed diets rich in omega-6 FA had lower viable embryos than those fed WF.
Introduction
Supplemental fatty acids (FA) are often used to increase the dietary energy density and minimise negative energy balance (NEB), which has been related to low fertility in cows (Butler Citation2005). Additionally, certain FA have benefits that are independent of changes on the energy status of cows (Santos et al. Citation2008). Unsaturated FA of omega-3 and omega-6 series are associated with prostaglandins metabolism in dairy cows (Leroy et al. Citation2014), wherein the pathways involving prostaglandins production from omega-3 and omega-6 FA were reviewed by Wathes et al. (Citation2007).
Feeding diets rich in omega-6 FA to late pregnant ewes enhanced placental and endometrial prostaglandins production by increasing arachidonic acid (precursor of prostaglandins synthesis) concentration in blood (Emes et al. Citation2004; Cheng et al. Citation2005). On the other hand, omega-3 FA supplementation decreased endometrial prostaglandins production and increased the lifespan of corpus luteum (CL), which would benefit embryo maintenance in a dairy cow (Thatcher et al. Citation2006). However, the findings of omega-6 and omega-3 FA supplementation on oocyte and embryo quality in bovine have varied from positive effects (Cerri et al. Citation2009; Marei et al. Citation2009) to negative effects (Bilby et al. Citation2006; Marei et al. Citation2010).
Fatty acid supplementation increases blood cholesterol levels in dairy cows (Grummer & Carrol Citation1991), and cholesterol is a precursor for steroid hormones production (i.e. progesterone and oestradiol). Blood cholesterol concentration was positively associated with improvements on reproductive parameters including the expression of oestrus, days open and conception rate in dairy cows (Westwood et al. Citation2002). The objective of this study was to evaluate the effects of supplying diets rich in omega-3 [whole flaxseed (WF)] and omega-6 [whole raw soybean (WS) or calcium salts of fatty acids (CSFA)] FA during the transition period and early lactation on size and number of follicles, oocyte and embryo quality, and blood cholesterol concentrations of Holstein cows.
Materials and methods
The current study was authorised by the Bioethics Committee of School of Veterinary Medicine and Animal Science, University of Sao Paulo. The experiment was carried out in the Dairy Cattle Research Laboratory, Pirassununga Brazil. The Laboratory is located at 21° 57’ 28’’ south latitude, 47° 27′ 21″ east longitude, and 635 m of altitude.
Animals and experimental design
Forty-two multiparous Holstein cows were randomly assigned to receive one of four diets during the pre and post-partum periods: (1) control (CON, with no fat sources; n = 11), (2) whole flaxseed (WF, omega-3 FA source; n = 11), cows fed 60 g/kg (pre-) and 80 g/kg (post-partum) of WF, (3) whole raw soybeans (WS, omega-6 FA source; n = 10), cows fed 120 g/kg (pre-) and 160 g/kg (post-partum) of WS, and (4) calcium salts of unsaturated fatty acids (CSFA: Megalac®-E, Arm & HammerTM; Church & Dwight Co. Inc., Ewin, NJ, rumen-protected omega-6 FA source; n = 10), cows fed 24 g/kg (pre-) and 32 g/kg (post-partum) of CSFA. The experimental diets were supplied from 35 d before the expected calving date until 84 days in milk, and were formulated according to NRC (Citation2001) to meet the nutrient requirements of pre- and post-partum periods (Table ). Additionally, diets were formulated to have similar crude protein (CP) and neutral detergent fibre (NDF) contents. The management, housing, average milk yield, body condition score, body weight, and chemical analyses of dietary ingredients were described by Gandra et al. (Citation2016a).
Table 1. Ingredients, chemical composition and fatty acid profile of diets.
Follicle development
Cows’ ovaries were monitored daily after the morning milking from 14 to 84 days in milk using a 7.5 MHz linear rectal probe (Aloka SSD-500, Hitachi Aloka Medical America, Inc., Wallingford, CT). Visible follicles (≥2 mm diameter) were recorded in a drawn ovarian map of each cow. Afterwards, follicles were classified in subclasses according to their sizes as follows: class 1 (<3 mm), class 2 (3–5 mm), class 3 (6–9 mm), class 4 (10–15 mm), class 5 (>15 mm). Number and area of CL were also recorded.
In vitro fertilisation and embryo quality
Ovum pick-up (OPU) were performed on days 35 ± 7 and 60 ± 7 of lactation, according to the methods described by Pontes et al. (Citation2011) and embryos were produced according to Pontes et al. (Citation2009). OPU and in vitro embryo production were performed by In Vitro Brasil Ltda (Mogi Mirim, Brazil).
After each OPU, oocytes were classified as: grade I – more than three layers of compact cumulus cells, grade II – one layer of cumulus cells, grade III – denuded cell, atretic – oocyte containing dark cumulus cells, and degenerated – signs of cytoplasmic degeneration (Seneda et al. Citation2001). Atretic oocytes were discarded and the remaining oocytes were washed three times in TCM-199 HEPES containing gentamycin sulphate (Gibco Life Technologies, Grand Island, NY); then, oocytes were washed once in bicarbonate TCM-199 (Gibco Life Technologies) containing FCS, follicle stimulating hormone, oestradiol, pyruvate, and sulphate gentamycin. Cumulus oocytes complexes were separated after being cultured for 24-h in mineral oil. Frozen semen of a unique sire with known fertility was used for in vitro fertilisation. Sperm concentration was adjusted to 25.0 × 106 live sperm/mL and each fertilisation drop received 4 μL of sperm. Embryos were classified according to Wright (Citation1998). The drugs and medium culture used in the current experiment are described in Pontes et al. (Citation2011).
Blood metabolites
Blood samples were collected from coccygeal vessels in sterile vacutainers without clot activator before the morning feeding (07h00) on days −28, −21 and −7 relative to the expected calving date, and on days 7, 14, 21, 28, 56 and 84 of lactation. After clotting, blood samples were centrifuged 2000 × g during 15 min at 4 °C and serum was stored at −20 °C. Analyses of blood cholesterol and triglycerides were performed by colorimetric method using commercial kits (Bioclin®, Belo Horizonte, Brazil), and absorbance was determined using a semi-automatic spectrophotometer (SBA 200, CELM®, Sao Caetano do Sul, Brazil).
Statistical analyses
Data were submitted to MIXED procedure of SAS (version 9.2), after checking the normality of residuals and homogeneity of variances by UNIVARIATE procedure of SAS. Data of ovarian structures (number, class and area of follicle and CL) and blood metabolites were evaluated according to the model: Yijk = μ + Di + Tj + Di*Tj + ck + eijk, in which Yijk is the dependent variable, μ is the overall mean, Di is the fixed effect of diet, Tj is the fixed effect of time (week), Di*Tj is the fixed effect of diet by time interaction, ck is the random effect of cow, and eijk is the residual error. The data of metabolites from the first sampling of blood were used for covariate adjustment in the statistical analysis.
Data of oocyte and embryo quality were assessed using the following model: Yijk = μ + Di + Oj + Di*Oj + ck + eijk, in which Yijk is the dependent variable, μ is the overall mean, Di is the fixed effect of diet, Oj is the fixed effect of OPU day, Di*Oj is the fixed effect of diet by OPU day interaction, ck is the random effect of cow, and eijk is the residual error.
The Satterthwaite’s method (ddfm = satterth) was used to determine the degrees of freedom. The best covariance structure was based upon the smallest Akaike’s information criterion values. Orthogonal contrasts were performed to determine differences among diets: C1 (CON vs. WF + WS + CSFA), C2 (WF vs. WS + CSFA) and C3 (WS vs. CSFA). Significance level was set at 0.05. The first contrast was performed to describe the effect of FA supplementation. The second contrast was made to compare diets rich in omega-3 with diets rich in omega-6 FA. Finally, the third contrast was performed to compare two omega-6 FA sources, including WS (a natural rumen-protected fat) and CSFA (a commercially available rumen-protected fat).
Results
As expected, diets greatly differed in omega-6 and omega-3 FA concentrations. While the diet rich in omega-3 FA had 26.0 g/100 g of FA from C18:2 FA and 37.9 g/100 g from C18:3 FA, diets rich in omega-6 FA showed an average content of 45.3 g/100 g of FA from C18:2 FA and 3.1 g/100 g of FA from C18:3 FA, during the pre-partum period (Table ). Further, during the post-partum period the diet rich in omega-3 FA exhibited 27.2 g/100 g of FA from C18:2 FA and 36.9 g/100 g FA from C18:3 FA, meanwhile the diets rich in omega-6 FA had an average content of 48.5 g/100 g FA from C18:2 FA and 4.6 g/100 g FA from C18:3 FA.
Supplementation with omega-3 and omega-6 FA resulted in greater number of class 1 and total follicles (Table ). Neither diets rich in omega-3 nor those rich in omega-6 FA affected the number and area of CL in dairy cows. Regarding the comparison among omega-3 and omega-6 FA supplementation, no differences were detected on follicle development and CL traits (number and area). Although oocyte quality was not affected by experimental diets (Table ), the number of degenerated oocytes was greater at the first OPU compared to the second OPU (3.01 vs. 1.36, respectively; Figure ). No diet by time interaction effect was observed on oocyte quality. While diets rich in omega-3 or omega-6 FA had no effect on embryo quality compared to CON, feeding omega-6 FA sources resulted in lower number of viable embryos than omega-3 FA source, notably because of the lowest value of viable embryo for cows fed WS.
Figure 1. Degenerated oocytes of cows fed diets rich in omega-3 or omega-6 fatty acids during the transition period and early lactation. Diets: control (CON, with no fat sources); whole flaxseed (WF, diet rich in omega-3 FA), 60 and 80 g/kg of WF during the pre and post-partum, respectively; whole raw soybeans (WS, diet rich in omega-6 FA), 120 and 160 g/kg of WS during the pre and post-partum; and calcium salts of unsaturated fatty acids (CSFA, diet rich in omega-6 FA), 24 and 32 g/kg of CSFA during the pre- and post-partum.
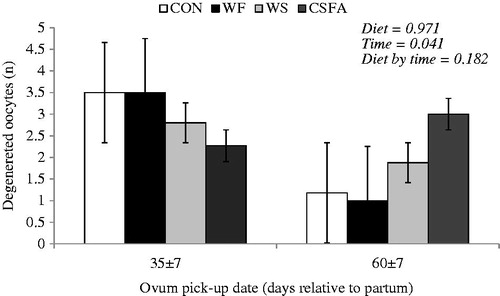
Table 2. Follicle and corpus luteum number of cows fed diets rich in omega-3 and omega-6 fatty acids during the transition period and early lactation (mean ± SEM).
Table 3. Oocyte and embryo quality, and blood metabolites of cows fed diets rich in omega-3 and omega-6 fatty acids during transition period and early lactation (mean ± SEM).
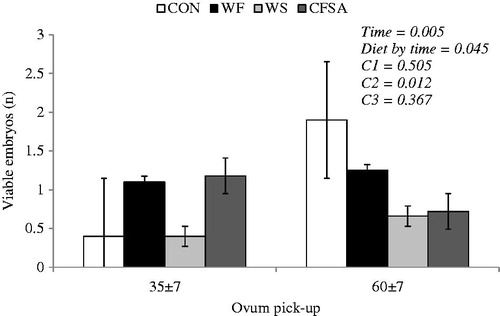
No time effect was observed on embryo quality, but a diet by time interaction effect was observed for viable oocytes number (Figure ). Both cows fed omega-3 and omega-6 FA sources had higher blood cholesterol concentration during the post-partum and greater blood triglyceride concentration during the pre-partum period.
Figure 2. Viable embryos of cows fed diets rich in omega-3 or omega-6 fatty acids during the transition period and early lactation Diets: control (CON, with no fat sources); whole flaxseed (WF, diet rich in omega-3 FA), 60 and 80 g/kg of WF during the pre and post-partum, respectively; whole raw soybeans (WS, diet rich in omega-6 FA), 120 and 160 g/kg of WS during the pre and post-partum; and calcium salts of unsaturated fatty acids (CSFA, diet rich in omega-6 FA), 24 and 32 g/kg of CSFA during the pre and post-partum. Orthogonal contrasts - C1: CON vs diets with supplemental fat, C2: WF vs. WS + CSFA, and C3: WS vs. CSFA.
Discussion
It is important to highlight that despite the fact of diets varied in a number of components, the differences in blood cholesterol and milk FA profile as well (Gandra et al. Citation2016b), suggest that the results of the current study are likely related to differences of the dietary omega-3 and omega-6 FA content in treatments.
Agreeing with the current study, several authors reported an increase on small follicle number when supplying diets rich in omega-3 (Zeron et al. Citation2002; Zachut et al. Citation2010) or omega-6 FA (Ponter et al. Citation2006), but the reasons for these effects were unclear. Although we did not assess hormonal blood profile, the greater number of total follicles when supplying omega-3 or omega-6 FA may be related to an increase on insulin-like growth factor I (IGF-I), which has a key hole on stimulus for follicular growth (Lucy Citation2000). The IGF-I has manifold effects on ovarian function including steroidogenesis, hormone release, and follicular growth, development and atresia (Cushman et al. Citation2001; Ptak et al. Citation2004). Earlier studies have reported that feeding cows with diets rich in omega-6 FA has an association with increased number of follicles (Robinson et al. Citation2002).
It is pertinent to point out that cows fed supplemental omega-3 and omega-6 FA showed higher energy balance (EB) than those fed CON during the post-partum (−1.7 vs. −5.2 Mcal/d; Gandra et al. Citation2016b), in which this effect was attributed to the differences in energy content of diets. The authors have shown that NEB during early lactation diminish gene transcription of IGF-I (Fenwick et al. Citation2008), thus it is expected that cows fed diets with higher energy density should exhibit better ovarian function. Spicer and Echternkapm (Citation1995) reported that IGF-I stimulates mitosis and this effect is observed only in small follicles. Further, blood glucose and insulin are found in low concentrations in cows during NEB (Butler Citation2001), and insulin enhances follicular responsiveness to LH (Frajblat Citation2000) resulting in recruitment of small follicles (Webb et al. Citation1999).
Differences on follicle development may also be partially explained by changes in blood cholesterol concentration and blood FA profile, mainly related to the concentrations of omega-3 FA. Wehrman et al. (Citation1991) diet-induced hyperlipidaemia in cattle and reported that an increase in serum cholesterol concentration was associated with folliculogenesis. Despite the fact that blood FA profile was not assessed in this study, we observed that cows fed either diets rich in omega-3 or diet rich omega-6 exhibited higher concentration of linolenic acid in milk compared to CON (Gandra et al. Citation2016b), suggesting greater availability of linolenic acid in blood. Other studies have reported an increase in number of small follicles when cows or ewes were fed omega-3 FA (Zeron et al. Citation2002; Ponter et al. Citation2006; Heravi Moussavi et al. Citation2007).
Although a greater number of follicles are desired in donor cows, the current experiment showed no differences on oocyte and embryo quality after feeding cows with diets rich in omega-3 or omega-6 FA. Therefore, supplementing cows with fat in order to increase the number and quality of embryos produced in vitro was not effective. The effects of supplemental omega-3 or omega-6 FA seems to be more evident on the follicle development, since a diet by time interaction effect was observed in total number of follicles. During all the experimental period, cows fed diets rich in omega-3 and omega-6 FA showed higher number of follicles than CON, notably during the first three weeks of lactation.
The number of degenerated oocytes at the first OPU was higher than the number of degenerated oocytes at the second OPU. At the first OPU cows showed an average energy balance of −4.0 Mcal/d while at the second OPU cows had energy balance of 1.2 Mcal/d (Gandra et al. Citation2016b). The early post-partum NEB may adversely impact follicle development and exert carryover effects on fertility (Britt Citation1992). Furthermore, cows with a more severe NEB produce oocytes with lower developmental capacity following in vitro maturation and fertilisation (Kruip et al. Citation2001).
The current experiment demonstrated that WS was detrimental to the number of viable embryos. To our knowledge, there is no experiment that has evaluated WS on oocyte and embryo quality, but studies have already reported adverse effects of omega-6 FA on in vitro oocyte development and consequently interfering on embryo quality. Marei et al. (Citation2010) supplemented a growth media with linoleic acid and reported inhibition of oocyte nuclear maturation. Fouladi-Nashta et al. (Citation2009) compared different sources of fat (calcium salts of palm oil, toasted soybean, and linseed) on oocyte and embryo quality of early to mid-lactating cows, and the authors found similar oocyte quality, but also reported negative effects of both toasted soybean and linseed supply on cleaved embryos after in vitro fertilisation. However, several studies reported better oocyte and embryo quality for cows fed diets rich in omega-3 FA in comparison with cows fed other sources of FA (Zeron et al. Citation2002; Bilby et al. Citation2006; Cerri et al. Citation2009). The mechanism by which polyunsaturated FA supplementation alters oocyte and embryo quality is related to their capacity to integrate phospholipid membrane altering its fluidity and affecting any process that is mediated by the membrane (Grammatikos et al. Citation1994).
In addition, cows fed diet rich in omega-3 FA showed higher blood glucose concentration compared to those cows fed WS during the early lactation (67.2 vs. 57.4 mg/dL, respectively; Gandra et al. Citation2016a). Glucose is an indispensable molecule for adequate oocyte maturation (Leroy et al. Citation2005) and the relative low glucose concentration of cows fed WS probably influenced the oocyte maturation and consequently the number of viable embryos. Fat supplementation frequently increases blood cholesterol concentrations (Spicer et al. Citation1993; Garcia-Bojalil et al. Citation1998), as reported in the current experiment. We expected that cholesterol would alter follicle development or oocyte quality through enhancement of steroidal hormones production. However, blood cholesterol had no effect on follicle and oocyte number and quality, agreeing with previous studies (Ferguson et al. Citation1990; Spicer et al. Citation1990).
Conclusions
Diets rich in omega-3 and omega-6 FA increase the number of small follicles, which is desired in OPU and in vitro fertilisation procedures. However, omega-3 and omega-6 FA supplementation does not ameliorate oocyte and embryo quality. Diets rich in whole raw soybeans may decrease the number of viable embryos.
Acknowledgements
The authors acknowledge the University of São Paulo and the Dairy Cattle Research Laboratory, for providing all the physical structure and staff necessary for this study.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article
Additional information
Funding
References
- Bilby TR, Block J, Amaral BC, Sa Filho O, Silvestre FT, Hansen PJ, Staples CR, Staples Thatcher WW. 2006. Effects of dietary unsaturated fatty acids on oocyte quality and follicular development in lactating dairy cows in summer. J Dairy Sci. 89:3891–3903.
- Britt JH. 1992. Influence of nutrition and weight loss on reproduction and early embryonic death in cattle. In: Proceedings of the XVII World Buiatric Congress 1992; p. 143–149
- Butler WR. 2001. Nutritional interactions with reproductive performance in dairy cattle. Anim Repr Sci. 60:449–457.
- Butler WR. 2005. Relationships of negative energy balance with fertility. Adv Dairy Technol. 17:35–46.
- Cerri RLA, Juchem SO, Chebel RC, Rutigliano HM, Bruno RGS, Galvão KN, Thatcher WW, Santos JEP. 2009. Effect of fat source differing in fatty acid profile on metabolic parameters, fertilization, and embryo quality in high-producing dairy cows. J Dairy Sci. 92:1520–1531.
- Cheng Z, Elmes M, Kirkup SE, Chin EC, Abayasekara DRE, Wathes DC. 2005. The effect of a diet supplemented with the n-6 polyunsaturated fatty acid linoleic acid on prostaglandin production in early- and late-pregnant ewes. J Endocrinol. 184:165–178.
- Cushman RA, Souza JC, Hedgpeth VS, Britt JH. 2001. Alteration of activation, growth, and atresia of bovine preantral follicles by long-term treatment of cows with estradiol and recombinant bovine somatotropin. Bio Repr. 65:581–586.
- Emes M, Tew P, Cheng Z, Kirkup SE, Abayasekara PC, Hanson MA, Wathes DC, Burdg GC. 2004. The effect of dietary supplementation with linoleic acid to late gestation ewes on the fatty acid composition of maternal and fetal plasma and tissues and the synthetic capacity of the placenta for 2-series prostaglandins. Biochimica Et Biophysica Acta. 1686:139–147.
- Fenwick MA, Fitzpatrick R, Kenny DA, Diskin MG, Patton J, Murphy JJ, Wathes DC. 2008. Interrelationships between negative energy balance (NEB) and IGF regulation in liver of lactating dairy cows. Dom Anim Endocrinol. 34:31–44.
- Ferguson JD, Sklan D, Chalupa WV, Kronfeld DS. 1990. Effects of hard fats on in vitro and in vivo rumen fermentation, milk production and reproduction in dairy cows. J Dairy Sci. 73:2864–2879.
- Fouladi-Nashta AA, Wonnacott KE, Gutierrez CG, Gong JG, Sinclair KD, Garnsworthy PC, Webb R. 2009. Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction. 138:771–781.
- Frajblat M. 2000. Metabolic state and follicular development in the postpartum lactating dairy cow. Ithaca (NY): Cornell University.
- Gandra JR, Barletta RV, Mingoti RD, Verdurico LC, Freitas Junior JE, Oliveira LJ, Takiya CS, Kfoury JR, Wiltbank MC, Rennó FP. 2016a. Effects of whole flaxseed, raw soybeans, and calcium salts of fatty acids on measures of cellular immune function of transition dairy cows. J Dairy Sci. 99:4590–4606.
- Gandra JR, Mingoti RD, Barletta RV, Takiya CS, Verdurico LC, Freitas JE, Paiva PG, Jesus EF, Calomeni GD, Rennó FP. 2016b. Effects of flaxseed, raw soybeans and calcium salts of fatty acids on apparent total tract digestibility, energy balance and milk fatty acid profile of transition cows. Animal. 1:1–8.
- Garcia-Bojalil CM, Staples CR, Risco CA, Savio JD, Thatcher WW. 1998. Protein degradability and calcium salts of long-chain fatty acids in the diets of lactating dairy cows: productive responses. J Dairy Sci. 81:1374–1384.
- Grammatikos SI, Subbaiah PV, Victor TA, Miller WM. 1994. Diverse effects of essential (n-6 and n-3) fatty acids on cultured cells. Cytotechnology. 15:31–50.
- Grummer RR, Carrol DJ. 1991. Effects of dietary fat on metabolic disorders and reproductive performance of dairy cattle. J Anim Sci. 69:3838–3852.
- Heravi Moussavi AR, Gilbert RO, Overton TR, Bauman DE, Butler WR. 2007. Effects of feeding fish meal and n-3 fatty acids on ovarian and uterine responses in early lactating dairy cows. J Dairy Sci. 90:145–154.
- Kruip TAM, Wensing T, Vos PLAM. 2001. Characteristics of abnormal puerperium in dairy cattle and the rationale for common treatments. Animal Sci Occas Publ. 26:63–79.
- Leroy JLMR, Vanholder T, Mateusen B, Christopher A, Opsomer G, Kruif A, Genicot G, Van Soom A. 2005. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Repr Res. 130:485–495.
- Leroy JLMR, Sturmey RG, Van Hoeck V, De Ble J, McKeegan PJ, Bols PEJ. 2014. Dietary fat supplementation and the consequences for oocyte and embryo quality: hype or significant benefit for dairy cow reproduction? Repr Domestic Anim. 49:353–362.
- Lucy MC. 2000. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J Dairy Sci. 83:1635–1647.
- Marei WF, Wathes DC, Fouladi-Nashta AA. 2009. The effect of linolenic acid on bovine oocyte maturation and development. Biol Reprod. 81:1064–1072.
- Marei WF, Wathes DC, Fouladi-Nashta AA. 2010. Impact of linoleic acid on bovine oocyte maturation and embryo development. Reproduction. 139:979–988.
- National Research Council (NRC). 2001. Nutrient requirements of dairy cattle. 7th Rev ed. Washington, DC: National Academy of Sci; p. 381.
- Ponter AA, Parsy AE, Saadé M, Mialot JP, Ficheux C, Duvaux-Ponter C, Grimard B. 2006. Effect of a supplement rich in linolenic acid added to the diet of post partum dairy cows on ovarian follicle growth, and milk and plasma fatty acid compositions. Reprod Nutr Dev. 46:19–29.
- Pontes JHF, Nonato-Júnior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros RR, Oliveira JA, Hasler JF, Seneda MM. 2009. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology. 71:690–697.
- Pontes JHF, Melo Sterza FA, Basso AC, Ferreira CR, Sanches BV, Rubin KCP, Seneda MM. 2011. Ovum pick up, in vitro embryo production, and pregnancy rates from a large-scale commercial program using Nellore cattle (Bos indicus) donors. Theriogenology. 75:1640–1646.
- Ptak A, Kajta M, Gregoraszczuk EL. 2004. Effects of growth hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured luteal cells collected from early, mature, and regressing porcine corpora lutea. Anim Repr Sci. 80:267–279.
- Robinson RS, Pushpakumara PGA, Cheng Z, Peters AR, Abayasekara DRE, Wathes DC. 2002. Effects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cows. Reproduction. 124:199–131.
- Santos JEP, Bilby TR, Thatcher WW, Staples CR, Silvestre FT. 2008. Long chain fatty acids of diet as factors influencing reproduction in cattle. Repr Domest Anim. 43:23–30.
- Seneda MM, Esper CR, Garcia JM, Oliveira JÁ, Vantini R. 2001. Relationship between follicle size and ultrasound-guided trans-vaginal recovery. Anim Repr Sci. 67:37–43.
- Spicer LJ, Tucker WB, Adams GD. 1990. Insulin-like growth factor-I in dairy cows: relationships among energy balance, body condition, ovarian activity, and estrous behavior. J Dairy Sci. 73:929–937.
- Spicer LJ, Echternkapm SE. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest Anim Endocrinol. 12:223–245.
- Spicer LJ, Vermon RK, Tucker WB, Wettemann RP, Hogue JF, Adams GD. 1993. Effects of inert fat on energy balance, plasma concentrations of hormones, and reproduction in dairy cows. J Dairy Sci. 76:2664–2673.
- Thatcher WW, Bilby TR, Bartolome JA. 2006. Strategies for improving fertility in the modern dairy cow. Theriogenology. 65:30–44.
- Webb R, Campbell BK, Garverick HA, Gong JG, Gutierrez CG, Armstrong DG. 1999. Molecular mechanisms regulating follicular recruitment and selection. J Repr Fert. 54:33–48.
- Weiss WP, Conrad H, Pierre NS. 1992. A theoretically-based model for predicting total digestible nutriente values of forage and concentrates. Anim Feed Sci Technol. 39:95–110.
- Westwood CT, Lean IJ, Garvin JK. 2002. Factors influencing fertility of Holstein dairy cows: a multivariate description. J Dairy Sci. 85:3225–3237.
- Wathes DC, Abayasekara DRE, Aitken RJ. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 77:190–201.
- Wehrman ME, Welsh TH Jr. Williams GL. 1991. Diet-induced hyperlipidemia in cattle modifies the intrafollicular cholesterol environment, modulate ovarian follicular dynamics, and hastens the onset of postpartum luteal activity. Biol Repr. 45:514–522.
- Wright J. 1998. Photomicrographic illustration of embryo codes. In: Stringfellow DA, Seidel SM, editors. Manual of the International Embryo Transfer Society. Savory: International Embryo Transfer Society; p. 161–170.
- Zachut M, Dekel I, Lehner H, Arielli A, Arav A, Livshitz L, Yakoby S, Moallem U. 2010. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J Dairy Sci. 93:529–545.
- Zeron Y, Sklan D, Arav A. 2002. Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Molecular Repr Dev. 61:271–278.
