Abstract
The hypothalamic–pituitary–adrenal (HPA) axis and the hypothalamic–pituitary–gonadal (HPG) axis systems are inversely related in humans and animals. Although livestock animals, such as sheep (Ovis aries), tend to be well adapted to their environment, it is known that the livestock production processes subject animals to a multitude of physical and psychological stressful stimuli that have the potential to elevate the HPA axis activity. Chronic stress is one of the major challenges in sheep production, as it is difficult to detect and can result in prolonged dysfunction of the HPA axis, causing downstream negative physiological effects such as immunosuppression, increased susceptibility to disease and reproductive dysfunction. The elevation of HPA axis activity during chronic stress has been suggested as the primary neuroendocrine mechanism underlying the aetiology of reproductive dysfunction in sheep. Research in sheep has demonstrated that glucocorticoids act on the HPG axis at the level of the hypothalamus and hypophyseal portal system to decrease gonadotrophin secretion and at the level of the pituitary gland to reduce responsiveness and sensitivity of gonadotroph cells and their receptors to GnRH. Sheep farming enterprises rely on the breeding efficacy of ewes to optimise lambing percentage and reproductive success in order to ensure a profitable business. This review discusses the influences of the HPA axis on the HPG axis and defines any significant reproductive function consequences caused by stress in ewes and places them into perspective for sheep management and productivity.
Introduction
It is already known that the livestock production process can produce a stressful environment for the animals (Dantzer & Mormède Citation1983), whereby animals are subjected to a multitude of stressful stimuli (Aggarwal & Upadhyay Citation2013). Although production animals (e.g. sheep) tend to be well adapted to aspects such as environmental conditions, feed availability and disease (Qureshi Citation2012), studies have demonstrated that stressors associated with animal production negatively affect reproductive physiology (Dobson et al. Citation2012). Sheep are prey animals and their natural response to stress is to mask any obvious behavioural signs to avoid predation (Stubsjøen et al. Citation2015). This makes prolonged or chronic stress difficult to detect in sheep farming practices (Aggarwal & Upadhyay Citation2013).
Stress in livestock animals is defined as the inability of the animal to cope with stressful stimuli or situations within its environment, whereby the animal fails to meet its genetic potential in fitness parameters such as growth rate, milk production and fertility (Dobson & Smith Citation2000). A stressor is a stimulus that disrupts homeostasis (Smith & Dobson Citation2002) and activates a variety of chemical and specific physiological processes and behavioural coping mechanisms to restore homeostasis and promote survival (Cizauskas et al. Citation2015). Acute stress is caused by a short-term negative situation, whereby the individual is able to quickly and completely recover (Trevisi & Bertoni Citation2009; Papargiris et al. Citation2011). In contrast, chronic stress is defined as a long lasting condition in response to a long-term stressor, whereby the individual may never recover (Trevisi & Bertoni Citation2009). Stress-induced reproductive dysfunction is more likely attributed to chronic stress rather than acute stress and involves neuroendocrine action at the level of the hypothalamus, pituitary glands and gonads. Chronic stress is one of the major challenges in livestock production, as it can result in immune suppression, increased susceptibility to disease and reproductive dysfunction, potentially leading to undesired fitness consequences and failure to meet full genetic potential (Wagenmaker et al. Citation2010). Macfarlane et al. (Citation2000) suggested that stress-like concentrations of cortisol may actually arrest follicular development prior to maturation, which could lead to stress related infertility in ewes. This information suggests that the effects of stress on reproductive performance in ewes may have severe consequences on fecundity and fertility and that the actual impact on lambing percentage and reproductive wastage.
This review aims to discuss the proposed mechanisms of stress-induced reproductive effects in ewes involving the hypothalamo–pituitary–adrenal and the hypothalamo–pituitary–gonadal (HPA–HPG) axes interplay. Furthermore, this review will define any significant reproductive fitness consequences caused by acute and chronic stress in ewes and place them into perspective for farm management and productivity.
Stress in the context of sheep reproduction: the HPA and HPG axis and their basic responses to stress, interactions and feedback mechanisms
Survival and growth remain the leading priority over productivity and reproduction (Qureshi Citation2012). Reproduction has been suggested as the last priority for nutritional partitioning; therefore, it is usually the first physiological function to be negatively affected by stress (Qureshi Citation2012). Exposure to stressful environmental stimuli activates and increases the secretion of glucocorticoid hormones such as cortisol within the HPA axis, which are the downstream effectors that regulate the physiological changes associated with the stress response (Adams et al. Citation1999). It has been postulated that glucocorticoids act on the HPG axis to induce reproductive suppression in two definitive ways (Smith & Dobson Citation2002): first, at the level of the hypothalamus and hypophyseal portal system to decrease gonadotrophin secretion, and second, at the level of the pituitary gland to reduce responsiveness and sensitivity of gonadotroph cells and their receptors to gonadotropin releasing hormone (GnRH) (Adams et al. Citation1999). In a study that examined the effects of inflammatory stress via the administration of a bacterial endotoxin lipopolysaccharide (LPS), on the reproductive system at the level of the pituitary in anoestrous ewes, it was found that inflammatory stress inhibited luteinising hormone (LH) release by reducing LHβ subunit transcription, resulting in decreased LH secretion from the anterior pituitary (Herman et al. Citation2010). The authors of this study suggested that these results were attributed to inflammatory stress affecting the HPG axis firstly at the level of the hypothalamus through the modulation of GnRH production and release, by decreasing GnRH secretion to the anterior pituitary and subsequently altering the expression of GnRH receptor genes and LHβ subunit transcription in the anterior pituitary, thus inhibiting LH release (Herman et al. Citation2010). Although it has not been fully elucidated, this insinuates that the influence of stressors on animals begins at the level of the hypothalamus and precedes a cascade of physiological changes that ultimately lead to reproductive inhibition in sheep. This issue will be discussed later on in this review.
Functional interactions between the HPA and HPG axes
Understanding how both the HPA axis and the HPG axis function individually is imperative in order to be able to understand and identify potential points of cross-talks and possible feedback mechanisms between the two neuroendocrine pathways. The HPA axis consists of three endocrine glands: the hypothalamus, the anterior pituitary and the adrenal glands (Chrousos Citation2000). The feedback interactions between these organs form the HPA axis, which is part of a neuroendocrine system that controls the physiological and behavioural responses to stress and can regulate other bodily processes such as glucose storage and release, immune function, digestion and reproduction (Chrousos Citation2000). Corticotrophin releasing factor (CRF) is secreted from the paraventricular nucleus (PVN) of the hypothalamus in response to stress (Tsigos & Chrousos Citation2002). Corticotrophin releasing factor is a peptide hormone and neurotransmitter, and functions to stimulate adrenocorticotrophic hormone (ACTH) synthesis and release from the anterior pituitary (Tsigos & Chrousos Citation2002). Adrenocorticotrophic hormone is a polypeptide trophic hormone that functions to stimulate the secretion of glucocorticoids from adrenal cortex cells in the adrenal glands (Chrousos Citation2000). Cortisol is a glucocorticoid steroid hormone and is produced in the adrenal glands in response to stress and low plasma glucose levels (Tsigos & Chrousos Citation2002). When cortisol is released during the stress response, it functions to increase plasma glucose via gluconeogenesis, suppress the immune system and increase fat, protein and carbohydrate metabolism to aid in the stress response (Chrousos Citation2000; Smith & Vale Citation2006).
The HPG axis governs reproductive function in animals and also plays a crucial role in development and ageing (Viau Citation2002). The three structures that comprise the HPG axis are the hypothalamus, the anterior pituitary and the gonads (Dismukes Citation2013). The most important function of the HPG axis is to regulate reproduction in females by controlling the ovarian or oestrous cycle to prepare the follicle in the ovary for ovulation (Dobson et al. Citation2012). Gonadotrophin releasing hormone is a decapeptide synthesised in specialised neurons within the hypothalamus and is responsible for initiating the synthesis and secretion of gonadotrophins, LH and follicle stimulating hormone (FSH), from the anterior pituitary (Nestor Citation2012). Follicle-stimulating hormone and LH synthesis and secretion are mediated by the amplitude and frequency of GnRH pulses and feedback interactions from gonadal hormones (Dobson et al. Citation2012). In female animals, gonadotrophin action is dependent upon the target structure on the ovary during the reproductive cycle, being either the corpus luteum or the dominant follicle (Nestor Citation2012). During the follicular phase of the female reproductive cycle, FSH is responsible for initiating follicular growth and maturation and producing oestrogen from the ovary (Breen & Karsch Citation2006). During the luteal phase of the female reproductive cycle, LH triggers ovulation and the development of the corpus luteum and stimulates progesterone production from the corpus luteum (Nestor Citation2012). Oestrogen is the primary female sex steroid hormone, which functions to promote sexual behaviour during oestrus and prepare the female reproductive tract to facilitate pregnancy and ovulation (Papargiris et al. Citation2011). Progesterone, another important sex steroid hormone, is responsible for maintaining pregnancy and the corpus luteum to prevent future ovulations (Li et al. Citation2010; Papargiris et al. Citation2011).
The HPA and HPG axes are parallel structures (Figure ) that undergo a biochemical cascade of events that begin and end at the hypothalamus of the brain (Dismukes Citation2013). The end products of both axes, such as cortisol, oestrogen and progesterone, regulate these biochemical processes by conferring negative feedback on the hypothalamus and the anterior pituitary, which are the same structures in the brain responsible for initiation of the cascade (Pierce et al. Citation2008). The anatomical structures that constitute both pathways are found in the central nervous system and peripheral tissues and include the hypothalamus, anterior pituitary gland, the gonads and the adrenal glands (Pierce et al. Citation2008).
Figure 1. Diagram of the hypothalamic–pituitary–adrenal axis (HPA-axis) and hypothalamic–pituitary–gonadal axis (HPG-axis) loop in the female animal, detailing feedback and interactions between the hormones and structures involved in both pathways. Oestrogen and progesterone confer positive/negative feedback onto the hypothalamus to regulate the HPG-axis. Cortisol confers negative feedback onto the hypothalamus to regulate the HPA-axis. Cortisol also acts at the level of the anterior pituitary (P = pituitary) and reproductive organs to impede reproductive function. Hypothalamic kisspeptin (KNDy cells) are believed to modulate the synthesis of gonadotropin releasing hormone (GnRH) within the hypothalamus. Gonadotrophin inhibitory hormone (GnIH) neurons are assumed to act both directly on GnRH neurons within the hypothalamus and project to the median eminence to mediate pituitary function via G-protein coupled receptors on gonadotroph cells.
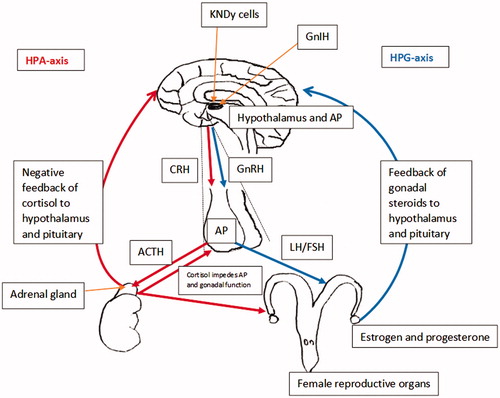
There are many notable similarities between the HPA axis and HPG axis (Figure ), the most significant being that the hypothalamus projects to and stimulates the anterior pituitary via hypophyseal portal vessels (Dismukes Citation2013). Interestingly, both CRF and GnRH are synthesised and secreted from neurons located in the preoptic area (POA) of the hypothalamus (Dismukes Citation2013). Studies have suggested that CRF receptors may be involved in regulating GnRH gene expression in the hypothalamus by regulating GnRH mRNA levels and thus inhibiting GnRH pulse generation (Smith & Vale Citation2006).
Due to the parallel structure of both HPA–HPG axes (Figure ), it is highly probable that there is a great deal of communication across and within the common structures of the HPA and HPG axes (Viau Citation2002). The primary interactions between the HPA and HPG axes occur via the negative feedback interactions of the end products produced by both axis, such as cortisol and oestrogen, the regulatory actions of the intermediaries CRF, ACTH, GnRH, LH and FSH and the involvement of other molecules such as gonadotrophin-inhibitory hormone (GnIH), inhibin and kisspeptin (Smith & Vale Citation2006).
HPA–HPG axes interplay on GnRH
In ewes, the cells that synthesise GnRH are located in the POA and the arcuate nucleus of the hypothalamus (Dobson et al. Citation2012). Gonadotrophin-releasing hormone axons project from the arcuate nucleus and POA to the median eminence, which constitutes a diffuse neural network that forms the GnRH pulse generator (Li et al. Citation2010). The pulsatile neuropeptide secretion from GnRH neurons is dependent on intracellular signalling mechanisms that lead to the pulsatile release of GnRH from the hypothalamus (Dobson et al. Citation2012). The GnRH pulse generator also constitutes the pulsatile release of LH from the anterior pituitary (Li et al. Citation2010). Gonadotrophin-releasing hormone synthesis and secretion is usually assessed by monitoring pulsatile LH secretion, as the frequency of LH pulses is correlated to the activity of the GnRH pulse generator, with the amplitude of LH pulses reflecting pituitary responsiveness to GnRH (Clarke Citation2014). It is most likely that stress induced suppression of the HPG axis begins through the inhibition of GnRH secretion, which subsequently alters the downstream effects of GnRH on LH and FSH release from the pituitary (Clarke Citation2014).
Glucocorticoids are assumed to suppress GnRH by acting at a central level (Figure ). Stress-like levels of cortisol could either reduce pituitary responsiveness to GnRH or inhibit hypothalamic synthesis and secretion of GnRH itself. Breen and Karsch (Citation2006) found that stress-like levels of cortisol directly reduced reproductive neuroendocrine function in ovariectomised ewes by reducing pituitary responsiveness to both endogenous and exogenous GnRH pulses, instead of inhibiting GnRH itself. Furthermore, the suppressive action of cortisol on pituitary responsiveness to GnRH is supported during the presence of oestradiol (Oakley et al. Citation2009). For example, Pierce et al. (Citation2009b) set out to determine if cortisol acts directly at the pituitary or indirectly via the hypothalamus in the presence of oestradiol to inhibit pituitary responsiveness to GnRH in ovariectomised ewes that had undergone surgical hypothalamo–pituitary disconnection. The results determined that oestradiol influences the suppressive effect of cortisol on pituitary responsiveness to GnRH in ewes, where LH pulses were reduced during cortisol infusion in the presence of oestradiol (Pierce et al. Citation2009b). Hypothalamic regulation of the anterior pituitary was eliminated in this experimental model, which suggests that cortisol is able to act directly on the pituitary to suppress LH secretion and hypothalamic GnRH does not influence the ability of cortisol to reduce pituitary responsiveness to GnRH. This suggests that the frequency and amplitude of GnRH pulses do not appear to influence the efficacy of cortisol to reduce pituitary responsiveness to GnRH. On the contrary, in another study that assessed the effects of psychosocial stress on ewe reproduction by isolation, blindfold and predator stress, it was found that psychosocial stress inhibited pulsatile LH secretion in ovariectomised ewes by both decreasing GnRH pulse amplitude and pituitary responsiveness to GnRH (Pierce et al. Citation2008).
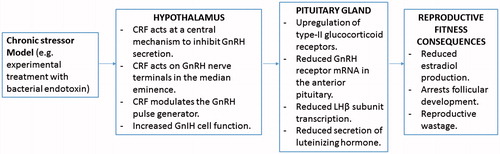
Figure 2. Diagram summarising the main neuroendocrine changes occurring in the animal (sheep model) due to direct exposure to chronic stressor, and the culminating effects on reproductive fitness of the animal (this summary diagram is based on the published literature).
Glucocorticoids exert their physiological actions by binding to specific receptors on target cells (Ralph & Tilbrook Citation2016a,Citationb). Glucocorticoid binding sites occur mainly in pituitary cytosols in both neonatal and adult sheep (Rose et al. Citation1985). Type I glucocorticoid receptors regulate the basal activity of the HPA axis and are located in the hypothalamus and other structures of the brain (Ralph & Tilbrook Citation2016b). Type II glucocorticoid receptors are utilised during the stress response when glucocorticoid concentration exceeds basal levels and localise in the hypothalamus and on corticotroph cells in the pituitary (Ralph & Tilbrook Citation2016b). It has been postulated that pituitary responsiveness to GnRH is mediated via type II glucocorticoid receptors, as it was found that type II glucocorticoid receptors are present on gonadotroph cells in the pituitary, suggesting that type II receptors within the pituitary may be responsible for reducing pituitary responsiveness to GnRH via the direct actions of cortisol (Dobson et al. Citation2012). The short-term effects of type II glucocorticoid receptors on gonadotrophs within the pituitary are suspected to inhibit GnRH-receptor affinity and disrupt GnRH stimulation of LH release and long term by suppressing GnRH receptor synthesis (Dobson et al. Citation2012). On the contrary, type II glucocorticoid receptor immunoreactivity determined that there was no colocalisation between GnRH neurons and glucocorticoid receptors in the hypothalamus of ewes, suggesting that type II glucocorticoid receptors do not act directly on GnRH neurons in the hypothalamus, but rather via an indirect neuronal pathway on GnRH cells in the ewe (Dufourny & Skinner Citation2002).
There are cells within the hypothalamus that synthesise the neuropeptide kisspeptin, called KNDy cells, that are named after the three neuropeptides that they express, kisspeptin, neurokinin B and dynorphin (Dobson et al. Citation2012), and are implicated in the control of GnRH secretion in the ewe (Ralph et al. Citation2016). Kisspeptin from KNDy cells stimulates the release of GnRH from hypothalamic GnRH neurons, and oestrogen regulates GnRH synthesis by acting on kisspeptin neurons (Kageyama Citation2013). Kisspeptin is believed to play a crucial role in the transduction of stress-induced reproductive dysfunction, as the expression of kisspeptin is downregulated by stress (Kageyama Citation2013). Hypothalamic KNDy cells have oestrogen, progesterone and type II glucocorticoid receptors (Dobson et al. Citation2012), suggesting that they are a target for cortisol as well as oestrogen and progesterone. In addition, KNDy cells have axons that enable direct projections with GnRH cell bodies and terminals within the hypothalamus and are believed to be positioned in manner that enables them to control GnRH and LH release in different situations (Lehman et al. Citation2010; Dobson et al. Citation2012). As previously mentioned, kisspeptin from KNDy cells is believed to modulate the synthesis of GnRH, where the action of kisspeptin is regulated by oestradiol. It is postulated that stress suppresses GnRH in a similar manner, whereby glucocorticoids act on type II glucocorticoid receptors on KNDy cells, suppressing the expression of kisspeptin and, therefore, the synthesis of GnRH (Nestor Citation2012; Ralph et al. Citation2016).
Gonadotrophin-inhibitory hormone is a hypothalamic peptide that inhibits gonadotrophin synthesis and secretion in a variety of animal species including sheep (Kirby et al. Citation2009). G-protein coupled receptors are the receptors for GnIH (Ubuka et al. Citation2013). Gonadotrophin-inhibitory hormone neurons act by projecting to the median eminence, where they can mediate pituitary function via G-protein coupled receptors expressed in gonadotroph cells (Ubuka et al. Citation2013). Hypothalamic GnRH neurons also express G-protein coupled receptors, in which GnIH is able to bind to and inhibit gonadotrophin synthesis and secretion by decreasing the activity of GnRH neurons and inhibit the effects of GnRH on gonadotrophs (Ubuka et al. Citation2013). Hypothalamic GnIH neurons are believed to act directly with GnRH neurons to suppress HPG axis function. Most recent research in sheep has demonstrated that both acute and chronic stressors increased the GnIH cell function in the hypothalamus and their input to GnRH cells in ewes (Clarke et al. Citation2016).
HPA–HPG axes interplay on LH and FSH
The inhibition of GnRH production in the hypothalamus and reduced pituitary responsiveness to GnRH disrupts the synthesis and secretion of LH and FSH in the anterior pituitary (Dismukes Citation2013). However, the exact mechanisms and potential actions of the HPA axis intermediaries and secretory products have not been defined yet, specifically not in sheep. It has been shown in ewes that psychosocial (Pierce et al. Citation2008), transportation (Dobson et al. Citation1999) and inflammatory (Herman et al. Citation2010) stressors disrupt pulsatile LH secretion and delay the preovulatory LH surge. The blocking or blunting of the preovulatory LH surge results in the delay or cessation of ovulation, which can impair fertilisation. Stress during the late follicular phase of the oestrous cycle is most likely to impact the LH surge and thus affect ovulation, compared with stress during the early follicular phase or luteal phase (Ralph et al. Citation2016). One study found that the administration of stress-like concentrations of cortisol during the luteal and follicular phase suppressed the preovulatory LH surge in ewes (Breen & Karsch Citation2004). Inflammatory stress caused by bacterial endotoxin in ewes, delayed and blocked the LH surge during the follicular phase. The timing of a stressor relative to the onset of the LH surge appears to be important in how a stressor affects the LH surge (Dobson et al. Citation1999).
Information detailing the effects of stress and glucocorticoid hormones on LH receptors in the reproductive organs of sheep is scarce. A study in cattle that examined the direct effects of cortisol on LH receptor numbers in cultured bovine granulosa cells, found that the addition of cortisol significantly decreased the number of LH receptors on granulosa cells (Kawate et al. Citation1993), demonstrating the direct inhibitory effects of cortisol on LH receptor content. This data insinuate that glucocorticoids from stress may be able to act directly on the reproductive organs to reduce LH receptor content and further alter gonadal hormone synthesis and secretion.
The actions of oestrogen within the HPG axis are critical for the generation of the LH surge (Nestor Citation2012). In a study that examined the effects of cortisol on the action of oestrogen to induce the preovulatory LH surge in ovariectomised ewes, it was determined that cortisol was able to disrupt the oestrogen-induced LH surge, by either blocking the LH surge or suppressing LH surge amplitude (Pierce et al. Citation2009b). This study also found that cortisol caused an increase in the latent period between the oestrogen stimulus and the LH surge in the ewes (Pierce et al. Citation2009b). In sheep, oestrogen stimulates the hypothalamus to initiate GnRH secretion and stimulates the pituitary to enhance pituitary responsiveness to GnRH (Pierce et al. Citation2009a,Citationb). The effect of cortisol on the LH surge may involve action by attenuating GnRH production or by disrupting the actions of oestrogen at the level of the pituitary or hypothalamus.
Hypothalamic GnRH stimulates the synthesis of FSH, but secretion does not require the input of GnRH to gonadotroph cells in the pituitary (Clarke Citation2014). In a study where ewes underwent hypothalamo–pituitary disconnection to eliminate the influence of endogenous GnRH on the pituitary, the cessation of pulsatile GnRH leads to the inhibition of pulsatile LH secretion, but had no effect on FSH secretion (Clarke Citation2014). In another study, cyclic ewes that were treated with 2 mg/d of dexamethasone had no change in plasma FSH concentration, but plasma FSH response to exogenous GnRH was significantly reduced (Phillips & Clarke Citation1990). Sex steroids may play a significant role in how stress impacts LH and FSH secretion. For example, a study found that prolonged cortisol infusion did not affect LH pulse frequency in ovariectomised ewes deprived of gonadal hormones, but lowered LH pulse frequency in ewes that were supplied with oestrogen and progesterone (Li et al. Citation2010). There are very few studies that describe how CRF and ACTH affect LH and FSH secretion in sheep. In the ewe, the administration of CRF either dramatically increased or had no effect on LH pulse frequency (Li et al. Citation2010). In contrast, in another study, a CRF antagonist was unable to prevent hypoglycaemic stress from inhibiting LH synthesis and secretion in sheep (Li et al. Citation2010). In earlier studies, exogenous ACTH was found to suppress plasma LH concentration and reduce LH response to exogenous GnRH in wethers and rams (Mohamed & Cox Citation1988; Mohamed et al. Citation1988). Van Lier et al. (Citation1999) found that exogenous ACTH suppressed LH pulse frequency in male castrated rams, which was correlated with an increase in plasma cortisol, but had no effect on plasma LH concentration.
Influences of acute and chronic stress on HPA–HPG axes interplay
Acute stress
Acute stress requires a short-term physiological response to avoid a negative outcome and the effects of acute stress on reproductive function tend to be relatively short lived (Coburn et al. Citation2010). Transportation is an efficient way to induce acute stress in ruminants in an experimental setting (Maziero et al. Citation2011). In a study conducted on sheep that examined the effects of transport stress on LH surge and secretion, both ovariectomised and intact ewes demonstrated a delay in the LH surge and a reduced LH concentration (Dobson et al. Citation1999). The resumption of normal LH profile in the next follicular phase of the ewes indicated that acute stress from transport leads to only temporary disruption of the HPG axis (Dobson et al. Citation1999). In another study, there was no significant difference observed in oestrus expression between transported ewes at different stages of oestrus and non-transported ewes (Orihuela et al. Citation2002).
In terms of reproductive behaviour, the acute 5-h infusion of cortisol reduced sexual receptive behaviour in ewes, where cortisol acted by inhibiting the action of oestrogen to induce sexual receptive behaviour (Papargiris et al. Citation2011). The action of acute stress to inhibit sexual receptive behaviour could have relevant implications for reproductive success as it may reduce the chance of the ewe being mounted by the ram in field conditions (Papargiris et al. Citation2011). However, both acute and chronic stress-like levels of cortisol had no effect on sexual attractivity and proceptivity behaviours in this study (Papargiris et al. Citation2011). On the contrary, another study found that the repeated exposure of ewes to acute psychosocial stressors over two oestrous cycles did not alter the incidence, timing, amplitude or duration of the preovulatory LH surge (Wagenmaker et al. Citation2010). This study also found that ewes subjected to a variety of acute stressors during the follicular phase, such as food denial, unfamiliar noises, exercise and transportation, had no effect on the LH surge (Wagenmaker et al. Citation2010). There was no difference in the mean latent period to the LH surge in the stressed ewes compared with the control ewes of this study (Wagenmaker et al. Citation2010). This suggests that the type or intensity of the acute stressor may determine the effect of reproductive dysfunction in sheep; however, it contradicts the effects of transportation stress on the preovulatory LH surge observed in other research.
Chronic stress
Research has shown that peripheral administration of CRF has no effect on LH secretion, but central injection of CRF inhibits LH secretion, suggesting that a central mechanism is involved in regulating GnRH neuronal activity in the hypothalamus and downstream LH release from the pituitary (Kageyama Citation2013). Furthermore, CRF inhibits the release of GnRH into the hypophyseal portal circulation both in vitro and in vivo, confirming that CRF acts via a central mechanism within the hypothalamus (Polkowska & Przekop Citation1997; see Figure ). In ewes, stress-induced suppression of GnRH neuronal activity can be initiated by CRF release after stressful stimulation (Polkowska & Przekop Citation1997). Corticotrophin-releasing factor has direct anatomical connections between CRF axon terminals and the dendrites of GnRH secreting neurons (Rivier & Rivest Citation1991). It has been suggested that CRF inhibits the release of GnRH at the level of neurosecretory terminals in the median eminence of the hypothalamus, where CRF acts directly on GnRH nerve terminals (Rivier & Rivest Citation1991). A study that examined the effects of CRF infusions in ewes during the late follicular phase of the oestrous cycle determined that CRF induced a decrease in GnRH stores in the nerve terminals of the median eminence, which was believed to be responsible for blocking the GnRH/LH surge (Polkowska & Przekop Citation1997). In this study, immunohistochemistry of the hypothalamus found that GnRH neurons were most concentrated in the medial POA of the hypothalamus and GnRH neurons and cell bodies were concentrated near the median eminence (Polkowska & Przekop Citation1997). The blockade of the preovulatory LH surge in CRF treated ewes in this study could be attributed to diminished GnRH stores in GnRH nerve terminals of the median eminence (Polkowska & Przekop Citation1997). The decrease in hormonal stores in GnRH nerve terminals in the median eminence (Polkowska & Przekop Citation1997) suggests that CRF may not block the biosynthesis of GnRH, but may instead inhibit GnRH release and transportation from GnRH cell bodies to GnRH nerve terminals via the GnRH pulse generator. The suppression of GnRH release via CRF infusion did not occur after acute stress when CRF concentration is highest, but did so after prolonged stimulation with CRF when HPA axis activity was at normal levels (Polkowska & Przekop Citation1997), suggesting that chronic stimulation of the hypothalamus with CRF is needed to modulate GnRH release.
In ewes, the predominant effect of chronic stress on the HPG axis is the suppression of pituitary responsiveness to GnRH (Pierce et al. Citation2008). In ovariectomised ewes, treatment with bacterial endotoxin marginally suppressed GnRH secretion, but significantly disrupted LH pulsatility, demonstrating that immune stress is able to block LH pulses without inhibiting GnRH, therefore uncoupling GnRH and LH (Williams et al. Citation2001). In this study, bacterial endotoxin also suppressed LH amplitude in the presence of exogenous GnRH (Williams et al. Citation2001), further confirming that stress acts at the level of the pituitary by inhibiting pituitary responsiveness to GnRH. The mechanisms that govern stress-induced reduction in pituitary responsiveness to GnRH have not been fully elucidated, but it is believed that it involves modulation of GnRH receptors and the colocalisation of CRF receptors with gonadotroph cells within the anterior pituitary.
Tissue concentration of GnRH receptors in the anterior pituitary is considered to be a determinant of pituitary/gonadotroph responsiveness to GnRH (Daley et al. Citation1999b). Stress-like concentrations of cortisol (intravenous cannula delivery into left jugular vein at 3.6 mg/50 kg per hour continuous infusion for a period of 7) did not affect gonadotroph responsiveness or GnRH receptor and GnRH receptor mRNA in the pituitary of ovidectomised sheep, which was correlated with no significant change in GnRH secretion from the hypothalamus (Daley et al. Citation1999b). Endotoxin injection significantly decreased the level of GnRH receptor mRNA by 80% in the anterior pituitary of anoestrous ewes, which was correlated with a decrease in LH secretion (Herman & Tomaszewska-Zaremba Citation2010). In another study, GnRH receptor mRNA was significantly reduced in the anterior pituitary of follicular phase ewes that underwent foot shock stimulation, which was associated with a decrease in plasma LH concentration (Ciechanowaska et al. Citation2007). On the contrary, a similar experiment found that both short and prolonged foot shock stimulation caused an increase in GnRH receptor mRNA in the pituitary of anoestrous ewes (Łapot et al. Citation2007). Interestingly, GnRH infusion significantly increased GnRH receptor mRNA in the pituitary and plasma LH concentration of anoestrous ewes (Parhar et al. Citation2012), suggesting that increased GnRH concentration can augment the biosynthesis of GnRH receptors in the anterior pituitary. Hypothalamic GnRH is a crucial regulator of GnRH receptor biosynthesis (Herman & Tomaszewska-Zaremba Citation2010). Decreased GnRH receptor gene expression results in a reduction in GnRH receptors in the membrane of gonadotroph cells (Herman & Tomaszewska-Zaremba Citation2010), which could very well explain stress-induced suppression of LH secretion from the anterior pituitary.
Fitness consequences of stress: follicular development and ovarian function
Cortisol is believed to affect follicular development and ovarian function by either acting at the level of the hypothalamus and pituitary to limit the amount of LH and FSH to support folliculogenesis and ovulation, or by acting directly at ovarian loci to impair follicular maturation, ovulation and oestrogen secretion (Daley et al. Citation1999a). Serum oestrogen concentration is used to estimate follicular maturation (Carson et al. Citation1981). This is because large preovulatory follicles synthesise and secrete oestrogen and little oestrogen is produced by small atretic follicles (Daley et al. Citation1999a). An early study found that ovine follicles larger than 3.5 mm in diameter are able to synthesise and secrete oestradiol (Huet et al. Citation1997), which suggests that an alteration in oestrogen secretion could indicate that follicular development may be impaired in the presence of significantly elevated cortisol. In a study that examined follicular maturation and oestrogen production in sheep receiving stress-like levels of cortisol (intravenous delivery in right and left jugular veins at 0.1 mg/kg per hour 5 d before and 5 d after vaginal pessary removal) during the late luteal and early follicular phase of the oestrous cycle, oestrogen secretion was suppressed (Macfarlane et al. Citation2000). The suppression of oestrogen during cortisol infusion demonstrated that stress-like levels of cortisol arrest follicular development prior to follicular maturation (Macfarlane et al. Citation2000). In a similar study in ewes that underwent vaginal pessary synchronisation of the oestrous cycle, it was found that serum oestrogen increased in control ewes that were treated with saline solution and oestrogen levels were either blocked or attenuated in ewes that received stress-like levels of cortisol (Daley et al. Citation1999a). In this study, oestrogen secretion was suppressed in all ewes that received 0.1 mg/kg per hour of exogenous cortisol and in only 50% of ewes that received 0.08 mg/kg per hour of exogenous cortisol, which suggests that cortisol concentration or duration may be a factor that determines if follicular development will be adversely affected (Daley et al. Citation1999a). In a second experiment by Daley et al. (Citation1999a), ewes that were administered cortisol and exogenous episodic GnRH had a significant increase in oestrogen secretion, the same as control ewes in the previous experiment. In ewes that received cortisol without exogenous episodic GnRH, oestrogen secretion was attenuated (Daley et al. Citation1999a), suggesting that stress-like concentrations of cortisol can potentially arrest or delay follicular development and that episodic GnRH is able to override the cortisol-induced delay in follicular maturation and ovulation.
There is little information that defines the mechanisms that govern the effects of stress-like concentrations of cortisol on follicular maturation and ovulation in sheep; however, it may also involve action via glucocorticoid or LH receptors in the ovary. Glucocorticoid receptors have been localised in the ovarian follicles of humans, rats and cattle (Tetsuka Citation2007), indicating that glucocorticoids can act directly at the level of the reproductive organs. The expression of LH receptors in granulosa and theca cells in the ovary is essential for follicular maturation (Tetsuka Citation2007). A study in cattle that examined the direct effects of cortisol on oestradiol secretion and LH receptor numbers in cultured bovine granulosa cells found that the addition of cortisol caused a significant decrease in the amount of oestradiol secreted by granulosa cells and a significant decrease in the number of LH receptors on granulosa cells (Kawate et al. Citation1993). This demonstrates the direct inhibitory effects of cortisol on LH receptor content and oestradiol secretion, indicating that cortisol from stress may be able to inhibit follicular maturation and ovulation at the level of the reproductive organs. However, if cortisol acts via glucocorticoid receptors on ovarian cells to inhibit ovarian function or by reducing LH receptors in the ovary, remains undetermined.
Embryo and foetal development, fecundity and offspring birth weight
Livestock animals experience stressful situations frequently throughout pregnancy. Little is known about the adverse effects of stress during gestation in farm animals; however, other research confirmed that stress following conception in sheep can result in early embryonic loss and reduced implantation rate (Edey Citation1966; Dixon et al. Citation2007). Embryonic loss was significantly higher in ewes subjected to environmental stress after mating (Doney et al. Citation1976). Similarly, heat stress during pregnancy in mice disrupted meiotic maturation of ova, increased preimplantation losses and significantly increased early embryonic mortality (Baumgartner & Chrisman Citation1987).
There are very few research works that describe the impact of stress on foetal development, fecundity and birth weight in livestock animals. In a study that examined the effects of repeated stress throughout pregnancy on lamb birth weight and physiology, ewes that were exposed to isolation and dogs birthed lambs that had heavier birth weights and higher basal cortisol levels than lambs born to non-stressed mothers (Roussel et al. Citation2004). In another study, nutritionally stressed ewes gave birth to lambs with significantly lower birth weights than lambs born to non-nutritionally stressed mothers (Meza-Herrera et al. Citation2015). This study also found that nutritionally stressed ewes had fewer placental cotyledons, significantly smaller cotyledon diameter and produced smaller litter sizes than non-nutritionally stressed ewes (Meza-Herrera et al. Citation2015). Another study in sheep found that chronic heat stress during gestation lowered placental weight by 54%, which was correlated with low foetal weight (Bell et al. Citation1989), suggesting that heat stress adversely affects foetal growth. Similarly, in cattle, calves born to heat stressed mothers had lower birth weights and weaning weights compared with calves born to non-heat-stressed mothers (Tao et al. Citation2012). Furthermore, embryonic and foetal mortality from stress has the potential to reduce lambing rate and litter size, which could contribute to economic loss in the sheep farming industry.
Conclusions
This review set out to investigate reproductive-stress endocrine axis interplay and stress-induced reproductive dysfunction in sheep and the potential mechanisms and consequences for sheep production. Elevated HPA axis activity due to stress can cause reproductive dysfunction and lead to reproductive fitness consequences in sheep, by interacting with and modulating molecular and cellular aspects of the HPG axis (Figure ). The impact of HPA–HPG axes interplay on the reproductive fitness potential of sheep can impede fertility, fecundity, ovulation rate, ova quality, offspring birth weight and increase embryonic and offspring mortality. This has major implications for economic loss in the sheep farming industry, where farmers rely on the reproductive success of ewes in order to ensure a profitable enterprise. Stress acts firstly at the level of the hypothalamus to decrease GnRH synthesis and secretion, and secondly at the level of the anterior pituitary gland to reduce pituitary responsiveness to GnRH and modulate gonadotrophin secretion via a number of potential mechanisms. The mechanisms of reproductive-stress endocrine axis interplay appear to involve more than just the action of glucocorticoids to modulate HPG axis function, but instead a series of complex interactions with hormones such as GnIH and CRF, colocalisation of CRF receptors with gonadotroph cells and GnRH, and GnRH receptor gene expression modulation. Future areas of potential research on reproductive-stress endocrine axis interplay that could provide a valuable insight into sheep reproductive efficacy, may involve examining GnIH–GnRH interaction in sheep and examining the effects of chronic stress and elevated glucocorticoids on lambing percentage and fecundity.
Acknowledgements
This review paper forms the literature review for Master of Animal Science thesis submitted by SP and supervised by EN. The authors would like to thank the two anonymous reviewers for their comments on earlier versions of this article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams TE, Sakurai H, Adams BM. 1999. Effect of stress-like concentrations of cortisol on estradiol-dependent expression of gonadotropin-releasing hormone receptor in orchidectomized sheep. Biol Reprod. 60:164–168.
- Aggarwal A, Upadhyay R. 2013. Heat stress and animal productivity. New Delhi, India: Springer.
- Baumgartner AP, Chrisman CL. 1987. Embryonic mortality caused by maternal heat stress during mouse oocyte maturation. Anim Reprod Sci. 14:309–316.
- Bell AW, McBride BW, Slepetis R, Early RJ, Currie WB. 1989. Chronic heat stress and prenatal development in sheep: I. Conceptus growth and maternal plasma hormones and metabolites. J Anim Sci. 67:3289–3299.
- Breen KM, Karsch FJ. 2004. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 145:692–698.
- Breen KM, Karsch FJ. 2006. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol. 27:233–245.
- Carson RS, Findlay JK, Clarke IJ, Burger HG. 1981. Estradiol, testosterone, and androstenedione in ovine follicular fluid during growth and atresia of ovarian follicles. Biol Reprod. 24:105–113.
- Chrousos GP. 2000. The HPA axis and the stress response. Endocr Res. 26:513–514.
- Ciechanowaska M, Łapot M, Malewiski T, Misztal T, Mateusiak K, Przekop F. 2007. Effect of stress on the expression of GnRH and GnRH receptor (GnRH-R) genes in the preoptic area-hypothalamus and GnRH-R gene in the stalk/median eminence and anterior pituitary gland in ewes during follicular phase of the estrous cycle. Acta Neurobiol Exp. 67:1–12.
- Cizauskas CA, Turner WC, Pitts N, Getz WM. 2015. Seasonal patterns of hormones, macroparasites, and microparasites in wild African ungulates: the interplay among stress, reproduction, and disease. PLoS ONE. 10:e0120800.
- Clarke IJ, Bartolini D, Conductier G, Henry BA. 2016. Stress increases gonadotropin inhibitory hormone cell activity and input to GnRH cells in ewes. Endocrinology. 157:4339–4350.
- Clarke IJ. 2014. Interface between metabolic balance and reproduction in ruminants: focus on the hypothalamus and pituitary. Horm Behav. 66:15–40.
- Coburn S, Salman M, Rhyan J, Keefe T, McCollum M, Aune K, Spraker T, Miller L. 2010. Comparison of endocrine response to stress between captive-raised and wild-caught bighorn sheep. J Wildlife Manage. 74:532–538.
- Daley CA, Macfarlane MS, Sakurai H, Adams TE. 1999a. Effect of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Reproduction. 117:11–16.
- Daley CA, Sakurai H, Adams BM, Adams TE. 1999b. Effect of stress-like concentrations of cortisol on gonadotroph function in orchidectomized sheep. Biol Reprod. 60:158–163.
- Dantzer R, Mormède P. 1983. Stress in farm animals: a need for reevaluation. J Anim Sci. 57:6–18.
- Dismukes A. 2013. Coupling of the HPA and HPG axes [MS dissertation]. New Orleans (LA): University of New Orleans.
- Dixon AB, Knights M, Winkler JL, Marsh DJ, Pate JL, Wilson ME, Dailey RA, Seidel G, Inskeep EK. 2007. Patterns of late embryonic and fetal mortality and association with several factors in sheep. J Anim Sci. 85:1274–1284.
- Dobson H, Fergani C, Routly JE, Smith RF. 2012. Effects of stress on reproduction in ewes. Anim Reprod Sci. 130:135–140.
- Dobson H, Smith RF. 2000. What is stress, and how does it affect reproduction? Anim Reprod Sci. 60–61:743–752.
- Dobson H, Tebble JE, Phogat JB, Smith RF. 1999. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil. 116:1–8.
- Doney JM, Smith WF, Gunn RG. 1976. Effects of post-mating environmental stress or administration of ACTH on early embryonic loss in sheep. J Agric Sci. 87:133–136.
- Dufourny L, Skinner DC. 2002. Type II glucocorticoid receptors in the ovine hypothalamus: distribution, influence of estrogen and absence of co-localization with GnRH. Brain Res. 946:79–86.
- Edey TN. 1966. Nutritional stress and pre-implantation embryonic mortality in Merino sheep. J Agric Sci. 74:187–192.
- Herman AP, Romanowicz K, Tomaszewska-Zaremba D. 2010. Effect of LPS on reproductive system at the level of the pituitary of anestrous ewes. Reprod Domest Anim. 45:e351–e359.
- Herman AP, Tomaszewska-Zaremba D. 2010. Effect of endotoxin on the expression of GnRH and GnRHR genes in the hypothalamus and anterior pituitary gland of anestrous ewes. Anim Reprod Sci. 120:105–111.
- Huet C, Monget P, Pisselet C, Monniaux D. 1997. Changes in extracellular matrix components and steroidogenic enzymes during growth and atresia of antral ovarian follicles in the sheep. Biol Reprod. 56:1025–1034.
- Kageyama K. 2013. Regulation of gonadotropins by corticotropin-releasing factor and urocortin. Front Endocrinol (Lausanne). 4:12.
- Kawate N, Inaba T, Mori J. 1993. Effects of cortisol on the amounts of estradiol-17β and progesterone secreted and the number of luteinizing hormone receptors in cultured bovine granulosa cells. Anim Reprod Sci. 32:15–25.
- Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. 2009. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 106:11324–11329.
- Łapot M, Ciechanowaska M, Melewski T, Misztal T, Mateusiak K, Przekop F. 2007. The effect of stress on the expression of GnRH and GnRH receptor genes in the discrete regions of the hypothalamus and pituitary of anestrous ewes. Reprod Biol. 7:55–71.
- Lehman MH, Coolen LM, Goodman RL. 2010. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 151:3479–3489.
- Li XF, Knox AM, O’Byrne KT. 2010. Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female. Brain Res. 1364:153–163.
- Macfarlane MS, Breen KM, Sakurai H, Adams BM, Adams TE. 2000. Effect of duration of infusion of stress-like concentrations of cortisol on follicular development and the preovulatory surge of LH in sheep. Anim Reprod Sci. 63:167–175.
- Maziero RRD, Martins AC, Mollo MR, Martin I, Bastos MR, Ferreira JCP, Rumpf R, Sartori R. 2011. Ovarian function in cows submitted to acute stress during proestrus. Livest Sci. 138:105–108.
- Meza-Herrera CA, Vicente-Pérez A, Osorio-Marín Y, Girón-Gómez BS, Beltran-Calderon E, Avendaño-Reyes L, Correa-Calderon A, Macías-Cruz U. 2015. Heat stress, divergent nutrition level, and late pregnancy in hair sheep: effects upon cotyledon development and litter weight at birth. Trop Anim Health Prod. 47:819–824.
- Mohamed FHA, Cox JE, Moonan V. 1988. Studies of pituitary-adrenal-testis interaction in sheep. I. The effects of repeated injections of adrenocorticotrophic hormone during the breeding season. Theriogenology. 29:849–857.
- Mohamed FHA, Cox JE. 1988. Studies of pituitary–adrenal–testis interaction in sheep. II. The effects of repeated injections of adrenocorticotrophic hormone outside the breeding season. Theriogenology. 29:859–865.
- Nestor CC. 2012. Evidence of a role for three neuropeptides that mediate steroid negative feedback on gonadotropin releasing hormone/luteinizing hormone secretion in the ewe: Kisspeptin, neurokinin B and orphanin FQ [PhD dissertation]. Morgantown, WV: West Virginia University.
- Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. 2009. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 150:341–349.
- Orihuela A, Sánchez-Mejorada H, Toledo M. 2002. Effect of short transport during di-oestrus and pro-oestrus on cortisol levels and oestrous behaviour of sheep. J Agric Sci. 138:93–96.
- Papargiris MM, Rivalland ETA, Hemsworth PH, Morrissey AD, Tilbrook AJ. 2011. Acute and chronic stress-like levels of cortisol inhibit the oestradiol stimulus to induce sexual receptivity but have no effect on sexual attractivity or proceptivity in female sheep. Horm Behav. 60:336–345.
- Parhar I, Ogawa S, Kitahashi T. 2012. RFamide peptides as mediators in environmental control of GnRH neurons. Prog Neurobiol. 98:176–196.
- Phillips DJ, Clarke IJ. 1990. Effects of the synthetic glucocorticoid dexamethasone on reproductive function in the ewe. J Endocrinol. 126:289–295.
- Pierce BN, Clarke IJ, Turner AI, Rivalland ETA, Tilbrook AJA. 2009a. Cortisol disrupts the ability of estradiol-17beta to induce the LH surge in ovariectomized ewes. Domest Anim Endocrinol. 36:202–208.
- Pierce BN, Hemsworth PH, Rivalland ETA, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ. 2008. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Horm Behav. 54:424–434.
- Pierce BN, Stackpole CA, Breen KM, Clarke IJ, Karsch FJ, Rivalland ETA, Turner AI, Caddy DJ, Wagenmaker ER, Oakley AE, Tilbrook AJ. 2009b. Estradiol enables cortisol to act directly upon the pituitary to suppress pituitary responsiveness to GnRH in sheep. Neuroendocrinology. 89:86–97.
- Polkowska J, Przekop F. 1997. The effect of corticotropin-releasing factor (CRF) on the gonadotropin hormone releasing hormone (GnRH) hypothalamic neuronal system during preovulatory period in the ewe. Acta Neurobiol Exp. 57:91–99.
- Qureshi M. 2012. Stress impedes reproductive physiology of dairy animals under subtropical conditions – a review. J Anim Plant Sci. 22:75–78.
- Ralph CR, Lehman MN, Goodman RL, Tilbrook AJ. 2016. Impact of psychosocial stress on gonadotrophins and sexual behaviour in females: role for cortisol? Reproduction. 152:R1–R14.
- Ralph CR, Tilbrook AJ. 2016a. The hypothalamo-pituitary-adrenal (HPA) axis in sheep is attenuated during lactation in response to psychosocial and predator stress. Domest Anim Endocrinol. 55:66–73.
- Ralph CR, Tilbrook AJ. 2016b. Invited review: the usefulness of measuring glucocorticoids for assessing animal welfare. J Anim Sci. 94:457–470.
- Rivier C, Rivest S. 1991. Effect of stress on the activity of the hypothalamic–pituitary–gonadal axis: peripheral and central mechanisms. Biol Reprod. 45:523–532.
- Rose JC, Kute TE, Winkler L. 1985. Glucocorticoid receptors in sheep brain tissues during development. Am J Physiol. 249:E345–E349.
- Roussel S, Hemsworth PH, Boissy A, Duvaux-Ponter C. 2004. Effects of repeated stress during pregnancy in ewes on the behavioural and physiological responses to stressful events and birth weight of their offspring. Appl Anim Behav Sci. 85:259–276.
- Smith RF, Dobson H. 2002. Hormonal interactions within the hypothalamus and pituitary with respect to stress and reproduction in sheep. Domest Anim Endocrinol. 23:75–85.
- Smith SM, Vale WW. 2006. The role of the hypothalamic–pituitary–adrenal axis in neuroendocrine responses to stress. Dialog Clin Neurosci. 8:383–395.
- Stubsjøen SM, Bohlin J, Dahl E, Knappe-Poindecker M, Fjeldaas T, Lepschy M, Palme R, Langbein J, Ropstad E. 2015. Assessment of chronic stress in sheep (part I): the use of cortisol and cortisone in hair as non-invasive biological markers. Small Ruminant Res. 132:25–31.
- Tao S, Monteiro AP, Thompson IM, Hayen MJ, Dahl GE. 2012. Effect of late-gestation maternal heat stress on growth and immune function of dairy calves. J Dairy Sci. 95:7128–7136.
- Tetsuka M. 2007. Actions of glucocorticoid and their regulatory mechanisms in the ovary. Anim Sci J. 78:112–120.
- Trevisi E, Bertoni G. 2009. Some physiological and biochemical methods for acute and chronic stress evaluation in dairy cows. Ital J Anim Sci. 8:265–286.
- Tsigos C, Chrousos GP. 2002. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 53:865–871.
- Ubuka T, Son YL, Bentley GE, Millar RP, Tsutsui K. 2013. Gonadotropin-inhibitory hormone (GnIH), GnIH receptor and cell signaling. Gen Comp Endocrinol. 190:10–17.
- Van Lier E, Regueiro M, Pérez-Clariget R, Andersson H, Kindahl H, Forsberg M. 1999. Effects of adrenocorticotrophin (ACTH) and progesterone on luteinising hormone (LH) secretion in recently castrated rams. Anim Reprod Sci. 55:115–126.
- Viau V. 2002. Functional cross-talk between the hypothalamic–pituitary–gonadal and -adrenal axes. J Neuroendocrinol. 14:506–513.
- Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. 2010. The estrous cycle of the ewe is resistant to disruption by repeated, acute psychosocial stress. Biol Reprod. 82:1206–1215.
- Williams CY, Harris TG, Battaglia DF, Viguié C, Karsch FJ. 2001. Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone 1. Endocrinology. 142:1915–1922.
