Abstract
A trial was carried out to study the head and skull characteristics in a crested chicken breed with cerebral hernia located in a frontal bony protuberance, an uncrested chicken hybrid strain and their relative crosses. Males and females from five genotypes were used: two Padovana breed varieties (two colour plumages: Padovana argentata, silver -PA- and Padovana camosciata, chamois -PC-), the relative cross (PCxPA), a commercial single comb medium-growing strain (Berlanda gaina [B]) and the cross between PC and B (PCxB). As regards skull dimensions B showed heavier, longer, and wider skull than Padovana group (p < .001); the cross PCxB had skulls heavier than Padovana purebreds (p < .05). In the Padovana purebreds the frontal bone height varied from 9.2 to 16.2 mm, whereas in the groups with B component the height ranged from 4.2 to 6.8 mm. The frontal bone height was higher in PC and PCxPA than in PA (p < .01), whereas B and PCxB did not show any bony protuberance. No correlation resulted in Padovana groups between the frontal bone height and the skull length and width. A positive relationship between frontal bone height and skull height was significant only in PCxPA. For the skull characters of PCxPA both the two genotypes seems to be involved and no predominant and relevant effect of only one genotype was seen. The PCxB cross showed relevant differences in the skull morphometry, particularly in the absence of frontal bony protuberance and in the presence of comb.
Introduction
The origin of Padovana chicken is still uncertain; probably, it came to Italy in the Fourteenth Century, brought from Poland (Holzer Citation2013). This breed, widespread in the past, reduced its consistence and almost disappeared after 1960. From 1997 the Padovana breed was recovered thanks to an association of breeders and amateur breeders of this breed (Pro Avibus Nostris) in Padua; afterwards Padovana chicken (www.gallinapadovana.net) was involved in a conservation and promotion programme (Baruchello and Cassandro Citation2003).
Padovana is a local breed of Veneto, a region in the northern east of Italy, where these chickens are raised, partly for ornamental purpose. The Padovana chicken is a crested chicken, characterised by a big tuft rising from a very pronounced bony hemispherical protuberance of the skull’s frontal bone as descripted by researchers in the last two centuries (Tegetmeier Citation1856; Darwin Citation1868) and improperly called ‘cerebral hernia’ (Ghigi Citation1939). The bony protuberance is adapted to contain part of the brain that results enlarged and upthrusted (Ghigi Citation1968; Desmond and Jacobson Citation1977). The surface of the bony protuberance is not continue, but it is interrupted by openings, variable for number and dimension (Darwin Citation1868; Ghigi Citation1916).
In Padovana breed, besides the frontal bony protuberance, two more cranial variations, in the nasal and pre-maxilla bones, may be observed (Figures and ). The frontal processes of the nasal bone are quite large; the nasal processes of pre-maxilla are not joined together to constitute a single bone tissue along the dorsal beak’s middle line, but leave an open space (Figures and ). Consequently the nostrils are partially without bone support, and are placed rather high on the beak profile, looking wide, flat and elastic (Davenport Citation1906). Moreover the space between the nostrils and the bone process supporting the comb is extremely limited so that the normal development of the comb is prevented. The comb is often absent or very small in females, and often irregular in shape, with knobs or V-shaped, especially in males. Besides the big bouffant tuft, constituted by extremely long feathers, Padovana also has a thick beard and whiskers.
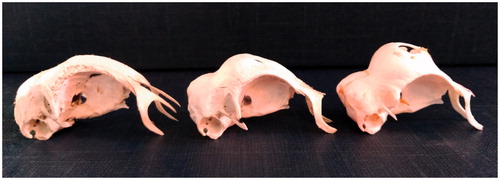
Figure 1. Skull of chicken with frontal bony protuberance (on the left, PCxPA) and without frontal bony protuberance (on the right, PCxB).
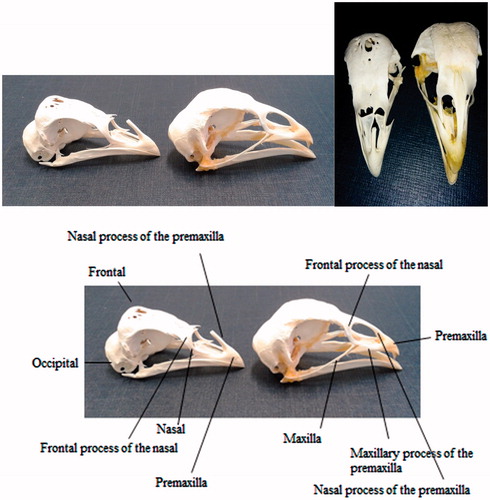
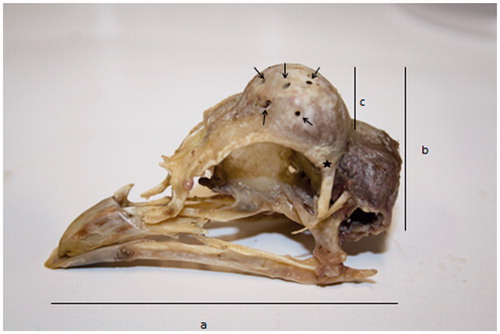
Figure 2. Skull of Padovana camosciata. Measurements: a = skull total length (skull length, comprehensive of the beck length); b = skull total height; c = frontal protuberance height. On the frontal protuberance little holes are indicated (arrows). The star indicates the point from which skull width was measured, from side to side, at eyes level.
The Padovana chicken is a laying breed, but also provides lean carcases with moderate meat yield and is particularly appreciated for flavour, succulence and tenderness of its meat (De Marchi et al. Citation2005; Verdiglione and Cassandro Citation2013; Cassandro et al. Citation2015), as it is the main ingredient of several famous traditional Paduan dishes, called ‘Gallina Padovana —Padovana hen’ (Gallina Padovana Citation2016). From 2000 the ‘Gallina padovana’ is a Slow Food Praesidium (Slow Food Citation2016; www.slowfood.it).
The price of Padovana chicken meat is not comparable to that of hybrid commercial meat as this breed is slow-growing and its production cycle is very long. The cross between Padovana chicken and commercial hybrid strains can be utilised to obtain medium growing chickens characterised by good physical (texture and grain size) and organoleptic (smell and flavour) characteristics (Cassandro et al. Citation2015).
The morphological characterisation of different chickens breeds can be performed by a morphometrical study of the head and of other parts of the body (neck, tail and tarsus) (Francesch et al. Citation2011). In this work, the morphological characterisation of the breeds was obtained by means of a skull osteometrical study (Baumel Citation1979; Hayashi et al. Citation1982; Samejima et al. Citation1988; Ino et al. Citation2008) and by comb and crest description.
The aim of this work was to study the head and skull characteristics in two Padovana chickens strains, Padovana camosciata (PC) and Padovana argentata (PA), in the relative cross (PCxPA), and in the cross between PC males and a single comb commercial strain females.
Materials and methods
Animals and measurements
Males and females from five different genotypes were used: two Padovana breed varieties (two colour plumages: Padovana argentata, silver -PA- and Padovana camosciata, chamois -PC-), the relative cross (PCxPA), a commercial single comb medium-growing strain (Berlanda gaina - B) and the cross between PC and B (PCxB).The plumage of PA consisted in white feathers with black edge, whereas PC had light brown feathers with white edge. Padovana breeders came from the Padovana nucleus flock of the Agricultural High School ‘Duca degli Abruzzi’ in Padova. Berlanda breeders came from ‘Avicola Berlanda’ (Avicola Berlanda Citation2016, Carmignano di Brenta, Padova, Italy). The eggs of the five genotypes were incubated at Agricultural High School ‘Duca degli Abruzzi’ in Padua; then, one-day-old chicks of all genotypes were housed in indoor pens (wood shavings litter) at the experimental farm of the Department of Agronomy, Food, Natural resources, Animals and Environment (Legnaro, Italy) (latitude 45°20′32″N, longitude 11°57′53″E) until the slaughtering age. Feed (commercial diet) and water were supplied ad libitum. The birds were reared assuring the CEASA (Citation2015) protocol in term of animal welfare (Ethical Committee for the Care and Use of Experimental Animals of the University of Padova, Italy).
After 180 days of rearing, when the chickens had reached a body weight suitable for meat production (B = 3066 g, PA = 1798 g, PC = 2000 g, PCxPA = 1970, PCxB = 2675 g), a total of 66 chickens, 35 males and 31 females, were weighed (20 g accuracy) and slaughtered after electrical stunning. At this age in all the genetic groups studied the sexual dimorphism can be evaluated by the shape of the feathers of head, neck and tail: the males showed lanceolate feathers of tuft (when present), lanceolate hackles of neck and sickles in tail, whereas the females showed more rounded and shorter feathers of tuft (when present) and neck and they were devoid sickles in tail. Birds with Padovana component (PA, PC, PCxPA and PCxB) showed no difference in plumage colour according to sex, but the colours were more polished and iridescent in the males.
The heads were collected after slaughtering, weighed and then frozen. Before freezing, comb and tuft were classified following a numerical code (Table ). Afterwards, heads were de-frozen, boiled (a single plastic bag for each head), de-pulped, therefore only bones remained. The skulls were weighed (0.01 g accuracy), then reconstructed (Figure ), and measured with a callipers (0.01 mm accuracy). The measures performed were the skull weight, the skull total length, comprehensive of beak length, from occipital bone to the beak point, the skull width, measured at the eyes level, the skull total height, from the ventral wall of the cranium (basisphenoide bone) to the top, and the height of the frontal bone, from the top of the orbital arch to the top of the head (frontal bone) (Figure ). In the Padovana genotypes the openings of the frontal bony protuberance were described, their number and size were recorded. With regard to the openings size, a numerical code was attributed (Table ).
Table 1. Comb, crest and frontal protuberance’s openings classification.
The measurement of skull length, skull width and beak length were performed as reported in previous works (Baumel Citation1979; Hayashi et al. Citation1982; Samejima et al. Citation1988; Ino et al. Citation2008) to classify different breeds of chicken. The measurements of skull height and hernia eight, as defined above, were used in this work because they are better suited to characterise this breed. Skull height and hernia eight measurement were detected by Ghigi (Citation1916) in the past.
Statistical analysis
On the data collected (weight, height, length and width of skull and frontal bone height) an ANOVA was performed with the GLM procedure (SAS Citation2010), according to the following linear model: Yijk = µ + Genotypei + Sexj + (Genotype*Sex)ij+ εijk where Yijk is the dependent variable; Genotypei is the fixed effect of the ith genotype of the bird (i = B, Padovana, PCxB); Sexj is the fixed effect of the jth sex of the bird (j = male, female); (Genotype*Sex)ij is the fixed interaction effect between genotype and sex; εijk is the random residual ∼N (0; σε2). On all the same data the sex effect for each genotype (Table ) and the genotype effect for each sex (Tables and ) were also evaluated by an ANOVA (SAS Citation2010) according to the following models: Yijk = μ + Sexi + εik where Yijk is the dependent variable; Sexi is the fixed effect of the ith sex of the bird (i = male, female); εik is the random residual ∼N (0; σε2) and Yijk = μ + Genotypei + εik where Yijk is the dependent variable; Genotypei is the fixed effect of the ith genotype of the bird (i = B, PA, PC, PCxPA, PCxB); εik is the random residual ∼N (0; σε2). Contrast estimates (±SE) allowed us to detect statistically significant differences between groups. Pearson correlations between head and skull parameters for each genotype were also calculated (SAS Citation2010). On the data of the number of openings and openings size, and for comb and crest the NPAR1WAY PROC and the test of Kruskall–Wallis were performed. Pearson correlations between skull parameters for each genotype were also calculated (SAS Citation2010). Spearman correlations between openings number and openings size, and comb and skull width, skull height and frontal bone height were calculated (SAS Citation2010).
Results and discussion
The data on the skull morphometry of the chickens belonging to the hybrid B, Padovana (PA, PC and PCxPA, together) and PCxB cross are showed in Table .
Table 2. Data on the skull morphometry of the three genetic groups.
Table 3. Sexual dimorphism (contrast estimates ± standard error) in skull characteristics of five genotypesTable Footnotea.
Genotype significantly affected all the studied parameters. The skull weight and the skull length of Padovana resulted significantly lower than those of B (p < .0001) and PCxB (p < .05). The skull width was higher for B chickens than Padovana group (p < .0001).
As far as height is concerned, in chickens the distance between the orbital arch of frontal bone and the top of the skull reach some mm as cerebral space. In the Padovana breed the height of the skull is greater than in an uncrested chicken due to the presence of a frontal bony protuberance, so the skull total height was higher (p < .0001) in Padovana group than B and PCxB. The frontal bone height was higher (p < .0001) in Padovana group than B and PCxB. In the groups with the sole Padovana component the height of frontal bone varied from 9.2 to 16.2 mm, whereas in the groups with B component the height ranged from 4.2 to 6.8 mm (Figure ). This result is in agreement with that obtained by Yoshimura et al. (Citation2012) in the cross crested Polish male × uncrested hybrid female.
The frontal bone height/skull width ratio (fbh/sw) was higher (p < .0001) in Padovana group than B and PCxB.
The effect of sex on skull morphometry according to the genotype is showed in Table . Sexual dimorphism was detected for many parameters in most genetic types. The skull data measurements were generally higher in males than in females and significant differences were detected (Figure ). Frontal bone height differed between sexes only in B (p < .001) and PCxB (p < .01), but not in the groups with the sole Padovana component. The fbh/sw ratio was higher in males than in females of B (p < .01) and PCxB (p < .05), whereas PA and PC showed and opposite trend with significant (p < .05) differences in PA birds. The PCxPA birds did not show differences for all the studied parameters.
Figure 4. Sexual dimorphism in the Padovana varieties, lateral view. PA (above) and PC (below), male (on the left) and female (on the right).
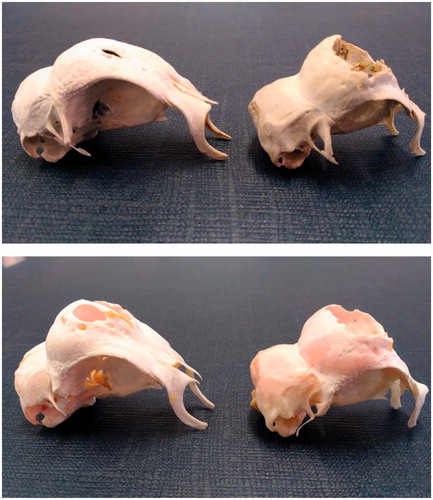
In Table some characters of the frontal bone and comb and crest, according to sex per genotype are shown. B and PCxB skull did not show any frontal bone openings. The frontal bony protuberance showed openings, variable for both number and size. In some birds only one big opening at the top of the skull was observed, independently of sex (PA female: 20.8 × 18 mm, PA female: 20.3 × 18 mm, PC male: 18.8 × 18.9 mm, PA: female 16.8 × 14.3mm, PC female: 14 × 14.9 mm) (Figures ), while in the other birds more openings were detected, from 2 to 7, from a minute dot to small, to medium or to large size (Figures ). No sexual effect was seen for the frontal bone openings, as their number and size, in PA, PC and PCxPA groups.
Figure 5. Frontal bone characteristics. Frontal–dorsal view. On the left skull without bony frontal protuberance (PCxB, female) is represented; in the middle skull with bony frontal protuberance with numerous little holes (PCxPA, male); on the right a skull with numerous holes of variable dimension on frontal bony protuberance (PCxPA, male). Note in PCxB the wide slot between the frontal processes of nasal bone, due to the lack of the nasal process of premaxilla removed together with the premaxilla.
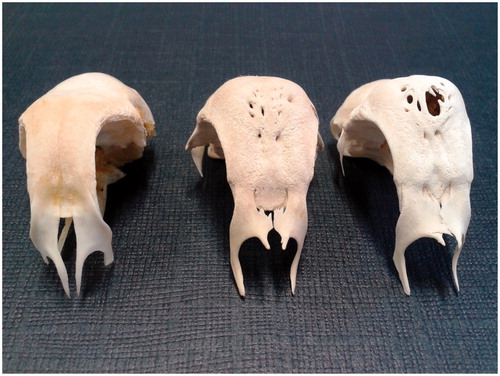
Figure 6. Padovana camosciata skulls (male), view from above. Frontal bone’s holes. In the figure skulls with holes, variables for number and dimensions, are represented. On the right only one big opening is located on the top of the skull.
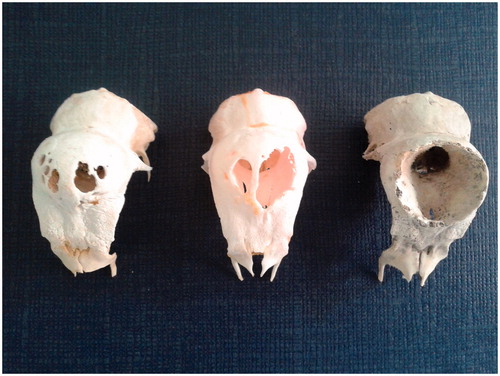
Figure 7. Padovana camosciata skulls, lateral view. In the skull on the right, characterised by a single big hole on the top of the head, the total skull height appears lower than the height of the skull on the left.
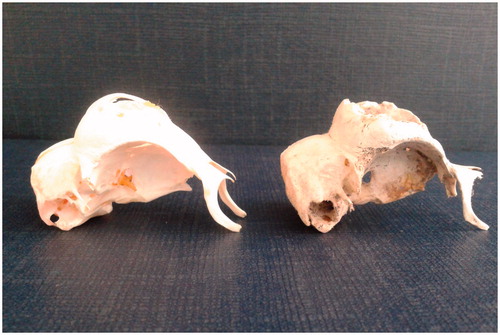
Table 4. Sexual dimorphism in frontal bone openings and comb and crest of the five genotypesTable Footnotea.
Table 5. Contrast estimates ± standard error for skull and head parameters for PA, PC and PCxPA chickens.
Table 6. Contrast estimates ± standard error for skull and head parameters for B, PC and PCxB chickens.
Comb size showed similar development in B males and females whereas it was significantly higher in males of PC, PCxPA and PCxB (p < .01), as well as in PA group (p < .05). In the Padovana females of this trial the comb resulted to be totally absent or of very small size, in presence of a well-developed crest atop the head. In the birds belonging to the cross PCxB the comb was sizeable; the prevailing type of comb was a single comb rising from a reduced base. Besides the comb, a partly developed crest was observed in the cross PCxB, placed caudal than the comb. The crest was absent when the comb was well developed, as occurred in B and in some specimens of PCxB. Crest showed similar development in all the Padovana birds, whereas in PCxB it was more developed (p < .01) in females than in males, together with a partly developed comb.
It is noteworthy that only in PA group some females showed little comb, whereas in PC and PCxPA groups no comb was detected in the female specimens. Many adult Galliformes show sexual dimorphism which may involve feathers length and shape, feathers colour, body weight and secondary sexual characters, so that, as a consequence, the phenotype quite differs between males and females. As indicated before, the crest was absent when the comb appeared well developed, as occurred in PCxB males. Adult males and females belonging to Gallus gallus are phenotypically quite different in most of existing breeds: the studied genotypes showed an evident sexual dimorphism both for body weight, feathers length of neck and tail, comb size and shape, and crest. It is well remembering that the birds of this trial were 26 weeks old, an age characterised by sexual dimorphism in chickens: in all the genotypes the body weight was notably higher in males than in females (B = 42%, PA = 29%, PC = 35%, PCxPA = 49% and PCxB = 39%).The skull weight, skull length, skull width are the variables more affected by sex as a consequence of different body dimensions between sexes, whereas for the skull bone protuberance no differences were detected between males and females, when it was higher than 6.00 mm, that is in the Padovana groups.
In Table the estimates of contrast of skull and head parameters between the two Padovana varieties and their cross in males and females are shown. In males, the comparison between PA and PC resulted in significant differences for skull height (p < .001), frontal bone height (p < .01) and fbh/sw ratio (p < .05), as PA was showing lower values. PCxPA cross showed lower skull height (p < .001) and length (p < .05) in comparison to PC, and an increase of frontal bone height (p < .001) and fbh/sw ratio (p < .001) in comparison to PA. No difference regarding the number of openings was detected in the contrast PA vs PC whereas PCxPA cross showed an increase in the number of openings than PC (p < .01) and PA (p < .05). As regards the opening size PA showed a decrease in the openings size in comparison to PC (p < .05), and PCxPA showed the same trend.
In females, as regards the comparison between PA and PC, a trend similar to males was observed for skull height (p < .001); PA showed lower skull length (p < .01) than PC. PCxPA females in comparison to PC showed a decrease in skull height (p < .01), less marked than in males, but a significant increase in the openings number (p < .05), as seen in the males. PCxPA females showed a higher skull weight (p < .01) and skull height and width (p < .05) than PA females.
In Table the estimates of contrast between B and PC and their cross in males and females are shown. In males, B showed higher skull weight (p < .05) and skull width (p < .001) but lower frontal bone height and fbh/sw ratio (p < .001) than PC. PCxB showed lower (p < .001) skull width in comparison to B. Comparing PCxB to PC, the cross showed lower skull height, frontal bone height and fbh/sw ratio (p < .001).
In females, a trend similar to that of males was observed between B and PC and between PCxB and B and PC, with exception of the skull width.
Crested chickens are characterised by an autosomal mutation (Cr) that promotes the development of elongated feathers on cranial skin that sprout from the head shaping the tuft. According to Wang et al. (Citation2012), the crest is promoted by the genomic sequence HOXC8 (Gene ID: 395711) that shows ectopic expression in cranial skin during embryonic development. The HOXC8 is natively expressed in the dorsal dermis (Kanzler et al. Citation1997), the ectopic expression of HOXC8 might reprogram the cranial dermis to act as dorsal dermis forming feather follicle. The crested phenotype shows a degree of sexual dimorphism, males exhibiting a more voluminous crest than females. The males crest is composed of long pointy feathers, while the females crest is rounded. Crest shows an autosomal incompletely dominant mode of inheritance, and is associated with cerebral hernia that someway gives rice to a malformation of the skull consisting in a bony protuberance in which hernia is located (Fisher Citation1934; Brandt Citation1936; Brothwell Citation1979; Frahm and Rehkamper Citation1998; Frahm et al. Citation2001; Wang et al. Citation2012). The presence of cranial protuberance is the result of adaptation to encephalic conformation of these birds. In crested chickens the brain is relatively larger, partly due to the significant enlargement of brain cavity, III and IV ventricles, partly to the enlargement of the optic tract, diencephalon, apicale and densocellulare hyperpallium, mesopallium and nidopallium (Krautwald Citation1910; Hutt Citation1949; Desmond and Jacobson Citation1977; Somes Citation1990; Frahm and Rehkamper Citation1998). The function of this enlargement is unknown (Melhorn and Rehkämper Citation2013) so that the responsible gene for the brain enlargement (Yoshimura et al. Citation2012). Cerebral hernia is closely associated with crest formation, but to date it’s still unknown if these features are controlled by the same gene or by separate loci (Cr and he). According to Yoshimura et al. (Citation2012) cerebral hernia is controlled by a single autosomal recessive gene (he), closely associated with crest formation. Crested individuals can be with or without hernia, but uncrested chickens with cerebral hernia were not shown (Yoshimura et al. Citation2012). It is reported that the cranial bony protuberance occurs in the crested species (Tegetmeier Citation1856; Darwin Citation1868; Brothwell Citation1979; Frahm and Rehkamper Citation1998; Yoshimura et al. Citation2012).
The bony surface of the cranial protuberance is not continuous, but presents openings coated by membranous structures. This is the result of an incomplete ossification occurred. The anatomical and physiological meaning of cranial openings is not known. The tuft atop the head can partially cover eyes so that the light stimulation, able to activate the hypothalamus, could be limited. Anyway this may not be a problem because in the birds, in addition to light that indirectly reaches the hypothalamus via the retina and then optic nerve, a different route can be made by the light, directly through the skull and cranial tissues (Lewis and Morris Citation2006). Avian skull and brain are very permeable to light so that a large number of photons penetrate deep into the brain and reach deep brain photoreceptors (DBPs) located within the hypothalamus (Menaker and Underwood Citation1976). The photoreceptors have been shown to sense the photoperiod and mediate seasonal reproduction (Dawson et al. Citation2001). Three DBPs were proposed to be the photo pigment involved in day length measurements, melanopsin, neuropsin/opsin 5 and vertebrate-ancient opsin (Halford et al. Citation2009; Davies et al. Citation2011; Garcıa-Fernandez et al. Citation2015; Kang and Kuenzel Citation2015). More knowledge is needed to clarify the role of frontal bony protuberance and skull openings on the physiology of brain and interactions involved with light penetration and transmission in crested birds for an adequate management of purebred and crossbred birds.
Correlations between skull parameters are shown in Table . Many significant and positive correlations were observed between many skull and head parameters. The skull height was significantly correlated to skull weight and to skull length in B (p < .01), PC (p < .01) and PCxB (p < .05). The frontal bone height was significantly and positively correlated to the skull length and skull width in B (p < .001) and PCxB (p < .05). The positive relationship between frontal bone height and skull height was significant only in B (p < .01) and PCxPA (p < .05).
Table 7. Correlations between skull and head parameters according to the genotypesTable Footnotea.
The positive and significant relationships between many skull dimensions reveal an adequate development of the skull total volume according to the somatic dimensions of the body and of the head for almost the genotypes considered. The relationship between total skull height and frontal bone height indicates that only in few genotypes an increase of the development of the total skull height corresponds to an increase of the frontal bone height. If this result is quite intuitive for B genotype that shows a frontal bone height with a mean value less than 6 mm (and only partially for PCxB), the result obtained by PCxPA that showed a mean frontal bone height of 14 mm, with many openings of little size, is relevant and might express a condition due to the crossing. In PA and PC this relationship was not evident because the frontal bone sometimes showed a large single opening at the top of the head so that it was not possible to measure the effective height of the frontal bone and of the skull (Figures and ).
The openings number showed a negative correlation to the openings size, statistically (p < .05) significant in PC and PCxPA (Table ).
As well as comb is concerned, given that only the Padovana males showed a different comb development according to each specimen, the data refers only to these groups and sex. Comb was positively correlated to skull height (p = .01), whereas no significant correlation was found between comb and frontal bone height. In B and PCxB genotypes no correlations were calculated as these genotypes have a comb completely developed and its size was not variable among the chickens.
The result can be important: if the comb is an important characters for choosing partners during the reproductive phase, as stated by Zuck et al. (Citation1992), and head appendages can be a signal for reproductive quality (Rizzi and Verdiglione Citation2015), the presence of a more evident comb in those genotypes with a reduced development of the comb due to their peculiar skull anatomy, may be considered in a genetic selection activity.
Conclusions
The results of this study add information on Padovana breed. The two Padovana varieties, PA and PC, characterised by different plumage colour, showed some somatic differences involving also the skull morphometry. Padovana camosciata at 26 weeks of age had a higher skull than PA genotype; the same skull was tendentially heavier and longer than PA. For the skull characters of PCxPA cross both the two genotypes seems to be involved and no predominant and relevant effect of only one genotype was seen. The PCxB cross, compared with Padovana genotype, showed relevant differences in the skull and head characteristics, particularly in the absence of hernia and in the presence of a more developed comb. The effect of genotype of the three Padovana groups was different for the number of openings and their size. As regards sexual dimorphism, PC, PA and PCxB males exhibited higher values for most of the considered skull parameters, but not for the bone openings characteristics, when present. The cross PCxPA did not show any sexual dimorphism with the exception of comb that resulted significantly more developed in males.
This research underlines that at about one hundred years after the study of Ghigi (Citation1916), Padovana chickens preserve the characteristics that mark this genotype. The frontal bony protuberance, the crest and the comb show dimensions and features very similar to those previously described by Ghigi.
Finally, if on the one hand the presence and characteristics of the frontal bone protuberance can be considered to recognise Padovana chickens and their cross, on the other hand for the recognising of crossbred chickens originating from the cross between Padovana and commercial hybrids, the genotype combination has to be considered time by time as the genetic transmission of the frontal bone protuberance characteristics and comb and crest could widely vary.
Acknowledgements
The Authors thank the Agricultural High School “Duca degli Abruzzi” in Padova for providing the Padovana breeders.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Avicola Berlanda. 2016. http://www.avicolaberlanda.it/Schede/gaina/gaina.html
- Baruchello M, Cassandro M. 2003. Avicoli veneti. In: Veneto Agricoltura, Settore Ricerca e Sperimentazione Agraria e Ittica, Editors. Progetto CO.VA. – Interventi per la conservazione e valorizzazione di razze avicole locali venete. http://www.venetoagricoltura.org
- Baumel JJ. 1979. Nomina Anatomica Avium. London: Academic Press; p. 53–121.
- Brandt AE. 1936. A note on dominant white and crest in poultry. J Hered. 27:79–82.
- Brothwell D. 1979. Roman evidence of a crested form of domestic fowl, as indicated by a skulls howing associated cerebral hernia. J Archaeol Sci. 6:291–293.
- Cassandro M, De Marchi M, Penasa M, Rizzi C. 2015. Carcass characteristics and meat quality traits of the Padovana chicken breed, a commercial line, and their cross. Ital J Anim Sci. 14:304–309.
- CEASA. 2015. http://www.unipd.it/comitato-etico-di-ateneo-la-sperimentazione-animale-ceasa
- Davenport CB. 1906. Inherìtance in Poultry. Washington: Publications of the Carnegie Institution.
- Davies WIL, Turton M, Peirson SN, Follett BK, Halford S, Garcia-Fernandez JM, Sharp PJ, Hawkins MW, Foster RG. 2011. Vertebrate ancient opsin photopigment spectra and the avian photoperiodic response. Biol Lett. 8:291–294.
- Darwin CR. 1868. The variation of animals and plants under domestication. 1st ed. Vol. 2, London: John Murray.
- Dawson A, King VM, Bentley GE, Ball GF. 2001. Photoperiodic control of seasonality in birds. J Biol Rhythms. 16:365–380.
- De Marchi M, Cassandro M, Lunardi E, Baldan G, Siegel PB. 2005. Carcass characteristics and qualitative meat traits of the Padovana breed of chicken. Int J Poult Sci. 4:233–238.
- Desmond ME, Jacobson AG. 1977. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol. 57:188–198.
- Fisher RA. 1934. Crest and hernia in fowls due to a single gene without dominance. Science. 80:288–289.
- Frahm HD, Rehkamper G. 1998. Allometric comparison of the brain and brain structures in the white crested polish chicken with uncrested domestic chicken breeds. Brain Behav Evol. 52:292–307.
- Frahm HD, Rehkamper G, Werner CW. 2001. Brain alterations in crested versus non-crested breeds of domestic ducks (Anas platyrhynchos f.d.). Poult Sci. 80:1249–1257.
- Francesch A, Villalba I, Cartañà M. 2011. Methodology for morphological characterization of chicken and its applicatio to compare Penedesenca and Emporadanesa breeds. Anim Genet Res. 48:79–84.
- Gallina Padovana. 2016. http://www.gallinapadovana.net/en/the-paduan-hen.php
- Garcıa-Fernandez JM, Cernuda-Cernuda R, Davies WIL, Rodgers J, Turton M, Peirson SN, Follet BK, Halford S, Hughes S, Hankins MW, et al. 2015. The hypothalamic photoreceptors regulating seasonal reproduction in birds: a prime role for VA opsin. Front Neuroendocrinol. 37:13–28.
- Ghigi A. 1916. Sulla eredità dell'ernia cerebrale dei polli in correlazione ad altri caratteri. Archivio Zoologico italiano. http://archive.org/stream/archiviozoologic08unio/archiviozoologic08unio_djvu.txt
- Ghigi A. 1939. Per l'Avicoltura Italiana. Zanichelli Bologna.
- Ghigi A. 1968. Trattato di avicoltura. UTET, Torino. p. 156–159.
- Halford S, Pires SS, Turton M, Zheng L, Gonzalez-Menendez I, Davies WL, Peirson SN, Garcıa-Fernandez JM, Hankins MW, Foster RG. 2009. VA opsin-based photoreceptors in the hypothalamus of birds. Curr Biol. 19:1396–1402.
- Hayashi Y, Nishida T, Fujioka T, Tsugiyama I, Mochizuchi K, Tomimoto M. 1982. Measurement of the skull of jungle and domestic fowls. Nippon Juigaku Zasshi. 44:1003–1006.
- Holzer F. 2013. Sulle orme della Gallina Padovana dal gran Ciuffo. Campodarsego: La Grafica Faggian.
- Hutt FB. 1949. Variations in the plumage: variations in the length of feathers: crest, Cr. In: Genetic of the fowl: the classic guide to poultry breeding and chicken genetics. New York (NY): McGraw-Hill Book Company Inc; p. 127–128.
- Ino Y, Oka T, Nomura K, Watanabe T, Kawashima S, Amano T, Hayashi Y, Okabe A, Uehara Y, Masuda T, et al. 2008. Breed differentiation among Japanese native chickens by specific skull features determined by direct measurements and computer vision techniques. Br Poult Sci. 49:273–281.
- Kang SW, Kuenzel WJ. 2015. Deep-brain photoreceptors (DBPs) involved in the photoperiodic gonadal response in an avian species, Gallus gallus. General Comparative Endocrinol. 211:106–113.
- Kanzler B, Prin F, Thelu J, Dhoually D. 1997. CHOXC-8 and CHOXD-13 expression in embryonic chick skin and cutaneous appendage specification. Dev Dyn. 210:274–287.
- Krautwald F. 1910. Die Haubeder Hühner und Enten. Ihre Ursache, Entstehung und Vererbung [Inaugural-Dissertation]. Bern: University of Bern.
- Lewis P, Morris T. 2006. Poultry lighting: the theory and practice. Andover (UK): Northcot Ed.
- Melhorn J, Rehkämper G. 2013. Some remarks on bird’s brain and behavior under theconstraints of domestication. ISRN Evolution Biol. 2013:460580.
- Menaker M, Underwood H. 1976. Extraretinal photoreception in birds. Photophysiology. 23:299–306.
- Rizzi C, Verdiglione R. 2015. Testicular growth and comb and wattles development in three Italian chicken genotypes reared under free-range conditions. Ital J Anim Sci. 14:266–271.
- Samejima M, Ito S, Fujioka T. 1988. Principal component analysis of measurements in the skull of red jungle fowl and 12 breeds of domestic folws. I. Cranium. Jpn Poult Sci. 25:222–236.
- SAS. 2010. SAS/STAT, user’s guide, version 9.2. Cary (NC): SAS Inst. Inc.
- Slow Food. 2016. http://www.fondazioneslowfood.com/it/presidi-slow-food/gallina-padovana
- Somes RG. Jr. 1990. Mutations and major variants of muscles and skeleton in chickens. In: Crawford RD, editor. Poultry breeding and genetics. Amsterdam: Elsevier B.V; p. 209–237.
- Tegetmeier WB. 1856. On the remarkable peculiarities existing in the skulls of the feather-crested variety of the domestic fowl, now known as the Polish. Proc Zool Soc Lond. 1856:366–368.
- Verdiglione R, Cassandro M. 2013. Characterization of muscle fiber type in the pectoralis major muscle of slow-growing local and commercial chicken strains. Poult Sci. 92:2433–2437.
- Yoshimura K, Kinoshita K, Mizutani M, Matsuda Y, Saito N. 2012. Inheritance and developmental pattern of cerebral hernia in the crested Polish chicken. J Exp Zool B Mol Dev Evol. 318:613–620.
- Wang Y, Gao Y, Imsland F, Gu X, Feng C, Liu R, Song C, Tixier-Boichard M, Gourichon D, Li Q, et al. 2012. The crest phenotype in chicken is associated with ectopic expression of HOXC8 in cranial skin. PLoS One. 7:e34012.
- Zuck M, Ligon JD, Thornhill R. 1992. Effect of experimental manipulation of male secondary sex characters on female mating preference in red jungle fowl. Anim Behav. 44:999–1006.