?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A two level factorial experiment (3 × 3) was designed to evaluate the effect of dietary inclusion level of probiotic and breed on productive performance, immune status and egg quality characteristics in laying hens reared under high ambient temperature. A total of 216, 32-week-old laying hens representing three different breeds (White Leghorn, Saudi black and Saudi brown) were randomly assigned to three dietary treatments (0, 200 and 400 g/t feed) in a 90-d experiment. The current results indicated that the inclusion of probiotics in layer diets did not appear to cause any adverse effects on egg production traits compared with non-treated hens. Dietary supplementation with probiotics at 400 ppm level significantly (p < .05) increased shell thickness compared to non-treated laying hens. Moreover, an improvement in eggshell quality and breaking strength in hens fed a diet containing probiotics was observed under high ambient temperature. Also, plasma cholesterol and triglyceride were reduced in laying hens fed a diet supplemented with probiotics. Furthermore, probiotic inclusion significantly increased IgM immunoglobulin concentration in laying hens fed a diet containing either 200 or 400 ppm compared with untreated hens. Concerning breed effect, Saudi black laying hens recorded significantly (p < .02) higher egg mass compared with the other breeds. Also, both Saudi chicken breeds exhibited significantly higher cell-mediated response and IgM concentration compared with Leghorn hens.
Introduction
Probiotics as feed additives are increasingly used in animal and poultry feeding in commercial manner. Improved growth performance, reduced mortality and enhanced immunocompetence broiler chickens are well defined (Attia et al. Citation2011). The effectiveness of probiotic administration in laying hens may depend on several factors, including microbial species composition, supplemental dose, method and frequency of administration, diet composition, bird’s age, genotype (breed) and environmental stress factors (Mikulski et al. Citation2012). It is well known that heat stress in laying hens has a severe negative impact on eggshell quality. Many investigators have reported that dietary probiotic supplementation could reduce heat stress in birds (Männer and Wang Citation1991; Zulkifli et al. Citation2000). It has been shown that probiotic supplementation is more beneficial during stressful conditions (Jin et al. Citation1997).
The inclusion of probiotic in the diet has been found to improve egg production and food conversion ratio in several studies (Panda et al. Citation2008; Youssef et al. Citation2013; Chung et al. Citation2015). Furthermore, giving probiotics to laying hens has been found to improve eggshell quality and reduced the number of damaged eggs (Mikulski et al. Citation2012; Zhang et al. Citation2012). Likewise, Zhang et al. (Citation2013) reported that the dietary supplementation of 0.01% probiotic improved egg production and egg quality. Conversely, some contradictorily results have been reported on the effects of probiotic supplementation on egg yield and feed efficiency (Zarei et al. Citation2011; Afsari et al. Citation2014; Sobczak and Kozłowski Citation2015).
The supplementation of probiotics to diet of laying hens may play an important role in altering the lipid metabolism of chickens. Many investigators have pointed out that probiotics could reduce the cholesterol content of egg yolk (Li et al. Citation2006; Mikulski et al. Citation2012) and serum (Kalavathy et al. Citation2003).
Probiotics have been used to stimulate the immune system in poultry. Numerous studies have concluded that using probiotics as feed additives exerts a beneficial influence on immune response and health status in both layer- and meat-type strain of chickens (Willis and Reid Citation2008; Vila et al. Citation2009; Shivaramaiah et al. Citation2011). Fong et al. (Citation2015) observed an improved immune response in the cells of healthy subjects treated with Lactobacillus ramnosus in vitro. An increase in serum bactericidal activity was observed in laying hens given a diet supplemented with probiotics (Forte et al. Citation2016). Therefore, the present study aimed at evaluating productive performance, immune status and egg quality of laying hens from different chicken breeds fed a diet supplemented with varied concentrations of probiotic under high ambient temperature.
Materials and methods
Experimental design, birds and dietary treatments
A total of 216, 36-week-old laying hens were allocated in a two-level factorial experimental design (3 × 3), consisting of three concentrations of dietary probiotic (0, 200 and 400 g/t feed) containing 4 × 109 cfu/g of Bacillus subtilis and three different breeds (White Leghorn, Saudi black and Saudi brown). Each subgroup had eight replicates (experimental cages) of three hens each. The birds were placed in wire cages (60 cm × 45 cm × 43 cm, L × W × H) under lighting schedule of 17 h/d light cycle. The average of high ambient temperature and relative humidity during the whole experimental period was 34 ± 1.5 °C and 55%, respectively. Feed and water were provided ad libitum throughout the 90-d experimental period. The basal diet was formulated to contain approximately 16.6% crude protein and 2875 ME kcal/kg in a typical layer diet (Table ). Three probiotic levels (0, 200 and 400 ppm) were mixed with diet every week. Conventional breeding and management procedures were applied throughout the experimental period. All birds were provided with similar environmental and hygienic conditions. The care and handling of the laying hens were in accordance with regulations of animal care committee of Qassim University.
Table 1. Ingredients and composition of the basal diet.
Laying performance
Feed intake was recorded on a replicate basis for the whole experimental period. Feed conversion ratio (FCR) was calculated at the end of the experiment on the basis of the amount of feed consumed in gram divided by egg mass in gram. Damaged eggs, including broken, cracked and shell-less eggs, were recorded as they occurred. All eggs were collected and recorded on a daily basis. Eggs were individually weighed and egg mass was calculated for the whole experimental period.
Egg quality
At 48 weeks of age, 48 eggs from each dietary treatment per breed were collected to assess internal and external egg quality characteristics. Egg width and egg length were measured in mm using electronic digital Vernier calliper (±0.01 mm). Egg shape was calculated according to the following formula:
Following collection, the breaking strength for intact eggs was determined in kg/cm2 using Egg Force ReaderTM, Orka Food Technology Ltd, St. Paul, MN. Also, egg weight, Haugh unit and yolk colour were measured automatically using Egg AnalyzerTM manufactured by Orka Food Technology Limited, St. Paul, MN. The liquid contents were put aside and the shell plus membranes were washed under running water to remove adhering albumen. The wet eggshell was left for 24 h at room temperature for drying and then weighed to the nearest 0.01 g. The relative weight of dry eggshell was calculated on the basis of egg weight. To measure shell thickness, pieces from three different regions (two poles and equator) of each eggshell with intact membranes were measured with a dial gauge micrometer to the nearest 0.01 mm.
Concentrations of plasma immunoglobulins
The effects of probiotic administration and breed type on the humoural status of the bird by quantifying IgA, IgM, and IgY levels in blood plasma were measured. At the end of the experiment, 10 hens per sub-group were randomly chosen and blood samples were collected from the wing vein in heparinised tubes. Blood samples were subsequently stored in ice, centrifuged at 2500 × g for 10 min at 4 °C, and the harvested plasma was stored at −20 °C until antibody analyses. Plasma IgA, IgM and IgY concentrations were determined in appropriately diluted samples by a sandwich ELISA using microtitre plates and chicken-specific IgA, IgM, and IgY ELISA quantitation kits (GenWay Biotech Inc., San Diego, CA).
Cell-mediated immunity
Cell-mediated immunity in vivo was evaluated by injection of the mitogen phytohemagglutinin-P (PHA-P) into the wattle. At 48 weeks of age, 15 laying hens from each treatment per breed were randomly assigned. Each hen was intradermally injected in the right wattle with 100 µg phytohemagglutinin-P (Sigma Chemical Co., St. Louis, MO) in 0.1 mL of sterile saline. The initial wattle thickness was measured with a constant tension calliper before injection and at 24, 48 and 72 h after PHA-P injection. The resultant swelling response in the wattle was calculated as the difference between its thickness before and after injection.
Blood biochemical analysis
At the end of the experimental period, 10 blood samples from each treatment group per breed were withdrawn for blood biochemical analysis. Plasma was harvested as mentioned above. Plasma total protein, albumen, total cholesterol and triglyceride were spectrophotometerically determined using commercial kits (Stanbio Laboratory, Boerne, TX). The globulin was calculated as the difference between the total protein and albumen. Serum triiodothyronine (T3) and thyroxine (T4) concentrations were measured using commercial ELISA kits (BioCheck®, Foster City, CA).
Statistical analysis
Data were subjected to a two-way ANOVA with probiotic level and breed as fixed effects using JMP Ver. 11 (SAS Institute Citation2013). The model applied is as follows:
where Yijk is the observation taken on the kth individual, µ is the overall mean, Pi is a fixed effect of the ith probiotic level, Bj is a fixed effect of the jth breed, (PB)ij is the interaction between probiotic level and breed, eijk is the random error assumed to be independent normally distributed with mean = 0 and variance = σ2.
Data given in percentage were subjected to arcsine transformation before statistical analysis, however, the actual data are listed. All results are presented as mean and the pooled SEM. The significance of difference among the groups was assessed using Tukey’s test. Significance was set as p < .05.
Results
Egg production traits and damaged egg ratio as affected by dietary probiotic level and breed are shown in Table . At the beginning of the experiment, there were no statistically differences in the average body weight-laying hens between dietary treatments within each breed (data not shown). During the experimental period, no hen died in each treatment within each breed. As shown in Table , there was no statistically significant difference in egg production performance among dietary treatments. Insignificant decreased feed intake (p < .08) was observed in both groups fed a diet supplemented with probiotics compared to group receiving the control diet. The lowest feed intake (9789 g) was found in group that consumed diet containing 200 ppm probiotics, while the highest (10,406 g) was recorded in the control group. There was no significant difference in FCR between hens fed probiotic-supplemented diets and hens fed the control diet. Probiotic supplementation did not affect damaged egg ratio. However, non-significant reduction in damaged eggs was detected (22% and 39% in groups supplemented with 200 and 400 ppm probiotics, respectively). With respect to breed effect, the Saudi black laying hens recorded significantly (p < .02) higher egg mass compared with the other breeds. The worst performance was found in Saudi brown breed.
Table 2. Effect of probiotic dietary inclusion and breed on egg production traits.
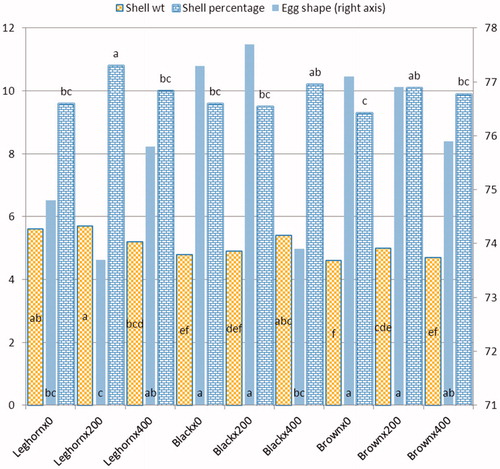
The effects of dietary probiotic supplementation and breed type on internal and external egg quality measurements of laying hens are presented in Table . No significant differences were found between the dietary groups with respect to yolk%, yolk colour and albumen quality expressed as Haugh unit score. Both probiotic levels significantly (p < .01) increased relative weight of eggshell compared with the control group. Likewise, shell thickness significantly (p < .05) improved in laying hens fed a diet containing 400 ppm probiotic when compared with those fed a basal diet. While the shell thickness of hens fed on 200 ppm probiotic was intermediate. Therefore, as a consequence, the inclusion of probiotic in both levels significantly (p < .05) increased eggshell strength compared with the control group (4.27, 4.17 and 3.82 kg/cm2, respectively). Regarding breed effect, it could be noticed that the native breeds (black and brown) laid eggs with significantly (p < .01) darker yolk colour than those of Leghorn hens. Also, they produced eggs with rounded shape compared with those of Leghorn. The brown breed recorded lower relative yolk and higher HU compared to the other two breeds. In terms of eggshell quality characteristics, the results revealed that there was no significant difference due to breed effect. As shown in Figure , a significant interaction between probiotic level and breed was observed for shell weight, shell thickness and egg-shape index. The highest shell weight and shell thickness were detected in Leghorn given 200 ppm probiotics and in black given 400 ppm probiotics.
Table 3. Effect of probiotic level and breed on internal and external egg quality characteristics of laying hens.
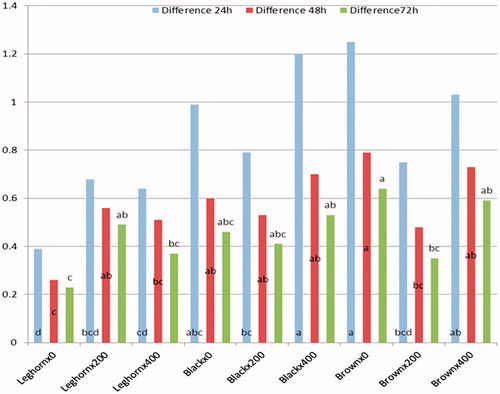
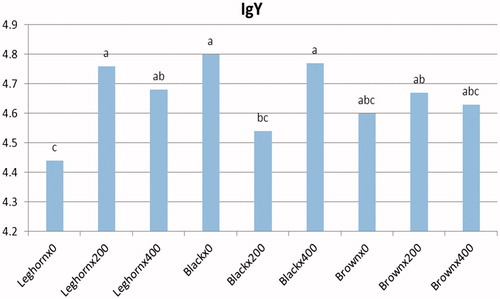
Cellular and humoural immune responses of the laying hens as affected by probiotic administration level and breed are shown in Table . The laying hens fed a diet with the level of 400 ppm inclusion resulted in a numerical improvement in cellular immune response (PHA-P) at all tested times compared with the other groups. Moreover, probiotic inclusion significantly (p < .01) increased IgM immunoglobulin concentration at both probiotic levels (200 and 400 ppm). On the contrary, this trend did not exist in both IgA and IgY immunoglobulins. In terms of breeds, the results obviously revealed that the Saudi native breeds (black and brown) had a significant increase in swelling response, particularly at earlier stage of PHA-P test. Concerning the interaction between breed and probiotic level, a significant effect on cell-mediated immunity was found. The best swelling responses at 24, 48 and 72 h were detected in black ×400 and brown ×0 (Figure ). The highest IgY concentration was detected in Leghorn× 200, black ×0 and black ×400 (Figure ).
Table 4. Effect of probiotic level and breed on cell mediated response and plasma immunoglobulins concentration of laying hens.
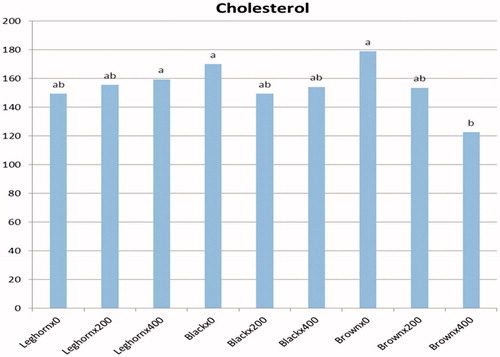
Biochemical blood plasma parameters as affected by probiotic concentration and type of breed in laying hens are presented in Table . Plasma total protein, albumen and globulin concentrations were not significantly affected due to dietary probiotic supplementation. On the basis of breed factor, there were significant differences among breeds for albumen and globulin, while no significant difference was observed in total protein. After 90-d of probiotic supplementation, a significant difference was found in blood cholesterol. As shown in Table , cholesterol level was significantly (p < .05) reduced by probiotic inclusion. This decrease was associated with increased dietary probiotic concentration. On the one hand, blood cholesterol level was significantly affected by the interaction between probiotic level and breed (Figure ). The lowest cholesterol level was found in brown breed fed a diet supplemented with 400 ppm probiotics. On the other hand, probiotic supplementation had no effect on serum triglyceride level. There were no significant differences in cholesterol and triglyceride due to breed effect. The statistical analysis revealed that there was no interaction between dietary treatment and breed for all blood parameters except for cholesterol level (Table ). In terms of thyroid hormones, no significant differences in blood plasma T3 and T4 concentrations were observed among treatments or breeds.
Table 5. Effect of probiotic level and breed on biochemical blood plasma parameters of laying hens.
Discussion
Laying performance
In congruent with our findings, it was speculated that FCRs were not affected by probiotics in some reports (Nahashon et al. Citation1994; Mohan et al. Citation1995; Tortuero and Fernández Citation1995). Similarly, numerous studies have concluded that probiotics used as feed additives did not affect egg production traits (Arpášová et al. Citation2012; Afsari et al. Citation2014; Sobczak and Kozłowski Citation2015). Beneficial effects on the damaged egg ratio resulting from probiotic supplementation are in agreement with the report of Balevi et al. (Citation2001), who indicated a similar probiotic supplementation. On the one hand, we suggest that the increase in shell thickness percentage and in turn, the reduction in number of damaged eggs in hens receiving probiotics may have been caused by increase calcium retention. This hypothesis was suggested by Balevi et al. (Citation2001). On the other hand, several studies confirmed that the inclusion of probiotic to the diet has been found to improve egg production and feed conversion ratio (Panda et al. Citation2008; Youssef et al. Citation2013; Chung et al. Citation2015). However, the difference between the present study and the previous reports may be related to differences in probiotic level, breed type and the hen’s age. Significant interaction between probiotic level and breed type in egg mass confirms that performance of laying hens receiving probiotics may be differ according to genotype or breed used in the current experiment.
Egg quality assessment
Our findings have been confirmed by many reports (Mikulski et al. Citation2012; Zhang et al. Citation2012; Sobczak and Kozłowski Citation2015). Conversely, Mahdavi et al. (Citation2005) and Mohebbifar et al. (Citation2013) did not find significant effects for dietary probiotic inclusion on egg quality. Probiotic supplementation seems to have a positive effect on the eggshell percentage. A positive effect of probiotic supplementation on eggshell quality characteristics has been reported (Li et al. Citation2006; Abdelqader et al. Citation2013). Świątkiewicz et al. (Citation2010) attributed the positive effects of probiotics on eggshell quality parameters to the increased intestinal availability of Ca. The beneficial effects on eggshell thickness and strength observed in the current study were directly associated with the reduction in the number of damaged eggs. Under heat stress, it is well known that the eggshell strength is impaired and in turn, the incidence of cracked eggs exceeded. Therefore, many researchers have focussed their investigations toward improving eggshell quality using genetic, nutritional and biological approaches. Probiotic supplementation is the effective applied procedure in birds suffering from several stressors. Insignificant results of eggshell quality due to breed effect are in consistence with the results of Najib and Al-Yousef (Citation2014) and Al-Homod (Citation2016).
Immune response
Inflammatory response to PHA-P injected in wattle, as an indication for cell-mediated immunity, did not differ significantly as affected by probiotic supplementation level. Immunoglobulin (chicken IgY, IgA and IgM) levels in serum are indicative of the humoural immune status of chickens (Mountzouris et al. Citation2010). As well established, IgM is a potent complement activating antibody and since complement is needed for the generation of a normal antibody response (Parmentier et al. Citation2004). Generally, enhancing immunity resulting from probiotic administration has been reported by many investigators (Fong et al. Citation2015; Attia et al. Citation2017). Laying hens received a diet containing multi strains of probiotics exhibited a higher antibody production against SRBC compared with non-treated hens during high environmental temperature (Asli et al. Citation2007). Higher antibody production against Newcastle disease virus (NDV) was observed in the group fed a diet supplemented with Lactobacillus acidophilus compared to the control group (Forte et al. Citation2016). Under heat stress, dietary supplementation of probiotics improved humoural immunity against Newcastle disease virus and infectious bursal disease virus (Sohail et al. Citation2010). Contrarily, probiotic supplementation did not affect specific antibody synthesis to ND vaccine antigen administered via drinking water (Balevi et al. Citation2001). This non-stimulation of humoural immunity by probiotic may be attributable to the non-host specific strains, species or even genera of the microorganisms. Perdigon et al. (Citation1990) showed that treating lagers with Lactobacillus supplementation increased cellularity of Peyer’s patches and this indicated a stimulation of the mucosal immune system, which responds to antigenic stimuli by secreting immunoglobulin (IgA). Diet supplemented with 0.1% Lactobacillus acidophilus induced the best immune response compared with 0.05% Bacillus subtilis in laying hens (Forte et al. Citation2016). The improvement in immune response found in Saudi chicken breeds compared with Leghorn chickens is in agreement with the results of Fathi et al. (Citation2017). Likewise, Saudi laying hens significantly (p < .01) recorded higher IgM concentration compared to Leghorn counterparts. In consistent with our results, Osei-Amponsah et al. (Citation2013) confirmed that the local Ghanaian ecotypes of chickens were superior to exotic breeds in terms of their ability to respond to SRBC antigens. In a comparison of humoural immunity due to breed, Fathi et al. (Citation2017) reported that there was no significant difference among breeds (Saudi, Leghorn and Lohmann) for antibody levels against Newcastle disease virus vaccine. Regarding the other types of immunoglobulins (IgA and IgY), the current results did not show significant differences among breeds. Contrary to our results, Mountzouris et al. (Citation2010) reported that the use of probiotics may modulate the systemic immune system by increasing the total levels of serum IgG in broilers and be indicative of the overall humoural immune status of the bird. Koenen et al. (Citation2002) also explored the effects of probiotics in the systemic humoural immune response, and found that different Lactobacillus spp. increase the levels of IgG in laying hens.
Blood plasma biochemical concentrations
The present findings are in agreement with those of Dimcho et al. (Citation2005), who found that probiotic inclusion did not affect the serum total protein concentration of chickens. Also, Alkhalf et al. (Citation2010) postulated that the serum concentrations of total protein and albumin were not significantly affected by dietary probiotic supplementation. The levels of total protein, albumin and globulin obtained in the present study are within the normal physiological values reported by Meluzzi et al. (Citation1992) and Attia et al. (Citation2011, Citation2016). It is generally known that blood plasma total protein plays key roles in the maintenance of colloid osmotic pressure, as a rapid substitute for indispensable amino acids, assuring glucose through gluconeogenesis, in transport of minerals and hormones, in forming enzymes and the immune system in the organism. Therefore, blood plasma proteins have an exceptional significance in homeostasis maintenance. Moreover, albumen serves as the most favourable source of amino acids for synthesis of tissue proteins in the period of quick somatic growth of birds, especially under feed restricted conditions (Yaman et al. Citation2000; Filipoviae et al. Citation2007).
Reducing blood cholesterol level due to probiotic inclusion has also been reported in broilers fed a diet supplemented with prebiotic and probiotic (Sohail et al. Citation2010) and layers supplemented with probiotic (Kurtoglu et al. Citation2004; Sobczak and Kozłowski Citation2015). On the one hand, manipulation of enteric microflora with probiotics may also play an important role in altering the lipid metabolism of chickens as various studies have shown that probiotics could reduce cholesterol content in egg yolk and serum (Jin et al. Citation1998; Kalavathy et al. Citation2003; Li et al. Citation2006). On the other hand, Kurtoglu et al. (Citation2004), Zarei et al. (Citation2011) and Mohebbifar et al. (Citation2013) reported that the dietary probiotic supplementation did not affect serum cholesterol or triglyceride. Insignificantly decreased serum triglyceride level is in consistency with Zhang et al. (Citation2012) who reported that using Bacillus subtilis at level of 400 g/t of diet significantly (p < .05) reduced serum triglyceride level (1760.6 mg·dL−1) compared with untreated group (2158.8 mg·dL−1). Our results concerning blood plasma T3 and T4 concentrations in laying hens fed a diet supplemented with probiotics are in agreement with Chotinsky and Mihaylov (Citation2013), who noted that the quantity of T4 did not change significantly, while blood serum T3 concentration increased with the supplementation of Lactobacillus acidophilus in the diets of broiler chickens. Plasma thyrotrophin levels were not significantly affected due to supplementation of Lactobacillus acidophilus in the diets of broiler chickens (Chotinsky and Mihaylov Citation2013). On the contrary, Sohail et al. (Citation2010) revealed that dietary probiotic supplementations significantly increased (p < .05) serum T4 concentration without affecting T3 concentration. Also, Aluwong et al. (Citation2013) reported that there was highly significant (p < .05) difference in T4 level for probiotic treated group when compared with the control one. According to breed effect, it could be noticed that there were no significant differences in plasma T3 and T4 concentrations among breeds. The thyroid gland is an endocrine organ found in all vertebrates. The thyroid hormones are primarily involved in energy production by increasing the metabolic rate. It is well known that thyroid activity is important in controlling metabolic rate (Reyns et al. Citation2002). Bobek et al. (Citation1976) showed that T3 is the main thyroid hormone regulating oxygen consumption (bio-oxidation processes in cells), particularly in young chickens. Klandorf et al. (Citation1981) confirmed that T3 is, in chickens, a metabolically more active substance than T4.
Conclusions
In conclusion, diet supplementation with probiotics did not improve laying performance. A beneficial effect resulting from probiotic supplementation on eggshell quality was observed without affecting internal egg quality traits under high ambient temperature. Also, plasma cholesterol and triglyceride reduced in laying hens fed a diet supplemented with probiotics. Furthermore, probiotic inclusion significantly increased IgM immunoglobulin concentration in laying hens fed a diet containing either 200 or 400 ppm compared with unsupplemented hens. Concerning breed effect, Saudi black laying hens recorded significantly higher egg mass compared with the other breeds. Also, both Saudi chicken breeds exhibited higher cell-mediated response and IgM concentration compared with Leghorn hens.
Disclosure statement
The authors alone are responsible for the content and writing of this article.
References
- Abdelqader A, Irshaid R, Al-Fataftah AR. 2013. Effects of dietary probiotic inclusion on performance, eggshell quality, cecal microflora composition, and tibia traits of laying hens in the late phase of production. Trop Anim Health Prod. 45:1017–1024.
- Afsari M, Mohebbifarn A, Torki M. 2014. Effects of dietary inclusion of olive pulp supplemented with probiotics on productive performance, egg quality and blood parameters of laying hens. Annu Res Rev Biol. 4:198–211.
- Al-Homod AH. 2016. Characterization of Saudi chicken breeds for productive performance and egg quality [master’s thesis]. Qassim University, Saudi Arabia.
- Alkhalf A, Alhaj M, Al-Homidan I. 2010. Influence of probiotic supplementation on blood parameters and growth performance in broiler chickens. Saudi J Biol Sci. 17:219–225.
- Aluwong T, Hassan F, Dzenda T, Kawu M, Ayo J. 2013. Effect of different levels of supplemental yeast on body weight, thyroid hormone metabolism and lipid profile of broiler chickens. J Vet Med Sci. 75:291–298.
- Arpášová H, Haščík P, Kačániová M, Gálik B. 2012. The effect of probiotic preparation enriched with selenium on performance parameters of laying hens. Anim Sci Biotechnol. 45:17–23.
- Asli MM, Hosseini SA, Lotfollahian H, Shariatmadari F. 2007. Effect of probiotics, yeast, vitamin e and vitamin c supplements on performance and immune response of laying hen during high environmental temperature. Int J Poult Sci. 6:895–900.
- Attia YA, Abd El-Hamid E, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, Sahin K, Abou-Shehema BM. 2016. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. Springerplus. 5:1619.
- Attia YA, Al-Khalifa H, Ibrahim MS, Abd Al-Hamid AE, Al-Harthi MA, El-Naggar A. 2017. Blood hematological and biochemical constituents, antioxidant enzymes, immunity and lymphoid organs of broiler chicks supplemented with propolis, bee pollen and mannan oligosaccharides continuously or intermittently. Poult Sci. 96:4182–4192.
- Attia YA, Zeweil HS, Alsaffar AA, El-Shafy AS. 2011. Effect of non-antibiotic feed additives as an alternative to flavomycin on broiler chickens production. Archiv Geflügel. 75:S40–S48.
- Balevi T, Ucan US, Coskun B, Kurtoglu V, Cetingul IS. 2001. Effect of dietary probiotic on performance and humoral immune response in layer hens. Br Poult Sci. 42:456–461.
- Bobek S, Jastrzebski M, Pietras M. 1976. Age-related changes in oxygen consumption and plasma thyroid hormone concentration in the young chicken. Gen Comp Endocrinol. 31:169–174.
- Chotinsky D, Mihaylov R. 2013. Effect of probiotics and Avotan on the level of thyroid hormones in the blood plasma of broiler chickens. Bulg J Agric Sci. 19:817–821.
- Chung SH, Lee J, Kong C. 2015. Effects of multi strain probiotics on egg production and quality in laying hens fed diets containing food waste product. Int J Poult Sci. 14:19–22.
- Dimcho D, Boicheva S, Simeonova T, Vlaikova T. 2005. Effect of feeding Lactina probiotic on performance, some blood parameters and caecal microflora of mule ducklings. Trakia J Sci. 3:22–28.
- Fathi MM, Al-Homidan I, Motawei MI, El-Zarei MF, Abou-Emera OK. 2017. Evaluation of genetic diversity of Saudi native chicken populations using microsatellite markers. Poult Sci. 96:330–336.
- Filipoviae N, Stojeviae Z, Milinkoviae-Tur S, Ljubiae BB, Zdelar-Tuk M. 2007. Changes in concentration and fractions of blood serum proteins of chickens during fattening. Vet Arhiv. 77:319–326.
- Fong FLY, Kirjavainen P, Wong VHY, El-Nezami H. 2015. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J Funct Foods. 13:71–79.
- Forte C, Acuti G, Manuali E, Casagrande Proietti P, Pavone S, Trabalza-Marinucci M, Moscati L, Onofri A, Lorenzetti C, Franciosini MP. 2016. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult Sci. 95:2528–2535.
- Jin LZ, Ho YW, Abdullah N, Jalaludin S. 1997. Probiotics in poultry: modes of action. World’s Poult Sci J. 53:351–368.
- Jin LZ, Ho YW, Abdullah N, Jalaludin S. 1998. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult Sci. 77:1259–1265.
- Kalavathy R, Abdullah N, Jalaludin S, Ho YW. 2003. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br Poult Sci. 44:139–144.
- Klandorf H, Sharp PJ, Newcomer WS. 1981. The influence of feeding patterns on daily variation in the concentrations of plasma thyroid hormones in the hen. IRC Med Sci. 9:82.
- Koenen ME, Heres L, Claassen E, Boersma WJ. 2002. Lactobacilli as probiotics in chicken feeds. Biosci Microflora. 2:209–216.
- Kurtoglu V, Kurtoglu F, Seker E, Coskun B, Balevi T, Polat ES. 2004. Effect of probiotic supplementation on laying hen diets on yield performance and serum and egg yolk cholesterol. Food Addit Contam. 21:817–823.
- Li L, Xu CL, Ju C, Ma Q, Hao K, Jin ZY, Li K. 2006. Effects of a dried Bacillus subtilis culture on egg quality. Poult Sci. 85:364–368.
- Mahdavi AH, Rahmani HR, Pourreza J. 2005. Effect of probiotic supplements on egg quality and laying hens performance. Int J Poult Sci. 4:488–492.
- Männer K, Wang K. 1991. Effectiveness of zinc bacitracin on production traits and energy metabolism of heat-stressed hens compared with hens kept under moderate temperature. Poult Sci. 70:2139–2147.
- Meluzzi A, Primiceri G, Giordani RA, Fabris G. 1992. Determination of blood constituents reference values in broilers. Poult Sci. 71:337–345.
- Mikulski D, Jankowski J, Zdunczyk Z, Juskiewicz J, Slominski B. 2012. The effect of different dietary levels of rapeseed meal on growth performance, carcass traits, and meat quality in turkeys. Poult Sci. 91:215–223.
- Mohan B, Kadirvel R, Bhaskaran M, Natarajan A. 1995. Effect of probiotic supplementation on serum/yolk cholesterol and on egg shell thickness in layers. Br Poult Sci. 36:799–803.
- Mohebbifar A, Kashani H, Afsari M, Torki M. 2013. Effects of commercial prebiotics and probiotics on performance of laying hens, egg traits and some blood parameters. Annu Rev Res Biol. 3:921–934.
- Mountzouris KC, Tsitrsikos P, Palamidi I, Arvaniti A, Mohnl M, Schatzmayr G, Fegeros K. 2010. Effects of probiotic inclusion levels in broiler nutrition on growth performance, nutrient digestibility, plasma immunoglobulins, and cecal microflora composition. Poult Sci. 89:58–67.
- Nahashon SN, Nakaue HS, Synder SP, Mirosh LW. 1994. Performance of single comb white leghorn layers fed corn–soybean meal and barley–corn–soybean meal diets supplemented with a direct-fed microbial. Poult Sci. 73:1712–1723.
- Najib H, Al-Yousef YM. 2014. Egg size of Saudi local layers as affected by line of the bird (body weight at sexual maturity) and dietary fat level. Int J Poult Sci. 13:442–448.
- Osei-Amponsah R, Boa-Amponsem K, Kayang BB, Naazie A. 2013. Characterization of primary immune response in Ghanaian local, Sasso T-44 and broiler chickens to sheep red blood cell antigens. Anim Genet Res. 53:51–55.
- Panda AK, Rao SSR, Raju MVLN, Sharma SS. 2008. Effect of probiotic (Lactobacillus sporogenes) feeding on egg production and quality, yolk cholesterol and humoral immune response of White Leghorn layer breeders. J Sci Food Agric. 88:43–47.
- Parmentier H, Lammers KA, Hoekman JJ, Reilingh GV, Zaanen ITA, Savelkoul HFJ. 2004. Different levels of natural antibodies in chickens divergently selected for specific antibody responses. Dev Comp Immunol. 28:39–49.
- Perdigon G, Nader De Macias MEN, Alvarez S, Oliver G, Pesce De Ruiz Holgado AA. 1990. Prevention of gastrointestinal infection using immunological methods with milk fermented with Lactobacillus casei and Lactobacillus acidophilus. J Dairy Res. 57:255–264.
- Reyns GE, Janssens KA, Buyse J, Kuhn ER, Darras VM. 2002. Changes in thyroid hormone levels in chicken liver during fasting and refeeding. Comp Biochem Physiol B Biochem Mol Biol. 132:239–248.
- SAS Institute. 2013. JMP Version 11. User’s Guide, SAS Institute Inc., Cary, NC.
- Shivaramaiah S, Pumford NR, Morgan MJ, Wolfenden RE, Wolfenden AD, Torres-Rodríguez A, Hargis BM, Téllez G. 2011. Evaluation of Bacillus species as potential candidates for direct-fed microbials in commercial poultry. Poult Sci. 90:1574–1580.
- Sobczak A, Kozłowski K. 2015. The effect of a probiotic preparation containing Bacillus subtilis ATCC PTA-6737 on egg production and physiological parameters of laying hens. Ann Anim Sci. 15:711–723.
- Sohail MU, Ijaz A, Yousaf MS, Ashraf K, Zaneb H, Aleem M, Rehman H. 2010. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult Sci. 89:1934–1938.
- Świątkiewicz S, Koreleski J, Arczewska A. 2010. Laying performance and eggshell quality in laying hens fed diets supplemented with prebiotics and organic acids. Czech J Anim Sci. 55:294–306.
- Tortuero F, Fernández E. 1995. Effects of inclusion of microbial cultures in barley-based diets fed to laying hens. Anim Feed Sci Technol. 53:255–265.
- Vila B, Fontgibell A, Badiola I, Esteve-Garcia E, Jiménez G, Castillo M, Brufau J. 2009. Reduction of Salmonella enterica var. enteritidis colonization and invasion by Bacillus cereus var. toyoi inclusion in poultry feeds. Poult Sci. 88:975–979.
- Willis WL, Reid L. 2008. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult Sci. 87:606–611.
- Yaman MA, Kita K, Okumura J. 2000. Different responses of protein synthesis to refeeding in various muscles of fasted chicks. Br Poult Sci. 41:224–228.
- Youssef AW, Hassan IM, Ali IM, Mohamed MA. 2013. Effect of probiotics, prebiotic and organic acids on layer performance and egg quality. Asian J Poult Sci. 7:65–74. http://docsdrive.com/pdfs/academicjournals/ajpsaj/0000/54857-54857.
- Zarei M, Ehsani M, Torki M. 2011. Dietary inclusion of probiotics, probiotics and symbiotic and evaluating performance of laying hens. Am J Agric Boil Sci. 6:249–255.
- Zhang ZF, Cho JH, Kim IH. 2013. Effects of Bacillus subtilis UBT-MO2 on growth performance, relative immune organ weight, gas concentration in excreta, and intestinal microbial shedding in broiler chickens. Livest Sci. 155:343–347.
- Zhang ZF, Zhou TX, Ao X, Kim IH. 2012. Effects of β-glucan and Bacillus subtilis on growth performance, blood profiles, relative organ weight and meat quality in broilers fed maize soybean meal based diets. Livest Sci. 150:419–424.
- Zulkifli I, Abdulllah N, Azrin NM, Ho YW. 2000. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br Poult Sci. 41:593–597.