Abstract
The current study investigated the efficacy of propolis supplementation (bee glue; BG) on ameliorating overcrowding and heat stress-induced impairment of growth performance, immunity, some stress indicators, and jejunum morphology in broilers. Three hundred eighty 21-d-old Ross 308 male broilers were randomly divided into 6 treatments. From d 22, the birds were either kept at thermoneutral zone (22 °C) or subjected to cyclic heat stress by exposing them daily to 32 °C for 10 h (from 0800 to 1800) and 22 °C from 1800 to 0800. The 2 levels of stocking density include the experimental groups with low stocking density (LSD; 10 chickens/m2) and those with a HSD (18 chickens/m2). A basal diet supplemented with BG (4 g/kg of diet) was fed to 2 groups, one with HSD and the other with LSD. The heat stress resulted in a significant decrease in broiler performance, coefficient of ileal digestibility (CIAD) of nitrogen (N), apparent metabolisable energy (AME), relative weight of lymphoid organs, morphometric indices, and increased mRNA levels of heat shock protein 70 (HSP70) in breast muscle. Overcrowding stress reduced performance parameters, heterophil:lymphocyte (H:L) ratio but increased levels of IgA, IgM, nitric oxide (NO), corticosterone (CS), and HSP70. Growth performance, CIAD of N, AME, relative lymphoid organs weight, and jejunum villus height were increased by BG supplementation in the chickens stocked at HSD than those housed at LSD. In addition, BG supplementation reduced blood NO and CS concentrations, and mRNA levels of HSP70 but elevated H:L ratio in heat-stressed broiler compared those reared under thermoneutral zone. It was concluded that dietary use of BG as a feed supplement can reduce some of the detrimental effects of heat stress and HSD in broilers.
Introduction
In the modern broiler chicken production systems, different environmental factors can result in stress. Ambient temperature is an important factor in poultry production since it affects the performance and causes economic problems (Bartlett and Smith Citation2003; Attia et al. Citation2006, Citation2011; Attia and Hassan Citation2017). Poultry have to protect their body temperatures against the changes in a ent temperatures. Ambient temperatures above the thermoneutral (TN) zone of chickens have been related to oxidative stress (Attia et al. Citation2016), as reflected in elevated lipid peroxidation products in blood and tissues, causing protein and DNA oxidation. Typically an upregulation of heat shock proteins (HSPs) is observed upon heat stress (Bongiovanni et al. Citation2007). Stocking density is considered as an important stress factor in broiler production because it is generally effective on health, welfare, well-being, and performance (Gomes et al. Citation2014). Although various stocking densities, depending on the production system and country, are used, a high stocking density (HSD) can be stressful and has detrimental effects on broiler performance and physiological parameters (Cengiz et al. Citation2015).
Several parameters are measured as stress biomarkers in broilers, including plasma levels of corticosterone (CS), glucose, cholesterol, and nitric oxide (NO); the heterophil:lymphocyte (H:L) ratio; and lymphoid organs’ weight (Munck et al. Citation1984). Reduction in the numbers of lymphocytes and monocytes and increase in the numbers of heterophils and H:L ratio have been reported for stressed broilers (Cengiz et al. Citation2015). HSPs are a family of proteins that are produced by cells in response to thermal and non-thermal stressors (Akbarian et al. Citation2014). Previous research in broilers showed that social isolation may elicit HSP70 expression.
The dietary application of natural antioxidants is considered an appropriate practical strategy to reduce the deleterious consequences of stressors in animals (Seven et al. Citation2012; Attia, Abd Al-Hamid, et al. Citation2014; Attia, El-Hanoun, et al. Citation2014; Attia et al. Citation2015). Propolis (bee glue; BG) is a resinous and balsamic substance that is collected from buds, leaves and similar parts of trees and plants like pine, oak, eucalyptus, poplar, chestnut, etc. by honeybees (Apis mellifera) and mixed with wax. Seven et al. (Citation2012) reported that dietary supplementation of BG is more effective than vitamin C on growth performance of broilers exposed to heat stress that can be attributed to positive effects of BG on reduction in the deleterious effects of oxidative stress. Propolis has strong antibacterial, antioxidant, antiviral, and anti-inflammatory, antifungal and immunorestorative properties, and cytostatic and hepatoprotective activities (Sforcin Citation2007; Attia et al. Citation2015). Due to the presence of important compounds such as flavonoids, phenolic constituents and terpenoid, the use of BG in broiler diet was recommended as a way to minimise the negative effects of heat stress (Attia, Abd Al-Hamid, et al. Citation2014; Hosseini et al. Citation2016).
The positive effects of BG on broiler health have already been reported, but to the best of our knowledge, no information is available concerning the potential effects of BG under the stress caused by HSD and high ambient temperature. Hence, the present study tested the hypothesis supplementation with BG, as a beneficial feed additive, could alleviate the detrimental effects of cyclic heat stress and overcrowding in finishing broilers.
Materials and methods
The trial was conducted in the Poultry Research Unit of the Animal Sciences Research Institute (ASRI). All the procedures carried out in this experiment were approved by the Animal Ethics Committee of Lorestan University, Khorramabad, Iran.
Preparation of BG
The propolis was mainly collected by the honey bee from Amygdalus scoparia L. (Karaj, Alborz, Iran) in the spring season and frozen (−24 °C) immediately. An amount of 30 g of the frozen samples was extracted with 70% ethanol. The extract was taken shaken at 250 Hz at room temperature for 48 h in the absence of bright light. After filtration, the extract was evaporated with a vacuum evaporator at 50 °C.
Birds, diets, and management
Three hundred eighty 1-d-old Ross 308 male broilers were first brooded in wood shaving bedding. All chickens received the same starter diet (Table ) until 21 d of age and were grown at the temperature recommended for Ross 308 broilers. At 21 d of age, the chickens were weighed and experimental units were composed of chickens with the same average weight. The chickens were distributed in a completely randomised design with six treatments, each of which was replicated 5 times. Two experimental groups were provided floor space 0.1 m2/bird denoted as low stocking density (LSD; 10 birds/m2) while rest of them were provided a floor space of 0.05 m2/bird regarded as HSD (18 birds/m2). The TN and heat treatment (HS) were divided into 2 separated rooms. Each room was controlled at a given temperature. The chickens were fed corn-soybean basal diets (CON) that were formulated for starter (d 0–21) and finisher (d 22–42) according to the NRC (Citation1994) recommendations (Table ). Treatments included CON-TN/LSD (no additive), BG-TN/LSD, CON-HS/LSD, BG-HS/LSD, CON-HS/HSD and BG-HS/HSD. In the experiment period, chickens were either kept at TN zone (22 ± 1 °C) or exposed to heat stress (33 ± 1 °C for 10 h, from 0800 to 1800 h, and 22 °C from 1800 to 0800 h). The RH in both temperature treatments was 70%. The chickens had free access to feed and water and a 24-h lighting plan was implemented throughout the study. The pens had dimensions of 1.2 m length × 0.85 m width × 0.7 m height. Nipple drinkers were used for drinking water and tube feeders were used for feeding the chicks throughout the study.
Table 1. Ingredients and calculated and analysed compositions of the basal diets.
Broiler performance parameters
Feed intake and body weight (BW) for each replicate were recorded from 22 to 42 d. Average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were further calculated.
Nutrient digestibility
For the determination of nutrient digestibility, 3 birds per replicate with BW similar to the average of the corresponding pen were selected and transferred to battery cages (3 birds in each) with a wire mesh bottom and excreta collection trays (60 × 30 × 30 cm, length × width × height) at 30 d of age. Each cage was equipped with feed and water cups placed outside the cage. Birds in each cage were offered the corresponding diet. A titanium dioxide (Ti) tracer was added as an indigestible marker at an inclusion rate of 3 g/kg. Feed intake and total excreta output of each pen were measured from 30 to 34 d of age. Daily collections from each cage were pooled, mixed in a blender and subsampled. Subsamples were lyophilised ground to pass through a 0.5-mm sieve and stored in airtight plastic containers at −4 °C pending analysis. On d 35, all of the chickens were killed by intravenous injection (1 mL per 2 kg live weight) of sodium pentobarbitone and then ileal digesta were collected from the ileum, according to the procedures described by Ravindran et al. (Citation2005). Digesta from chickens within a pen were pooled, lyophilised, ground to pass through a 0.5 mm sieve and stored at −4 °C until laboratory analysis.
Chemical analysis
Gross energy (GE) of diet and excreta samples were determined by adiabatic bomb calorimeter (Gallenkamp Autobomb, London, UK), standardised with benzoic acid. Diet and ileum Ti contents were analysed on a UV spectrophotometer following the method of Short et al. (Citation1996). The total N was measured by combustion (method 968.06; AOAC International Citation2005) using the Kjeldahl method (Kjeltec 1030 Autoanalyzer, Foss Tecator AB). Feeds and excreta samples were determined for content of dry matter (method 930.15; AOAC International Citation2005). Samples of the feed and excreta were also analysed for Ca and P concentrations by colorimetric methods after ashing the samples at 550 °C and acid digestion in 6.0 M HCl using standard procedures (method 968.08D; AOAC International Citation2005).
Calculations
The apparent metabolisable energy (AME) values of the diets were calculated using the following formula:
Ileal apparent nutrient digestibility coefficients (CIAD) were calculated using the following formula:
where (Diet component/Ti)d is the ratio of diet component to Ti in the diet, and (Diet component/Ti)i is the ratio of diet component to Ti in the ileal digesta.
Relative weights of lymphoid organs
After slaughtering the chickens at d 42 of the experiment, the weights of the spleen, thymus and bursa of Fabricius were recorded. Then, relative weights of lymphoid organs were calculated as percentage of live BW.
Immunoglobulin concentration and stress indicators
At 42 d of age (end of the experiment), a 4-mL blood sample was obtained from the wing vein of 2 broilers in each replicate (10 samples per treatment), and then it was collected into 2 tubes (2 mL in each tube). The first tube contained heparin as the anticoagulant. A blood smear was prepared, and the number of H and L were counted to a total of 60 cells, and then the H:L ratio was calculated. The rest blood samples were centrifuged (5000 × g) for 10 min at 4 °C. The collected plasma was stored at −20 °C until further analysis. The plasma IgG and IgM concentrations were measured using an enzyme-linked immuno sorbent assay - ELISA kit from Bethyl Laboratories (Montgomery, TX). The ELISA procedure was carried out according to the protocol of the manufacturer and absorbance was measured at 450 nm.
The serum of blood samples in the second tube was separated and used to measure concentrations of CS, NO, glucose and cholesterol. Serum CS and NO levels were determined using an RIA kit (Cayman Chemical Company, MI). Serum concentrations of glucose and cholesterol were measured using an automated chemistry analyser (Hitachi 902 Automatic Analyzer, Hitachi, Tokyo, Japan) using colorimetric methods and following the instructions of the manufacturer of the corresponding reagent kit (Zhongsheng Biochemical Co., Ltd., Beijing, China).
Jejunum morphology
Sections of approximately 5 cm in length were excised from the middle of the jejunum (from the distal portion of the duodenal loop to Meckel’s diverticulum). These samples were flushed with cold saline and immediately placed in formalin 70% fluid. Samples were transferred into 70% ethanol after 72 h. The variables measured were villus height, crypt depth, and then V/C ratio was calculated.
Quantitative real-time PCR (qRT-PCR)
The breast (pectoralis major) muscles without skin and adipose tissues were collected and quickly snap-frozen in liquid nitrogen that was further used to measure HSP 70 mRNA (HSP70) levels. Total RNAs were extracted from the homogenised tissues using TRIZOL reagent (Invitrogen Life Technologies, Carlsbad, CA). RNA concentration was quantified by spectrophotometer nano-drop (MD-1000) in wavelength of 250 nm. Complementary (c)DNA was synthesised from 1 μg of RNA samples with an MBI cDNA Synthesis Kit (Bio-Rad, Hercules, CA), according to the manufacturer’s recommended protocol. All primers were synthesised and purified by Sigma Company. The β-actin was used as reference gene to normalise the expression of target gene. The primer pairs for the amplification of HSP70 and β-actin cDNA fragments are listed in Table . Quantitative real-time PCR (qRT-PCR) was performed to determine the levels of inducible HSP70 mRNA. Two microliters of tenfold dilution reverse transcription products was used for PCR in a final volume of 25 μL containing 0.4–0.8 μM primers and 12.5 μL of QuantiTect SYBR Green master mix (Life Technologies, Cat # 4367659). Cycling parameters were as follows: 10 min at 95 °C, then 40 cycles of 95 °C for 30 s, annealing temperature for 30 s, and 72 °C for 30 s, and extension for 2 min at 72 °C. To confirm amplification specificity, the PCR products from each primer were subjected to a melting curve analysis and subsequent agarose gel electrophoresis. The final HSP70 concentrations were calculated as arbitrary unit of band density relative to total protein concentration of each sample.
Table 2. Primers used for quantitative real-time PCR.
Statistical analysis
The results are presented as means with the pooled standard error of the mean (SEM). Data were analysed by analysis of variance using the general linear model procedure of SAS (SAS Citation2001). The pen was an experimental unit for growth performance, whereas all selected birds from each replication were defined as the experimental unit for nutrient digestibility, blood metabolite, morphological and carcase measurements. Percentage data were transformed to arcsine square-root percentage for analysis. The corresponding means were compared by Tukey–Kramer’s test and statistical differences were declared at p < .05. Pairwise contrasts were used to show differences between the treatments.
Results
The effects of BG supplementation, heat stress and stocking density on the performance of broilers during d 22–42 are shown in Table . As expected, heat stress and HSD-depressed (p < .01) ADG and ADFI, and increased FCR. Compared with the basal diet, supplementation of BG increased ADG and ADFI (p < .01). Dietary addition of BG improved (p < .05) the growth performance in heat-stressed broilers housed at HSD than those kept at TN zone.
Table 3. Effect of propolis supplementation and stocking density on the growth performance of broilers under heat stress from d 22 to 42.
Heat stress reduced (p = .013) the CAID of N (Table ). In chickens exposed to heat stress, BG supplementation increased CAID of N (p = .029) and AME of feed (p = .001) compared to those fed basal diet. The CIAD of Ca and P were unaffected by the treatments (p > .05).
Table 4. Effect of propolis supplementation and stocking density on the coefficient of apparent ileal digestibility (CAID) of nitrogen (N), calcium (Ca) and phosphorus (P), and apparent metabolisable energy (AME) in broilers under heat stress at 35 d of age.
The effects of dietary BG supplementation, heat stress and stocking density on the immune status of broilers are given in Table . As compared with broilers in TN zone, heat-stressed broilers had a smaller spleen (p=.002), thymus (p = .006) and bursa of Fabricius (p < .001). In chickens subjected to heat stress, inclusion of BG significantly increased the relative weights of the spleen (p = .006) and bursa of Fabricius (p = .013) than those fed basal diet.
Table 5. Effect of propolis supplementation and stocking density on the immunity status of broilers under heat stress at 42 d of age.
As shown in Table , stocking density had significant effect on plasma immunoglobulin levels. The HSD increased significantly the plasma IgG (p = .022) and IgM (p = .016) levels compared with the LSD. At both heat stress and TN temperature, plasma IgG was significantly increased in chickens fed BG supplementation and stocked at LSD than those fed the unsupplemented diet (p = .002).
Broilers in heat stress had shorter (p < .001) villus height, deeper (p < .001) crypt depth and lower (p < .001) villus height to crypt depth ratio than those in the TN zone (Table ). Inclusion of BG increased (p = .014) villus height in the chickens housed at LSD and normal temperature, and in the chickens stocked at HSD and reared under heat stress, compared to those fed basal diet. Stocking density had no (p > .05) effect on the jejunum morphology.
Table 6. Effect of propolis supplementation and stocking density on the morphology of jejunum of broilers under heat stress at 42 d of age.
The effects of the BG, heat stress and stocking density on the stress indicators of broilers are shown in Table . Significant differences between the treatments were not observed for blood glucose and cholesterol. Compared with the broilers in TN zone, heat-stressed broilers had lower H:L ratio (p = .002). Chickens reared at HSD had a higher blood NO (p = .012) and CS (p = .017), and lower H:L ratio (p = .033) than those reared at LSD. Inclusion of BG supplementation reduced blood NO (p = .005) in the heat-stressed broilers, and decreased CS concentration (p = .010) and increased H:L ratio (p = .042) in the chickens housed in TN or heat stress rooms. RT-PCR analysis showed that heat stress and HSD increased the relative amount of HSP70 expression in the breast of broilers (Figure ). In the chickens exposed to heat stress, dietary BG downregulated the HSP70 expression (p < .05).
Figure 1. Effect of propolis and stocking density on heat shock protein 70 mRNA (HSP70) expression of chicken breast in heat-stressed broilers. Values are mean ± SE (n= 5). Within the graph, bars with different letters (a–c) are significantly different (p <.05). LSD: low stocking density (10 birds/m2), HSD: high stocking density (18 birds/m2), BG: basal diet + propolis (4 g/kg of feed)
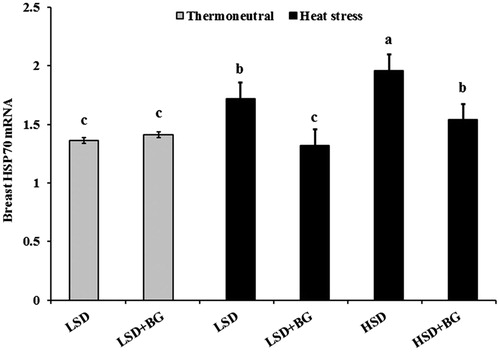
Table 7. Effect of propolis supplementation and stocking density on stress indicators in broilers under heat stress at 42 d of age.
Discussion
The results of the present study are in line with those reported by Abudabos et al. (Citation2013), Cengiz et al. (Citation2015) and Hosseini et al. (Citation2016) who indicated an impairment of performance parameters during stressful condition. The present study indicated that BG supplementation improved broiler performance. It has been well documented that BG can be used in animal feeds as a natural growth promoter and improved productive parameters in broilers reared under normal (Attia, Abd Al-Hamid, et al. Citation2014; Attia, El-Hanoun, et al. Citation2014, Attia et al. Citation2015) and high (Hosseini et al. Citation2016) environmental temperatures. This beneficial effect may be due to the bioactive substances of BG such as total flavonoids and benzoic acid that might have enhanced the animal metabolic process (Acikgoz et al. Citation2005; Attia, Abd Al-Hamid, et al. Citation2014; Attia, El-Hanoun, et al. Citation2014, Attia et al. Citation2015). The increase in feed intake and weight gain could be associated with the flavonoid content and palatable properties of BG diet (Attia, Abd Al-Hamid, et al. Citation2014).
Known effects of heat stress on the digestive function include modification of hypothalamic peptides involved in appetite regulation, a decreased passage rate of feed residue, decreased trypsin, chymotrypsin, and amylase activity, changes in intestinal morphology and nutrient absorption (Attia et al. Citation2006, Citation2011; Song et al. Citation2014; Attia et al. Citation2016; Attia and Hassan Citation2017). These effects can explain the poor feed efficiency and also contribute to the lower protein digestibility we observed in the chickens reared under high temperature. In current research, AME values tended to decrease in heat-stressed broilers. The lower energy efficiency found in the exposed chickens reflects the large energy demand for maintenance, especially for body temperature regulation (Houshmand et al. Citation2012). It is well established that thermoregulation in broilers is achieved by diverse mechanisms, including enhanced convective, radiant, and evaporative heat loss, in addition to greater air circulation in the air sacs (Lara and Rostagno Citation2013). In the present study, BG supplementation increased nutrient digestibility and AME values in the heat stress group. This may be due to palatability, antioxidant and immune stimulant properties (Seven et al. Citation2012). Although Acikgoz et al. (Citation2005) did not observe positive effects on digestibility of BG supplementation in broilers, Bonomi et al. (Citation2002) found that BG caused significant effects on these parameters in ducks. This inconsistency in the effectiveness of BG may be due to the dose used or relate to the study condition (stress or no stress conditions).
In the present study, the addition of BG to the diets increased relative weight of spleen, thymus, and bursa of Fabricius in broilers housed at HSD. Currently, there is a lack of sufficient literature to explain the effects of BG on lymphoid tissues in overcrowding stress. The increase in relative weight of lymphoid organs in the BG-supplemented diets may be an indication of the immune-booster effect of this additive (Sforcin Citation2007). Because BG is rich in bioflavonoids, phenolic acids, vitamins, and phytosterols, it promotes faster cell proliferation and differentiation in the immune system. It is well documented that dietary supplementation of BG induces T-lymphocyte formation and the division, proliferation and activity of thymus cells (Hosseini et al. Citation2016). Attia et al. (Citation2014) stated that BG can increase lymphocyte proliferation, and impact immune function and disease resistance.
Gomes et al. (Citation2014) found that stressors are able to enhance the immunoglobulin levels even at 24 h after subjection to the stressing conditions. In the present study, the levels of IgG and IgM in plasma were increased in the chickens housed at HSD. These results may be associated with decreased immunological memory, therefore leading to an increase in the susceptibility of chickens to pathogenic challenge. This may be a probable mechanism used by the immune system in an attempt to respond to overcrowding stress (Abudabos et al. Citation2013). Our findings showed that serum IgG concentration in the chickens fed dietary BG supplementation reared under both normal and high temperatures were higher than those fed the control diets, indicating that BG could modulate humoral immunity. Sforcin (Citation2007) stated that BG supplementation activates the immune system in chickens, increasing macrophage and natural killer cells (NK) activities, and raising cytokine levels. The cytokines increase activity of B-lymphocytes, which would be able to produce immunoglobulins. Hence, the increased levels of IgG in broilers fed dietary BG are probably related to the B-lymphocyte stimulation by cytokines (Hosseini et al. Citation2016).
The results of this study are consistent with the findings of Song et al. (Citation2014), who found that greater blood flow to the myocardium, turbinates, nasal mucosa, and respiratory muscles result in a decrease of gut blood flow. In addition, ischaemia of the enteric canal can cause epithelial shedding, leading to shortened villus height and deeper crypt depth. Hosseini et al. (Citation2016) reported that villus height is an important indicator of the digestive health of chickens and directly related to the absorptive capacity of the mucous membrane. The present study showed that BG resulted in greater villus height of the jejunum in the heat-stressed chickens stocked at HSD than in those housed at LSD. Hosseini et al. (Citation2016) found that dietary BG may stimulate the digestive and absorptive functions of broilers and may be helpful in explaining the improvement in the growth performance observed in this research.
The results of the current study indicated that CS and NO concentrations were higher in the chickens stocked at a high density than in those housed at a normal density. Overcrowding stress activates the HPA axis in the challenged broilers, increasing the CS and NO levels and releasing pro-inflammatory cytokines (i.e. IL-1, chemokines) (Cengiz et al. Citation2015). It is probable that overcrowding stress enhanced norepinephrine secretion by the adrenal medulla as reported for CS. Hosseini et al. (Citation2016) concluded that beneficial effects of BG may be more clear under stressful conditions, such as a HSD; therefore, the BG was expected to influence the effect of stress, and, therefore, its biomarkers. The higher H:L ratio and lower CS and NO concentrations observed in heat-stressed broilers fed diets supplemented with BG implies the positive influence of flavonoids on reducing stress in broilers (Seven et al. Citation2012). It seems that BG supplementation can improve the HPA axis and reduce the blood CS level, through the restoration of intestinal microbial ecology of broilers subjected to hot environment (Abdel-Mohsein et al. Citation2014). Nevertheless, the mechanisms mediating the suppressive effects of BG on inflammation are still unknown.
As found herein, HSP70 levels were raised in different tissues of broilers after thermal and non-thermal stressors such as overcrowding stress (Akbarian et al. Citation2014). Chickens reared at a high density and heat stress had higher HSP70 mRNA levels than those housed at a low density in a TN zone. Supplementation with BG caused a decrease in breast HSP70 mRNA level. Based on these findings, it can be concluded that the supplementation with BG had a protective effect against oxidative damage. This novel finding suggests that an unknown compound of BG might cause a similar effect of that observed for oestrogen on HSP70 mRNA induction through a nutritional mechanism (Bongiovanni et al. Citation2007). These findings were expected because increase in HSP70 mRNA level may be critical for protection from cellular damage attributed to environmental stressors such as heat stress and high density (Najafi et al. Citation2015). Bongiovanni et al. (Citation2007) investigated the effects of natural antioxidants against stressors and concluded that dietary supplementation of flavonoids may have a protective effect against acute heat stress.
Conclusions
It could be concluded that dietary use of BG as a beneficial additive may offer a nutritional strategy in broiler farming to overcome the deleterious effects of thermal or overcrowding stress. It seems that further studies on the mode of action of BG are needed to expand the knowledge on usage of such natural additives in industrial practice.
Acknowledgements
The authors gratefully appreciate the financial support provided by the Vice Chancellor in Research and Technology of the Lorestan University. Special thanks to Dr. Morteza Rezaei (Animal Science Research Institute of Iran) for his helpful discussions and technical assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdel-Mohsein HS, Mahmoud MAM, Mahmoud UT. 2014. Influence of propolis on intestinal microflora of Ross broilers exposed to hot environment. Adv Anim Vet Sci. 2:204–211.
- Abudabos AM, Samara EM, Hussein EOS, Al-Ghadi MQ, Al-Atiyat RM. 2013. Impacts of stocking density on the performance and welfare of broiler chickens. Ital J Anim Sci. 12:66–71.
- Acikgoz Z, Yucel B, Altan O. 2005. The effects of propolis supplementation on broiler performance and feed digestibility. Archiv Für Geflügelkunde. 69:117–122.
- Akbarian A, Michiels J, Golian A, Buyse J, Wang Y, De Smet S. 2014. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fed Origanum compactum and Curcuma xanthorrhiza essential oils. Poult Sci. 93:1930–1941.
- AOAC International. 2005. Official methods of analysis, 18th edition. Washington (DC): AOAC International.
- Attia YA, Abd El-Hamid Ael H, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, Sahin K, Abou-Shehema BM. 2016. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. Springerplus. 5:1619.
- Attia YA, Abd Al-Hamid AE, Ibrahim MS, Al-Harthi MA, Bovera F, El-Naggar A. 2014. Productive performance, biochemical and hematological traits of broiler chickens supplemented with propolis, bee pollen, and mannan oligosaccharides continuously or intermittently. Livest Sci. 164:87–95.
- Attia YA, Böhmer BM, Roth-Maier DA. 2006. Responses of broiler chicks raised under constant relatively high ambient temperature to enzymes, amino acid supplementations, or diet density. Arch Geflügelk. 70:80–91.
- Attia YA, Hassan SS. 2017. Broiler tolerance to heat stress at various dietary protein/energy levels. Europ Poult Sci. 81. DOI: 10.1399/eps.2017.171
- Attia YA, Bovera F, El-Tahawy WS, El-Hanoun AM, Al-Harthi MA, Habiba HI. 2015. Productive and reproductive performance of rabbits does as affected by bee pollen and/or propolis, inulin and/or mannan-oligosaccharides. World Rabbit Sci. 23:273–282.
- Attia YA, El-Hanoun AM, Bovera F, Monastra G, El-Tahawy WS, Habiba HI. 2014. Growth performance, carcass quality, biochemical and haematological traits and immune response of growing rabbits as affected by different growth promoters. J Anim Physiol Anim Nutr. 98:128–139.
- Attia YA, Hassan RA, Tag El-Din AE, Abou- Shehema BM. 2011. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Anim Nutr. 95:744–755.
- Bartlett JR, Smith MO. 2003. Effects of different levels of zinc on the performance and immunocompetence of broilers under heat stress. Poult Sci. 82:1580–1588.
- Bongiovanni GA, Soria EA, Eynard AR. 2007. Effects of the plant flavonoids silymarin and quercetin on arsenite-induced oxidative stress in CHO-K1 cells. Food Chem Toxicol. 45:971–976.
- Bonomi A, Bonomi BM, Quarantelli A, Sabbioni A, Superchi P. 2002. The use of propolis in ducks feeding. Riv Sci Aliment. 31:15–28.
- Cengiz Ö, Köksal BH, Tatlı O, Sevim Ö, Ahsan U, Üner AG, Ulutaş PA, Beyaz D, Büyükyörük S, Yakan A, et al. 2015. Effect of dietary probiotic and high stocking density on the performance, carcass yield, gut microflora, and stress indicators of broilers. Poult Sci. 94:2395–2403.
- Gomes AV, Quinteiro-Filho WM, Ribeiro A, Ferraz-de-Paula V, Pinheiro ML, Baskeville E, Akamine AT, Astolfi-Ferreira CS, Ferreira AJ, Palermo-Neto J. 2014. Overcrowding stress decreases macrophage activity and increases Salmonella Enteritidis invasion in broiler chickens. Avian Pathol. 43:82–90.
- Hosseini SM, Vakili Azghandi M, Ahani S, Nourmohammadi R. 2016. Effect of bee pollen and propolis (bee glue) on growth performance and biomarkers of heat stress in broiler chickens reared under high ambient temperature. J Anim Feed Sci. 25:41–50.
- Houshmand M, Azhar K, Zulkifli I, Bejo MH, Kamyab A. 2012. Effects of prebiotic, protein level, and stocking density on performance, immunity, and stress indicators of broilers. Poult Sci. 91:393–401.
- Lara LJ, Rostagno MH. 2013. Impact of heat stress on poultry production. Animals (Basel). 3:356–369.
- Munck A, Guyre PM, Holbrook NJ. 1984. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 5:25–44.
- Najafi P, Zulkifli I, Jajuli NA, Farjam AS, Ramiah SK, Amir AA, O'Reily E, Eckersall D. 2015. Environmental temperature and stocking density effects on acute phase proteins, heat shock protein 70, circulating corticosterone and performance in broiler chickens. Int J Biometeorol. 59:1577–1583.
- NRC. 1994. Nutrient requirements of poultry. 9th Rev. ed. Washington, DC: National Academy Press.
- Ravindran V, Hew LI, Bryden WL. 2005. Apparent ileal digestibility of amino acids in dietary ingredients for broiler chickens. J Anim Sci. 81:85–97.
- SAS. 2001. SAS User’s Guide. Version 8 ed. Cary (NC): SAS Inst. Inc.
- Seven I, Aksu T, Tatli O, Seven P. 2012. The effects of propolis and vitamin C supplemented feed on performance, nutrient utilization and carcass characteristics in broilers exposed to lead. Livest Sci. 148:10–15.
- Sforcin JM. 2007. Propolis and the immune system: a review. J Ethnopharmacol. 113:1–14.
- Short FJP, Gorton J, Wiseman J, Boorman KN. 1996. Determination of titanium oxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 59:215–221.
- Song J, Xiao K, Ke YL, Jiao LF, Hu CH, Diao QY, Shi B, Zou XT. 2014. Effect of a probiotic mixture on intestinal microflora, morphology, and barrier integrity of broilers subjected to heat stress. Poult Sci. 93:581–588.
