Abstract
To evaluate the effects of weaning age on intestinal mucosal morphology, expressions of tight junction proteins and inflammatory cytokines, as well as secretory IgA (sIgA) of Wuzhishan piglets, a total of 96 piglets at 1-day old with an average body weight of 0.56 ± 0.07 kg were randomly divided into four treatments with four replicates per treatment and 6 pigs per replicate. Piglets in those four treatments were weaned at the age of 21(21W), 28(28W), 35 (35W) and 42 (42W) days, respectively. Blood sample was collected for determining plasma diamine oxidase (DAO) and D-lactate at 28, 35, 42, 49 and 56 days old. The jejunum and ileum segments were collected for morphology analysis, and mucosa samples were collected for Real-time PCR and sIgA measurements at 56 days old. The results showed that: compared with the 21W groups, piglets in 35W and 42W groups significantly decreased DAO activity and D-lactate level at 28 to 35 days and 56 days old (p < .05), increased mRNA levels of occludin and zonula occluden-1 (ZO-1) in jejunal and ileal mucosa (p < .05) and claudin in ileal mucosa (p < .05) and reduced the sIgA contents in jejunum and ileum (p < .05) at 56 days old. By contrary, the intestinal mucosal morphology and mRNA levels of cytokines in jejuna and ileal mucosa at 56 days old were not significantly different among the treatments (p > .05). Results suggest that weaning at 35 or 42 days old may be beneficial to intestinal barrier function, whereas weaning at 21 days old promoted sIgA expression.
Introduction
Weaning is a stressful period in swine production. At weaning, pigs must rapidly adapt to the sudden psychological, environmental, social and nutritional changes (Boudry et al. Citation2004; Moeser et al. Citation2007). The combined effects of these stresses changed intestinal structures and functions. As a result, the digestive and absorptive capacity of small intestine is impaired, leading to poor growth performance such as reduced feed intake, slow growth rate and reduced feed conversion of post-weaning piglets (Hedemann and Jensen Citation2004). In addition, with the withdrawal of immunoregulatory and immunoprotective components of maternal milk, pigs at weaning often suffer from pathogenic microorganisms, resulting in increased incidence of diarrhoea (Pluske Citation2013; Rist et al. Citation2013). Moreover, weaning is associated with intestinal barrier dysfunction (van Beers-Schreurs et al. Citation1998; Smith et al. Citation2010a), increased expression of pro-inflammatory cytokines (Pié et al. Citation2004), decreased enzymatic activities (Montagne et al. Citation2007), diminished immune responses and disturbed intestinal microbiota (McLamb et al. Citation2013).
The weaning age has declined over time in modern swine production systems (Yang et al. Citation2016). Early weaning (21 d of weaning age or less) may shorten the cycle of sows farrowing, increase birth rate and reduce potential of transferring growth-depressing pathogens from sows to piglets (Butler et al. Citation2008). However, early weaning may also result in post-weaning diarrhoea, mainly related to substantial changes in the intestinal bacterial community (García et al. Citation2016). Pigs weaned later (five to seven weeks) could have more time to develop active immunity and improve intestinal barrier function, and hence increase post-weaning growth performance (Cera et al. Citation1988; Coffey et al. Citation2000; Van der Meulen et al. Citation2010). Furthermore, the demand for artificial antibiotics could be decreased if weaning is delayed (Do Citation2012). Main et al. (Citation2004) reported that increasing weaning age up to 21.5 d was effective in improving wean-to-finish growth performance of pig in multi production sites. Do (Citation2012) found that Berkshire pigs weaning at 32 days old was conclusively beneficial to growth performance. Moreover, it is noticeable that early weaning has significant and long-term effects on intestinal functions and post-weaning growth performance (Tokach et al. Citation1992; Smith et al. Citation2010a). Therefore, choosing suitable weaning age is very important for maintaining normal functions of small intestine and promoting growth and development of post-weaning piglets in swine production.
Wuzhishan pig, a miniature pig breed originated from Hainan Island of China, is famous for its typical body weight and size. Because of its similarity with human in anatomy, physiology and pathology, Wuzhishan pigs have been used as a promising means for applications in surgery, tissue engineering, and xenotransplantation (Hou et al. Citation2010; Fang et al. Citation2012). The Wuzhishan pigs are also used as food animals because of several excellent characteristics such as strong resistance, genetic stability, low-fat content, rich amino acid content and a thick back (Hou et al. Citation2015). To date, research on the effect of weaning age on Wuzhishan piglets has not been reported previously due to the backward breeding technology and other factors. The present study was conducted to investigate the effects of weaning age on intestinal morphology, expression of tight junction proteins and inflammatory cytokines, as well as intestinal mucus sIgA in Wuzhishan piglets.
Materials and methods
Animal, diets and experimental design
All experimental procedures were approved by the Animal Management Rules of the Ministry of Health of the People’s Republic of China and Institutional Animal Care and Use Committee of Chinese Academy of Tropical Agricultural Sciences, Hainan, China. A total of 96 piglets from12 litters, with an average weight of 0.56 ± 0.07 kg at 1-day old were randomly assigned into four treatments with 24 piglets per treatments (4 replicates per treatment and 6 pigs per replicate). Piglets in those four groups were weaned at the age of 21(21W), 28(28W), 35 (35W) and 42 (42W) days, respectively. All piglets had free access to feed from 8 days of age onward. Before weaning, piglets were kept with their sows in conventional farrowing pens and suckled. At weaning, the sows were moved away, and the piglets remained in the pens. After five days post-weaning, they were moved from farrowing pens to nursery pens and kept in groups of littermates. Weaning piglets were offered ad libitum access to water and standard nursery diet according to the Wuzhishan pig breeding technology discipline (DB46/T 88-2007) (The local standards of Hainan province Citation2007). Room lighting, temperature and humidity in the farrowing and nursery buildings were all met the requirement of piglets.
Sample collection and preparation
At 28, 35, 42, 49 and 56 days old, eight piglets per treatment (2 pigs per replicate, male and female in half) were randomly selected. Prior to morning feeding, a 5 mL blood sample was collected from each piglet from the anterior vena cava into tubes containing sodium heparin and centrifugation at 3000 × g for 15 min at 4 °C to obtain plasma. Plasma samples were transferred to Eppendorf tubes, snap-frozen in liquid nitrogen and stored at −80 °C for plasma DAO and D-lactate analyses.
At 56 days old, five piglets per treatment (2 pigs per replicate, male and female in half) were randomly selected and sacrificed by injection of sodium pentobarbital solution (50 mg/kg of body weight). Their small intestine was removed and flushed with normal physiological saline. The divided mid-jejunum (10 cm before the end of jejunal Peyer’s patches) and ileum (10 cm from the ileal-caecal junction) segments were fixed in 4% paraformaldehyde for 24 h for subsequent histological assays. Jejunal and ileal mucosa samples were scraped with a smooth glass rod, transferred to Eppendorf tubes, snap frozen in liquid nitrogen and stored at −80 °C for subsequent RNA isolation.
Chemical analysis
Plasma DAO activity and D-lactate level were determined by enzymatic spectrophotometry using reagent kits in accordance with the manufacturer’s instructions (Jiancheng Bioengineering Institute of Nanjing, Nanjing, Jiangshu, China).
Jejunal and ileal morphology and histology analysis
The jejunal and ileal histomorphology was determined as previously described (Xun et al. Citation2015). Briefly, following the fixing, the segments were embedded with paraffin following standard procedures and sectioned at 5 µm on a rotary microtome. Then, the sections were stained with haematoxylin and eosin and examined under a light microscope (Olympus) using an image processing system (version 1; Leica Imaging Systems Limited). A total of 10 well-oriented crypt-villus units were selected for each intestinal cross-section, and the means of these measurements was calculated.
Relative quantitative real-time PCR
Total RNA was isolated from jejunal and ileal tissues using RNAiso Plus (Takara, China) according to the manufacturer’s instructions. The quality and quantity of RNA samples were ensured by 1% agarose gel electrophoresis and the ratio of OD260nm to OD280nm ranging from 1.8 to 2.0. Synthesis of complementary DNA (cDNA) was performed using RT Reagents (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian China) according to the manufacturer’s instructions. The generated cDNA was used as PCR templates to evaluate gene expression using relative quantitative real-time PCR performed using SYBR Premix Ex Taq reagent (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) and PIKO-RT 96 Real-Time PCR Detection System (Thermo Fisher scientific, American). The sequences of primers were listed in Table . Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene to correct the variances in target gene transcript levels. The reaction was performed in a 10 μL system containing 1 μL cDNA template, 5 μL SYBR Green mix, 0.2 μL Rox, 3 μl distilled H2O, and 0.4 μL each of forward and reverse primers at the conditions of 95 °C for 7 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s and a final dissociation step from 60 to 95 °C at a heating rate of 0.2 °C per second. The results were presented as fold changes using the 2−ΔΔCT method as previously described (Xun et al. Citation2015).
Table 1. Sequences of primers used for real-time PCR.
Measurement of intestinal mucus secretory IgA
The intestinal mucus secretory IgA (sIgA) was determined by using an ELISA kit (Cusabio Biotech Company, Wuhan, China). Briefly, 0.1 g of jejunal or ileal mucus was suspended in 1 ml of 0.01 M PBS containing 2 mg/mL protease inhibitor in an Eppendorf tube, the supernatant was collected by centrifugation at 3500 ×g for 15 min at 4 °C and sIgA concentration was determined in accordance with the manufacturer’s instruction.
Statistical analysis
Statistical analysis was performed by the one-way ANOVA procedure of SPSS 17.0 (SPSS, Inc.). Differences among treatments were compared using Duncan’s multiple range tests. Statistical significance was set at p < .05.
Results
Intestinal morphology
Morphological measurements of small intestinal mucosa in pigs are shown in Table . The results showed no significant differences in villus height (p > .05), villus width (p > .05), crypt depth (p > .05) and villus hight:crypt depth ratio (p > .05) in jejunum and ileum among different treatment groups.
Table 2. Effect of weaning age on jejunal and ileal morphology of Wuzhishan piglets at 56 days old.
Intestinal mucosal permeability
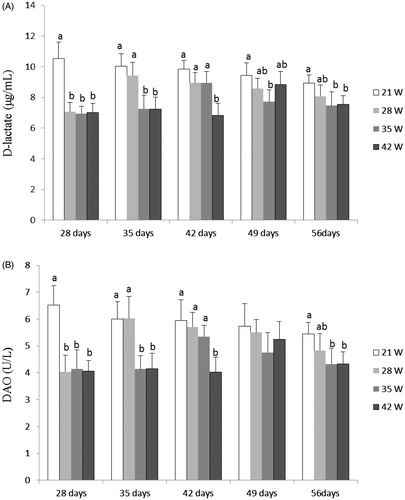
Figure shows the DAO activities and D-lactate levels in plasma. Compared with those in 21W group, D-lactate levels were significantly decreased in 35W at 28 to 35 days old and 49 to 56 days old and 42W groups at 28 to 35 days old and 56 days old (p < .05), and DAO activities were lower in 35W and 42W groups at 28 to 35 days old and 56 days old (p < .05). There was no significant difference (p > .05) in DAO activities and D-lactate levels between 35W and 42W groups except for piglets at 42 days old.
Figure 1. Effects of weaning age (21W, 28W, 35W, 42W) on plasma D-lactate level (A) and DAO activity (B) in piglets at 28, 35, 42, 49 and 56 day old (n = 8 pigs per treatment). a,b,cDifferent letters within a row indicate a significant difference (p < .05) between them. Values are means, with standard deviation represented by vertical bars.
Expression of tight junction proteins
Table shows the expression levels of occludin, ZO-1 and claudin mRNA in jejunal and ileal mucosa. Compared with those in 21W group, mRNA levels of occludin (p < .05) and ZO-1 (p < .05) in jejunal mucosa as well as occludin (p < .05), ZO-1 (p < .05) and claudin (p < .05) in ileal mucosa were higher in 35W and 42W groups, while mRNA level of claudin in jejunal mucosa was not affected by weaning ages (p > .05). No significant differences (p > .05) of these indicators were observed between 35W and 42W groups.
Table 3. Effect of weaning age on the relative expression levels of Claudin-1, Occludin and ZO-1 mRNA in the intestine mucosa of Wuzhishan piglets at 56 days old.
Expression of inflammatory cytokines
The mRNA levels of cytokines in jejuna and ileal mucosa are shown in Table . The mRNA levels of IL-1β, TNF-α, IL-10 and IL-6 in jejuna and ileal mucosa were not significantly different among all treatments (p > .05).
Table 4. Effects of weaning age on the relative expression levels of cytokines mRNA in intestinal mucosa of Wuzhishan piglets at 56 days old.
Intestinal mucus secretory IgA
Table presents the intestinal mucus sIgA concentration. The results showed that sIgA concentrations in jejunum and ileum were higher in 21W group than 35W and 42W groups (p < .05), but not significantly different between 35W and 42W groups (p > .05).
Table 5. Effects of weaning age on contents of intestinal mucus sIgA in Wuzhishan piglets at 56 days old (μg/g).
Discussion
Small intestine is the major site for digestion and absorption of nutrients, and intestinal mucosa plays an important role in this process. Villus height, crypt depth and villus hight: crypt depth ratio are indicators reflecting gut health status in piglets, and commonly used for evaluation of intestinal morphology (Liu et al. Citation2012; Han et al. Citation2013). During weaning, the intestine structure and morphology of piglets will undergo profound changes including villous shortening and crypt elongation, which reduce the nutrients digestion and absorption in weaned piglets (Barszcz and Skomiał Citation2011; Pluske Citation2013). In our experiment, intestinal morphology of piglets was measured at 56 days old and no differences were observed in villus height, villus width, crypt depth and villus hight:crypt depth ratio of jejunum and ileum among different treatments, implying that the intestinal morphology may have returned to preweaning values among different treatments. Similarly, Hu et al. (Citation2013) investigated the morphological changes of porcine jejunum after weaning and found that villus height and crypt depth returned to the preweaning value at 14 d postweaning (Hu et al. Citation2013). However, García et al. (Citation2016) reported that piglets weaned at 21 days old showed a sustained increase in villus length at 4, 20 and 40 d postweaning (García et al. Citation2016). This discrepancy might associate with swine breeds, weaning process and handling of pigs.
As the first defence barrier of intestine, small-intestinal mucosa is important in protecting against the entry of potential pathogens. Reports have confirmed that weaning could damage small-intestinal mucosal barrier due to the invasion of pathogenic microorganisms resulted from changes in living environment and nutrition (Wu et al. Citation1996). Plasma DAO activity and D-lactate level are useful markers to evaluate the extent of damage and repair of intestinal mucosa (Murray et al. Citation1993; Wu et al. Citation1996). These two markers are negatively related to the intestinal permeability, and will increase when intestinal permeability is damaged due to weaning or other stresses (Xiao et al. Citation2014). In the present study, DAO activities and D-lactate levels were significantly increased at 28 to 35 days old in 21W group compared with 35W and 42W groups. Moreover, these indicators were still at a higher level at 56 days old in 21W group, indicating that early weaning (21 d) resulted in lasting damage of intestinal integrity and increase of intestinal mucosa permeability in Wuzhishan piglets.
The intestinal barrier is mainly formed by the intercellular tight junctions together with a layer of columnar epithelium (Li et al. Citation2012). Prior studies have demonstrated that early weaning could cause sustained dysfunction of the intestinal barrier, which is characterised by decreased expression of tight junction proteins (Hu et al. Citation2013; Xiao et al. Citation2014). The mRNA levels of occludin and ZO-1 in jejunal mucosa as well as occludin, ZO-1, and claudin in ileal mucosa were lower in 21W group than 35W and 42W groups in this study. These results coincided with the increased intestinal mucosa permeability in 21 d weaned piglets, which may indicate sustained damage of intestinal mucosa integrity. Similar results were also founded by Smith et al. (Citation2010b) and Hu et al. (Citation2013) who reported that early weaning resulted in lasting impairment in the intestinal barrier.
Cytokines participate in immune and inflammatory responses and regulation of intestinal barrier integrity (Al-Sadi et al. Citation2009). Many pro-inflammatory cytokines, like TNF-α, IL-1β and IL-6, have adverse effects on intestinal mucosal integrity and epithelial function (Al-Sadi and Ma Citation2007; Liu et al. Citation2008). On the other hand, anti-inflammatory cytokines, such as IL-10, play an important role in protecting intestinal barrier function by attenuating the defects in tight junction permeability (Madsen et al. Citation1997; Howe et al. Citation2005). Some studies have investigated the expression of cytokines in the intestine of piglets after weaning. Pié et al. (Citation2004) reported that weaning resulted in up-regulation of inflammatory cytokines in the intestine of 28-day-old weaned piglets. Moreover, they also found that mRNA expressions of TNF-γ, IL-6 and IL-1β increased at 0 to 2 days post-weaning and then returned to the pre-weaning level at 2 to 8 days. Hu et al. (Citation2013) found that mRNA levels of TNF-α and IL-6 were increased at 3 d and 7 d post-weaning and returned to the pre-weaning levels at 14 d post-weaning, but no significant increase of IL-10 mRNA was observed during the 2 weeks after weaning in 21 d weaned piglets. The difference might be related to the weaning ages. We also analysed the levels of IL-1β, TNF-α, IL-6 and IL-10 in the intestine of 21 d, 28 d, 35 d and 42 d weaned piglets at 56 days old. The results showed that mRNA levels of IL-1β, TNF-α, IL-10 and IL-6 in jejunal and ileal mucosa were not significantly different among the treatments, indicating that intestinal inflammation, may have been restored in all piglets. Weaning transiently affected the intestinal inflammation at most 2 weeks after weaning (Hu et al. Citation2013). In future study, we will analyse the intestinal gene expressions of cytokines at different weaning ages within 2 weeks after weaning.
SIgA has long been considered to be the first protective barrier against adhesion and invasion of pathogenic microorganisms to the mucosa (Pabst Citation2012). During the early stage of breast feeding, intestinal sIgA is mainly provided by the colostrum and breast milk, whereas in post-weaned animals, sIgA synthesis predominantly depends on their own adaptive immune system (Kramer and Cebra Citation1995; Wagstrom et al. Citation2000). Prior study has found that shortening of breast feeding would increase post-weaning intestinal sIgA, leading to accelerated development of adaptive immune system, but negatively affects innate immunity (García et al. Citation2016). In the present work, the results showed that piglets weaned at 21 days old showed a significant increase of intestinal sIgA compared to that weaned at 35 and 42 days, suggesting that early weaning (21 days) may have positive effects on the adaptive immune system of Wuzhishan piglets. However, the adaptive immune system is not fully understood, and further studies are needed.
Conclusions
In summary, piglets weaned at 21 days old resulted in lasting impairment in intestinal barrier functions characterised by decreased mRNA expression of tight junction proteins and increased plasma DAO activities and D-lactate levels. Piglets weaned latter (35 and 42 days old) could alleviate the injury of intestinal barrier functions in Wuzhishan piglets. The mRNA levels of pro-inflammatory and anti-inflammatory cytokines were not different among different treatments, indicating that inflammatory responses may be transiently affected by weaning age. Early weaning (21 days old) increased the content of intestinal sIgA. Our study provides knowledge about advantages and disadvantages of weaning at different ages on intestinal barrier functions in Wuzhishan piglets.
Acknowledgements
The assistance of all staff at Animal Nutrition Laboratory in Tropical Crops Genetic Resources Institute of CATAS is highly appreciated.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Al-Sadi RM, Boivin M, Ma TY. 2009. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed). 14:2765–2778.
- Al-Sadi RM, Ma TY. 2007. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol. 178:4641–4649.
- Barszcz M, Skomiał J. 2011. The development of the small intestine of piglets-Chosen aspects. J Anim Feed Sci. 20:3–15.
- Boudry G, Péron V, Huërou-Luron IL, Lallès JP, Sève B. 2004. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of pigletintestine. J Nutr. 134:2256–2262.
- Butler J, Zhao Y, Sinkora M, Wertz N, Kacskovics I. 2008. Immunoglobulins, antibody repertoire and B cell development. Dev Comp Immunol. 33:321–333.
- Cera KR, Mahan DC, Cross RF, Reinhart GA, Whitmoyer RE. 1988. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J Anim Sci. 66:574–584.
- Coffey RD, Parker GR, Laurent KM. 2000. Feeding and managing the weanling pig. Frankfort, KY: University of Kentucky. Lexington and Kentucky State University. Cooperative Extension Service. ASC-149.
- Do CH. 2012. Estimation of weaning age effects on growth performance in berkshire pigs. Asian-Aust J Anim Sci. 25:151–162.
- Fang X, Mou Y, Huang Z, Li Y, Han L, Zhang Y, Feng Y, Chen Y, Jiang X, Zhao W, et al. 2012. The sequence and analysis of a Chinese pig genome. Gigascience. 1:16.
- García GR, Dogi CA, Ashworth GE, Berardo D, Godoy G, Cavaglieri LR, de Moreno de LeBlanc A, Greco CR. 2016. Effect of breast feeding time on physiological, immunological andmicrobial parameters of weaned piglets in an intensive breeding farm. Vet Immunol Immunopathol. 176:44–49.
- Han F, Hu L, Xuan Y, Ding X, Luo Y, Bai S, He S, Zhang K, Che L. 2013. Effects of high nutrient intake on the growth performance, intestinal morphology and immune function of neonatal intra-uterine growth-retarded pigs. Br J Nutr. 110:1819–1827.
- Hedemann MS, Jensen BB. 2004. Variations in enzyme activity in stomach and pancreatic tissue and digesta in piglets around weaning. Arch Anim Nutr. 58:47–59.
- Hou G, Wang D, Guan S, Zeng H, Huang X, Ma Y. 2010. Associated analysis of single nucleotide polymorphisms of IGF2 gene’s exon 8 with growth traits in Wuzhishan pig. Mol Biol Rep. 37:497–500.
- Hou GY, Zhou HL, Cao T, Xun WJ, Wang DJ, Shi LG, Guan S, Wang DF, Li M. 2015. Expression and variation of Myf5 and MyoD1 genes in different tissues of Wuzhishan pigs. Genet Mol Res. 14:3729–3735.
- Howe KL, Reardon C, Wang A, Nazli A, McKay DM. 2005. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am J Pathol. 167:1587–1597.
- Hu CH, Xiao K, Luan ZS, Song J. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 91:1094–1101.
- Kramer DR, Cebra JJ. 1995. Early appearance of natural mucosal IgA responses and germinal centers in suckling mice developing in the absence of maternal antibodies. J Immunol. 154:2051–2062.
- Li X, Akhtar S, Choudhry MA. 2012. Alteration in intestine tight junction protein phosphorylation and apoptosis is associated with increase in IL-18 levels following alcohol intoxication and burn injury. Biochimica Et Biophysica Acta (BBA) - Molecular Basis of Disease. 1822:196–203.
- Liu Y, Chen F, Odle J, Lin X, Jacobi SK, Zhu H, Wu Z, Hou Y. 2012. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J Nutr. 142:2017–2024.
- Liu YL, Huang JJ, Hou YQ, Zhu HL, Zhao SJ, Ding BY, Yin YL, Yi GF, Shi JX, Fan W. 2008. Dietary arginine supplementation alleviates intestinal mucosal disruption induced by Escherichia coli lipopolysaccharide in weaned pigs. Br J Nutr. 100:552–560.
- Madsen K, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. 1997. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 113:151–159.
- Main RG, Dritz SS, Tokach MD, Goodband RD, Nelssen JL. 2004. Increasing weaning age improves pig performance in a multisite production system. J Anim Sci. 82:1499–1507.
- McLamb BL, Gibson AJ, Overman EL, Stahl C, Moeser AJ. 2013. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLos One. 8:e59838.
- Moeser AJ, Ryan KA, Nighot PK, Blikslager AT. 2007. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am J Physiol Gastrointest Liver Physiol. 293:413–421.
- Montagne L, Boudry G, Favier C, Le Huërou-Luron I, Lallès JP, Sève B. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br J Nutr. 97:45–57.
- Murray MJ, Barbose JJ, Cobb CF. 1993. Serum D(-)-lactate levels as a predictor of acute intestinal ischemia in a rat model. J Surg Res. 54:507–509.
- Pabst O. 2012. New concepts in the generation and functions of IgA. Nat Rev Immunol. 12:821–832.
- Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr. 134:641–647.
- Pluske JR. 2013. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. 4:1–7.
- Rist VT, Weiss E, Eklund M, Mosenthin R. 2013. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets inrelation to gut health: a review. Animal. 7:1067–1078.
- Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JE, Blisklager AT, Moeser AJ. 2010a. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 298:352–363.
- Smith MG, Jordan D, Chapman TA, Chin JJ, Barton MD, Do TN, Fahy VA, Trott DJ. 2010b. Antimicrobial resistance and virulence gene profiles in multi-drug resistant enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhea. Vet Microbiol. 145:299–307.
- The local standards of Hainan province. 2007. Wuzhishan pig breeding technology discipline (DB46/T 88-2007), Hainan, China.
- Tokach MD, Goodband RD, Nelssen JL, Kats LJ. 1992. Influence of weaning weight and growth during the first week postweaning on subsequent pig performance. Kansas State University Swine Day Report of Progress 15-17.
- van Beers-Schreurs HMG, Nabuurs MJA, Vellenga L, Kalsbeek-van der Valk HJ, Wensing T, Breukink HJ. 1998. Weaning and the weanling diet influence the villous height and crypt depth in the small intestine of pigs and alter the concentrations of short-chain fatty acids in the large intestine and blood. J Nutr. 128:947–953.
- Van der Meulen J, Koopmans S, Dekker R, Hoogendoorn A. 2010. Increasing weaning age of piglets from 4 to7 weeks reduces stress, increases post-weaning feed intake but does not improve intestinal functionality. Animal. 4:1653–1661.
- Wagstrom A, Yoon K, Zimmerman J. 2000. Immune components in porcine mammary secretions. Viral Immunol. 13:383–397.
- Wu G, Meier SA, Knabe DA. 1996. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J Nutr. 126:2578–2584.
- Xiao K, Song ZH, Jiao LF, Ke YL, Hu CH. 2014. Developmental changes of TGF-β1 and smads signaling pathway in intestinal adaption of weaned pigs. PLoS One. 9:e104589.
- Xun W, Shi L, Zhou H, Hou G, Cao T, Zhao C. 2015. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int Immunopharmacol. 27:46–52.
- Yang H, Xiong X, Wang X, Tan B, Li T, Yin Y. 2016. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS One. 11:e0150216.
