Abstract
In this study, we tested the effects of Yucca schidigera extract (YSE) on the growth performance in broilers, and furthermore, its effects on antioxidative enzyme activities and corresponding gene in the liver of broilers. A total of 128 14-day-old broiler chickens were randomly assigned to four treatments: maize-soybean meal as the basal control diet and the basal diet containing 100, 200, or 300 mg/kg of YSE, respectively, in this study. Each treatment was consisted of four replicate pens with eight broilers per pen. The experiment lasted 28 days. Average daily gain (ADG), average feed intake (AFI) and feed efficiency (FE) were recorded during grower period (d 15 to d 28) and finisher period (d 29 to d 42), respectively. On day 28 and 42, liver samples were collected to analyse superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) activities, malondialdehyde (MDA) concentrations, total antioxidant capacity (T-AOC), and gene expressions of SOD, CAT, GPx. The results showed that during grower period, there was no difference on growth performance, while CAT activity and its gene expression were increased at 200 mg/kg YSE level. During finisher period, 100 mg/kg YSE supplementation enhanced ADG, and 100 and 200 mg/kg YSE groups improved FE. T-AOC was improved at both 200 and 300 mg/kg. 300 mg/kg supplementation of YSE enhanced GPx and SOD activity, and decreased MDA concentration. GPx gene expression was up-regulated at 300 mg/kg level. In conclusion, YSE promoted growth performance in broilers as well as exhibited liver antioxidative ability during finisher period.
Introduction
Oxidative stress is caused by increased ROS (reactive oxygen species) in cells. The major ROS in cells are superoxide radical anion (O2•−), hydrogen peroxide (H2O2) and the highly reactive hydroxyl radical (OH•−) (Sen et al. Citation2010). Several antioxidative enzymes in cells act as the defence system to prevent the formation of reactive species such as catalase (CAT), superoxide dismutase (SOD) and glutathion peroxidase (GPx) (Sanders et al. Citation2004).
Modulating dietary treatments of livestock such as adding antioxidants is one of the effective means to relieve oxidative stress potentials among various methods (Sen et al. Citation2010). Yucca schidigera (YS), prevalent in the deserts of the south-western United States and northern Mexico, is considered highly for its pharmaceutical values (Cheeke et al. Citation2006; Patel Citation2012). Yucca shidigera extract (YSE) was applied as dietary additives for livestock primarily for ammonia and odour control due to the presence of saponin (Cheeke Citation2000; Piacente et al. Citation2005; Ayasan Citation2013). But two other main active components, resveratrol and yuccaols, which possess biological functions, were identified in YS besides steroidal saponins (Patel Citation2012). Resveratrol is well known to be an effective scavenger of hydroxyl, superoxide radicals, as well as inhibiting ROS formation in cells. It also protects cell from lipid peroxidation in membranes and DNA damage caused by ROS (Leonard et al. Citation2003). Phenolic constituents such as yuccaols in YS which structurely related to resveratrol, also possess radical scavenging activity (Piacente et al. Citation2004, Citation2005; Patel Citation2012).
Alagawany et al. (Citation2016) reported that YSE supplementation improved SOD and reduced glutathione (GSH) level, and reduced MDA concentration in serum of laying hens. Another experiment observed increased GPx and CAT in rabbits with YSE supplementation (Ashour et al. Citation2014). Ince et al. (Citation2013) demonstrated that dietary YSE incorporation, in a dose-dependent manner, ameliorated arsenic-induced oxidative stress, lipid peroxidation, and increased antioxidant enzyme activities in mice. In another study, YSE treatment to rats was found to decrease blood and tissue malondialdehyde, and increased the glutathione in blood and various tissues (Cigerci et al. Citation2009).
Many reports have also reported that dietary YSE incorporation could produce positive effects on the average weight (Sahoo et al. Citation2015), FE (Ayasan et al. Citation2005; Wang & Kim Citation2011), behaviour (Sahoo et al. Citation2015) and health of chicks (Alfaro et al. Citation2007). However, there was little information on that using YSE as a feed additive to ameliorate the potential negative effects of oxidative stress in broilers. The objective of the present study was to elucidate the effects of YSE on growth performance, liver antioxidant status in broilers, and find out whether they are related to each other.
Materials and methods
Ethics statement
The experiment was carried out in a poultry research facility located at the Inner Mongolia Agricultural University, Hohhot, China. All experimental procedures performed were approved by the Animal Research and Ethics Board of College of Animal Science, Inner Mongolia Agricultural University, Hohhot, China.
Animals, experiment design and treatments
One-day-old AA broilers were purchased from a commercial farm in Hohhot, Inner Mongolia, China. They were housed in electrically heated battery brooders until day 7 and transported to the experimental site until day 14. Then, 128 14-day-old chickens of uniform body weight were selected and were reared in stainless-steel wire cages. Their average initial body weight was 372.82 ± 6.35 g. Broilers were assigned to 4 dietary treatments. Each treatment was randomly divided into 4 equal replicates, with 8 chickens/cage (100 × 50 × 50 cm). The duration of experimental period was 28 days, and was divided into grower period (d 15 to d 28) and finisher period (d 29 to d 42). Experimental diets and water were available ad libitum during the entire experimental period. Before the experiment, the poultry facility was fumigated using methanal plus potassium permanganate to disinfect the environment. Poultry facilities had thermostatically controlled heater and lighting programme. Pens were equipped with a pan feeder, a manual drinker. Drinkers were daily washed to prevent faecal and microbial contamination.
YSE product and diets
YSE powder was purchased from Shanxi Yuanzhixing Biotechnology Co., Ltd (Xian, China). Sterodial saponin content of YSE was >7%. Diets were formulated to meet nutrients recommendation of Feeding Standard of Chicken, China (NY/T 33-2004) (Chinese Ministry of Agriculture Citation2004). Diets were fed in mash form. Diet treatments were as follows: (1) control diet (basal diet without supplement), (2) basal diet with 100 mg/kg of YSE, (3) basal diet with 200 mg/kg of YSE, and (4) basal diet with 300 mg/kg of YSE. The formulation and composition of the control diet is found in Table .
Table 1. Feed composition and nutrient content of the basal experimental diet.
Growth performances and sample collection
Average daily gain (ADG), average feed intake (AFI) were recorded during grower period and finisher period, and feed efficiency (FE) was calculated as feed intake/body weight gain. On day 28 and day 42, 2 chickens from each pen (8 per treatment) were randomly selected, weighed, stunned, and slaughtered by exsanguination. And liver tissues were collected based on the measures as described by Tufarelli et al. (Citation2016), and the samples were stored at −80 °C to measure the antioxidative parameters and gene expression.
Antioxidative enzyme activities
All reagents for these assays were purchased from Jiancheng Bioengineering Institute (Nanjing, China). MDA, T-AOC, GPx, T-SOD, and CAT activities in liver samples were measured according to the instructions of manufacture (Cao et al. Citation2015; Liao et al. Citation2015).
Total RNA extraction and reverse transcription
Total RNA was extracted from liver tissues by Trizol extraction method described by Trizol manufacturer (RNAiso Plus Kit, Takara Bio Inc, Kusatsu, Japan). The quantity and purity of RNA samples was measured by using Nano Drop spectroscopy (Thermo Scientific, Waltham, MA) with the ratio of absorbance at 260 nm and 280 nm (Mueller et al. Citation2012). Reverse transcription was performed by using a commercial complementary DNA synthesis kit according to manufacturer’s instructions included in the kit (Reverse Transcription System Kit, Takara Bio Inc, Kusatsu, Japan).
Quantitative real-time PCR
Primers sequences for β-Actin, SOD, CAT, and GPx were designed by Gene bank database sequences from BGI Tech (Shenzhen, China) corresponding to each quantified gene (Table ). Quantitative real-time PCR (RT-PCR) was performed by IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA) on 96-well plate with 20 μl of total reaction volume including 10 μl SYBR Premix Ex Taq TM П (Takara Bio Inc, Kusatsu, Japan), 0.4 μl of forward primer (10 pmol), 0.4 μl of reverse primer (10 pmol), 2 μl of cDNA, and 7.2 μl of nuclease-free water. SYBR Premix Ex Taq TM П was used as RT-PCR master mix and each reaction was run in duplicate. The following PCR programme was employed to amplify target mRNA in tissue extracts: an initial denaturation step at 95 °C for 30 s, followed by amplification for 40 cycles at 95 °C for 5s, and 55 °C for 20 s, and 72 °C for 20 s, and annealing step at 72 °C for 7 min, then extension at 95 °C for 20 s.
Table 2. Primers used for quantitative real-time PCR analysis of chicken mRNAs.
Statistical analyses
The results are expressed as mean ± SEM or mean ± SD. The statistical significance of data was evaluated by ANOVA procedure of SAS 9.2 (SAS Institute Citation2002). Data were analysed by Duncan’s multiple range test. Differences in the mean values were considered significant at p < .05, whereas .05 < p < .10 was considered to constitute a tendency.
Results
Growth performance
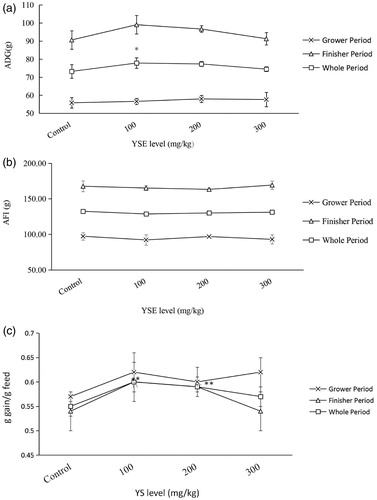
The influence of dietary YSE on growth performance in broilers was shown in Figure . During finisher period, ADG was increased with 100 mg/kg YSE in the diets (p = .034), FE was enhanced at 100 and 200 mg/kg YSE groups (p = .038), In the whole experimental period, there was a tendency of the increasing of ADG at 100 mg/kg level (p = .072), FE was enhanced at both 100 and 200 mg/kg groups (p = .011).
Figure 1. Effects of YSE on growth performance in broilers. The figure describes effects of dietary YSE on ADG (a), AFI (b), FE (c) in broilers. Statistical comparisons are made between control group and YSE-added groups. Results are expressed as means ± SD. Asterisks indicate significant differences according to different supplementing level of YSE (*p < .05).
Antioxidative activities and gene expressions in liver
The results of antioxidant enzymes activity in liver were shown in Table , the corresponding gene expressions were shown in Figure . During grower period, CAT activity was increased at 200 mg/kg YSE level, CAT gene expression was up-regulated with 200 mg/kg YSE addition (p = .003). MDA concentration was shown a decreasing tendency at 200 mg/kg level. During finisher period, T-AOC was improved at both 200 and 300 mg/kg, with 300 mg/kg group exhibiting the strongest effect. 300 mg/kg supplementation of YSE enhanced GPx and SOD activity, and decreased MDA concentration. GPx gene expression was up-regulated at 300 mg/kg level (p = .028).
Figure 2. Effects of YSE on gene expression in liver of broilers. Statistical comparisons are made between control group and YSE-added groups. Results are expressed as means ± SD. Asterisks indicate significant differences according to different supplementing level of YSE (*p < .05, **p < .01).
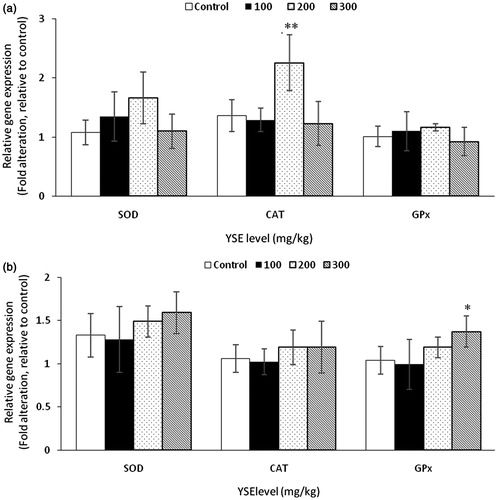
Table 3. Effects of YSE on antioxidative functions in liver.
Discussion
Growth performance
In the present study, we observed the better ADG and FE in broilers. The optimum dose in our study was in line with a study of Sahoo et al. (Citation2015) indicating that 125 mg/kg YSE increased the growth of broilers in 6th week by utilising lesser FI, better FCR, protein efficiency ratio, and energy efficiency ratio. However, current study only observed the improvement of growth performance in finisher period, but not in grower period. We proposed that it may be related to different energy requirements of broilers in different phase, thus the different changes of energy with YSE addition, as Kucukkurt & Dundar (Citation2013) indicated that YSE supplementation affects energy metabolism through modulating hormone secretions and depressing energy compounds in the organism.
Steroidal saponins are known as major physiologically active components of YS with broad-spectrum biological properties, and the growth-promoting effects of YSE has long been ascribed to them (Piacente et al. Citation2005). The positive influence of steroidal saponins on better absorption of nutrients in the intestinal tract (McAllister et al. Citation1998; Wang & Kim Citation2011) might be responsible for the better growth performance. However, YS bark contains polyphenols such as resveratrol, yuccaols A to E, which possess antioxidant, free-radical scavenging, and anti-inflammatory abilities (Piacente et al. Citation2004, Citation2005; Cheeke et al. Citation2006). It is hypothesised that YSE may exhibit its growth-promoting effects not only due to its saponin components. An experiment supplementing resveratrol to laying chickens might have supported this hypothesis at least to some extent (Zhang et al. Citation2014). In such study, the resveratrol level that significantly enhanced ADG fell on 400 mg/kg (Zhang et al. Citation2014), which is higher than the optimum doses observed in the present study (100 mg/kg YSE). We provided that the lower optimum dose observed in our study might be attributed to the composite effects of both saponin fractions and polyphenols. However, the claims must be scientifically verified with more emphasis on animal data.
Although there exists limited researches exploring toxicity of YSE in the application of animals, detecting of dioscin (Xu et al. Citation2012), a steroidal-saponin containing plants, provided us an implication of the potential toxic effects of YSE in livestock production if administered not appropriately. Whether or not the non-different performance at 300 mg/kg YSE levels in the present study was linked to the potential detrimental effects remains unknown, which needs to be investigated further more specifically.
Antioxidative activities and gene expressions in liver
MDA levels in the liver is proved to be a sensitive indicator of the lipid oxidative tendency (Shafey et al. Citation2015). SOD is an important substance that exists in various tissues and organisms, and is believed to protect cells from damage caused by superoxide radical (O2•−) (Kurutas Citation2016). In vitro study have proved the efficiency of phenolics from bark of YS in decreasing ROS production in blood platelets (Olas et al. Citation2005). In the current study, the decreases of MDA concentrations and the increases of SOD concentrations might be attributed to the YSE ability in terms of scavenging secondary reactive radicals or preventing formation of superoxide and hydrogen peroxide (Enginar et al. Citation2006). The results of our experiment showed that MDA concentration in finisher period was higher than grower period, which was in line with results of Liu et al. (Citation2011) who indicated that serum MDA levels at d 42 was higher than at d 21. It is assumed that oxidative damage in the liver might be more severe during finisher period than grower period, and the higher SOD in finisher phase may suggested that adaptive response may enhance the expression of antioxidant enzymes and compounds in response to free radical-mediated lipid peroxidation products (Kurutas Citation2016). In our study, the dose of YSE where SOD exhibited positive effects in finisher period was higher than grower period. We proposed that when the oxidative status is intensified such as in our cases, the optimum dose of YSE that exhibits antioxidative functions would be higher accordingly. The decreasing but not significant tendency of MDA during grower period may suggest that protective mechanisms in liver such as hepatic microsomes which have the ability of generating and degenerating TBARS (Venkatraman et al. Citation1998) expressed certain function to maintain MDA towards normal levels, or due to the different producing site of lipid peroxides as described in the article of Shafey et al. (Citation2015).
CAT catalyses the conversion of hydrogen peroxide to water and oxygen (Kurutas Citation2016). GPx acts as the catalyst to transform reduced glutathione (GSH) and H2O2 into oxidised glutathione (GSSG) and H2O (Kurutas Citation2016). We noticed that CAT was only promoted at grower period, while GPx was enhanced at finisher period but not in grower period. This demonstrated that in different phase, YSE might possess the ability to eliminate H2O2 primarily by either enhancing CAT or GPx, since CAT competes with GPx for hydrogen peroxide as a substrate (Molavian et al. Citation2015). According to Limón-Pacheco & Gonsebatt (Citation2009), CAT and GPx were not co-existed in the organism. CAT is primarily existed in subcellular organelles such as peroxisomes, while GPx are present in the cytoplasm and mitochondrial matrix. Mitochondria and the endoplasmic reticulum barely contain CAT. It is assumed that H2O2 might be produced in different sites in different growing period of broilers, thus CAT or GPx could serve their own H2O2-eliminating functions. CAT gene expression corresponded well with its enzyme activities in grower period, and GPx expression was in accordance with its enzyme activity in finisher period. These suggested that YSE could participate in the removal of H2O2 by up-regulating CAT expression during grower period and GPx expression during finisher period. In our study, although we did not detect the significant difference, the paralleling tendency between SOD expression and its enzyme activity during grower period gave us a hint of the potential of YSE to up-regulate SOD activity during grower period.
T-AOC, comprised of several key antioxidative enzymes and other mechanisms, is a reflection of total antioxidative ability within the body (Ahmad et al. Citation2012). In our experiment, YSE supplementation enhanced T-AOC in finisher period, proved that YSE exhibits certain antioxidant ability, and this was at least partially due to the enhancement of SOD and CAT/GPx both at transcript and translation levels based on our results. However, T-AOC was not increased in grower period. The enhancing but not significant tendency of SOD during grower period might contribute to the trend to some extent.
Alagawany et al. (Citation2016) reported that YSE up to 100 mg/kg could increase SOD, glutathione (GSH), and decrease MDA concentrations in laying hens. Another study observed increases of plasma GSH concentrations and T-AOC in 100 ppm YSE group (Aslan et al. Citation2005). In contrast, our results indicated that higher doses of YSE supplementation improved antioxidative functions in the liver of broilers. This inconsistency might be attributed to the physiological differences between broilers and laying hens. In our earlier study, the dose that stimulates growth and immune functions lies on 100 mg/kg level (Su et al. Citation2016), which was lower than optimum doses that express antioxidative functions in the present study. This again raised the question by other authors that whether removal of too many ROS by supplementation of antioxidants could upset the cell signalling pathways and actually increase the risk of chronic diseases (Niki Citation2012, Citation2014). It is well demonstrated that the main active substances to exhibit antioxidant ability in YSE are polyphenols (Patel Citation2012). Based on the study carried out in resveratrol, which resembles to yuccaols in structures (Piacente et al. Citation2005), rapid sulfatation and glucuronidation by theintestine seem to limit its systemic bioavailability (Walle et al. Citation2004). In addition, phenolics only occur in the external part of the trunk, not inside (Piacente et al. Citation2005). Thus, a margin of safety above the estimated requirements, as well as the economic efficiency, should be considered if the present data are used as a basis for formulation of broiler diets. Further tests need be focused on the effects of bark part of YSE on the antioxidative capacities of broilers.
Conclusions
In conclusion, 100 and 200 mg/kg dietary YSE promoted growth performance of broilers, while 200 and 300 mg/kg dietary YSE enhanced antioxidant capacities in the liver. And the effectiveness of YSE on growth performance and antioxidant system was mostly expressed during finisher period. The enhanced antioxidative capacities in liver may contribute to the better growth performance in broilers.
Implications
There are now studies available combining YSE with other additives such as caprylic acid (Wang & Kim Citation2011; Begum et al. Citation2015), coccidiostat (Alfaro et al. Citation2007), zeolite (Çabuk et al. Citation2004), yeast cell walls (Gurbuz et al. Citation2011), or coccidiosis vaccine (Alfaro et al. Citation2007), to explore their potentials in improving the performance of livestock. Our results showed that YSE alone exhibited greatest potentials in the finisher phase of broilers, which gives us a cue to explore the combining effects of YSE and other additives, to see if they could improve the productivity of animals in a wider range of time.
Acknowledgements
For their help during laboratory analysis and data analysis, the authors express deep appreciation to Fei Zhao, Hongyan Chen, Yuanqing Xu, Pengfei Zhang from College of Animal Science, Inner Mongolia Agricultural University, China.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahmad H, Tian JK, Wang JJ, Khan MA, Wang YX, Zhang LL, Wang T. 2012. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J Agr Food Chem. 60:7111–7120.
- Alagawany M, Abd El-Hack ME, El-Kholy MS. 2016. Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Environ Sci Pollut Res Int. 23:6774–6782.
- Alfaro DM, Silva AVF, Borges SA, Maiorka FA, Vargas S, Santin E. 2007. Use of Yucca schidigera extract in broiler diets and its effects on performance results obtained with different coccidiosis control methods. J Appl Poult Res. 16:248–254.
- Ashour EA, Alagawany M, Reda FM, Abd El-Hac ME. 2014. Effect of supplementation of Yucca schidigera extract to growing rabbit diets on growth performance, carcass characteristics, serum biochemistry and liver oxidative status. Asian J Anim Vet Adv. 9:732–742.
- Aslan R, Dundar Y, Eryavuz A, Bulbul A, Kuçukkurt I, Fidan AF, Akinci Z. 2005. Effects of various quantities of Yucca schidigera powder (Deodorase) added to diets on the performance, some hematological and biochemical blood parameters, and total antioxidant capacity of laying hens. Revue Méd Vét. 156:350–355.
- Ayasan T, Yurtseven S, Baylan M, Canogullarl S. 2005. The effects of dietary yucca schidigera on egg yield parameters and egg shell quality of laying Japanese Quails (Coturnix coturnix Japonica). Int J Poult Sci. 4:159–162.
- Ayasan T. 2013. Effects of dietary Yucca schidigera on hatchability of Japanese Quails. Indian J Anim Sci. 83:641–644.
- Begum M, Hossain MM, Kim IH. 2015. Effects of caprylic acid and Yucca schidigera extract on growth performance, relative organ weight, breast meat quality, haematological characteristics and caecal microbial shedding in mixed sex Ross 308 broiler chickens. Vet Med-Czech. 60:635–643.
- Çabuk M, Alçiçek A, Bozkurt M, Akkan S. 2004. Effect of Yucca schidigera and natural zeolite on broiler performance. Int J Poult Sci. 3:651–654.
- Cao LP, Ding WD, Du JL, Jia R, Liu YJ, Zhao CY, Shen YJ, Yin GJ. 2015. Effects of curcumin on antioxidative activities and cytokine production in Jian carp (Cyprinus carpio var. Jian) with CCl4-induced liver damage. Fish Shellfish Immun. 43:150–157.
- Cheeke PR. 2000. Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. J Anim Sci. 77:1–10.
- Cheeke PR, Piacente S, Oleszek W. 2006. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: a review. J Inflamm (Lond). 3:6.
- Chinese Ministry of Agriculture. 2004. Feeding Standard of Chicken, China (NY/T 33-2004). Hunan Feed. 4:19–27. (in Chinese)
- Cigerci IH, Fidan AF, Konuk M, Yuksel H, Kucukkurt I, Eryavuz A, Sozbilir NB. 2009. The protective potential of Yucca schidigera (Sarsaponin 30®) against nitrite-induced oxidative stress in rats. J Nat Med. 63:311–317.
- Enginar H, Avcu G, Eryavuz A, Kaya E, Kucukkurt I, Fidan AF. 2006. Effect of Yucca schidigera extract on lipid peroxidation and antioxidant activity in rabbits exposed to γ-radiation. Revue Méd Vét. 157:415–419.
- Ince S, Kucukkurt I, Turkmen R, Demirel HH, Sever E. 2013. Dietary Yucca schidigera supplementation reduces arsenic-induced oxidative stress in Swiss albino mice. Toxicol Ind Health. 29:904–914.
- Gurbuz E, Balevi T, Kurtoglu V, Oznurlu Y. 2011. Use of yeast cell walls and Yucca schidigera extract in layer hens’ diets. Ital J Anim Sci. 10:134–138.
- Kucukkurt I, Dundar Y. 2013. Effects of dietary Yucca schidigera supplementation on plasma leptin, insulin, iodated thyroid hormones and some biochemical parameters in rats. Revue Méd Vét. 164:362–367.
- Kurutas EB. 2016. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 15:71.
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi XL. 2003. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 309:1017–1026.
- Liao XD, Ma G, Cai J, Fu Y, Yan XY, Wei XB, Zhang RJ. 2015. Effects of Clostridium butyricum on growth performance, antioxidation, and immune function of broilers. Poult Sci. 94:662–667.
- Limón-Pacheco J, Gonsebatt M. 2009. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat Res. 674:137–147.
- Liu HZ, Luo P, Chen SH, Shang JH. 2011. Effects of squid ink on growth performance, antioxidant functions and immunity in growing broiler chickens. Asian-Aust J Anim Sci. 24:1752–1756.
- McAllister TA, Wang Y, Hristov AN, Olson ME, Cheeke PR. 1998. Applications of Yucca schidigera in livestock production. In Proc. 33rd. Pac. Northwest Anim. Nutr. Conf., Vancouver, British Columbia, Canada; p. 109–119.
- Molavian H, Tonekaboni AM, Kohandel M, Sivaloganathan S. 2015. The synergetic coupling among the cellular antioxidants glutathione peroxidase/peroxiredoxin and other antioxidants and its effect on the concentration of H2O2. Sci Rep. 5:1–8.
- Mueller K, Blum NM, Kluge H, Mueller AS. 2012. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic- and antioxidant enzymes in broiler chickens. Br J Nutr. 108:588–602.
- Niki E. 2012. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 586:3767–3770.
- Niki E. 2014. Antioxidants: basic principles, emerging concepts, and problems. Biomed J. 37:106–111.
- Olas B, Wachowicz B, Stochmal A, Oleszek W. 2005. Inhibition of blood platelet adhesion and secretion by different phenolics from Yucca schidigera Roezl. bark Nutrition. 21:199–206.
- Patel S. 2012. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat Prod Bioprospect. 2:231–234.
- Piacente S, Montoro P, Oleszek W, Pizza C. 2004. Yucca schidigera bark: phenolic constituents and antioxidant activity. J Nat Prod. 67:882–885.
- Piacente S, Pizza C, Oleszek W. 2005. Saponins and phenolics of Yucca schidigera Roezl: Chemistry and bioactivity. Phytochem Rev. 4:177–190.
- Sahoo SP, Kaur D, Sethi APS, Sharma A, Chandra M. 2015. Evaluation of Yucca schidigera extract as feed additive on performance of broiler chicks in winter season. Vet World. 8:556–560.
- Sanders LM, Henderson CE, Hong MY, Barhoumi R, Robert CB, Carroll RJ, Turner ND, Chapkin RS, Lupton JR. 2004. Pro-oxidant environment of the colon compared to the small intestine may contribute to greater cancer susceptibility. Cancer Lett. 208:55–161.
- SAS Institute. 2002. SAS user's guide: statistics. Cary (NC): SAS Institute.
- Sen S, Chakraborty R, Sridhar C, Reddy YSR, De B. 2010. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int J Pharm Sci Rev Res. 3:91–100.
- Shafey TM, Al-Batshan HA, Farhan AMS. 2015. The effect of dietary flaxseed meal on liver and egg yolk fatty acid profiles, immune response and antioxidant status of laying hens. Ital J Anim Sci. 14:428–435.
- Su JL, Shi BL, Zhang PF, Sun DS, Li TY, Yan SM. 2016. Effects of yucca extract on feed efficiency, immune and antioxidative functions in broilers. Braz Arch Biol Technol. 59:e16150035.
- Tufarelli V, Laudadio V, Casalino E. 2016. An extra-virgin olive oil rich in polyphenolic compounds has antioxidant effects in meat-type broiler chickens. Environ Sci Pollut Res. 23:6197–6204.
- Venkatraman JT, Angkeow P, Satsangi N, Fernandes G. 1998. Effects of dietary n-6 and n-3 lipids on antioxidant defense system in livers of exercised rats. J Am Coll Nutr. 17:586–594.
- Walle T, Faye H, DeLegge MH, Oatis JE, Walle UK. 2004. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 32:1377–1382.
- Wang JP, Kim IH. 2011. Effect of caprylic acid and Yucca schidigera extract on production performance, egg quality, blood characteristics, and excreta microflora in laying hens. Br Poult Sci. 52:711–717.
- Xu TT, Zhang S, Zheng LL, Yin LH, Xu LN, Peng JY. 2012. A 90-day subchronic toxicological assessment of dioscin, a natural steroid saponin, in Sprague-Dawley rats. Food Chem Toxicol. 50:1279–1287.
- Zhang CY, Tian YD, Yan FB, Kang XT, Han RL, Sun GR, Zhang HR. 2014. Modulation of growth and immunity by dietary supplementation with resveratrol in young chickens receiving conventional vaccinations. Am J Vet Res. 75:752–759.
