Abstract
This trial evaluates the feasibility of using passive injectable transponders (PITs) in field operations by testing identification procedures on 185 one-year-old Biellese ewes reared under nomadic farming conditions. Commercial PITs of 3.85 ± 0.05 mm × 31.2 mm were used at two application sites, the armpit and the retro-auricular region. The two application sites were compared taking into account the ease of injection, animal reaction, injection duration, inflammatory response, PIT readability at up to 12 months post-injection and PIT recovery at the slaughterhouse. The injection site influenced the reaction of the animal and the ease and duration of the injection, but it did not affect the palpation and the reading findings during the rearing period. The injection site also influenced the PIT retrieval at the slaughterhouse depending on the operator who recovered the PIT. The readability values observed at the end of the observation period for both injection sites (83% in the retro-auricular region and 79% in the armpit region) were too low to be suitable for long-term animal identification. In addition, the failed retrieval of PITs at slaughter, determined the destruction of 40 (22%) carcases to avoid any risks in the food chain. These findings suggest that the limited use of PITs is indicated when other methods of electronic identification cannot be employed, while a wider application of the device tested in the present study is not recommended in farming practices.
Background
The proper and functional identification of animals is essential for the creation of animal identification systems (Stanford et al. Citation2001; World Organisation for Animal Health Citation2014). When properly applied, electronic identification provides an opportunity to use information-based and electronically controlled livestock production systems, reducing labour costs and improving data accuracy (Eradus and Jansen Citation1999; Trevarthen and Michael Citation2008; Ait-Saidi et al. Citation2014). Electronic identification is foreseen as a powerful tool for the improvement of integrated frameworks designed for the monitoring of livestock by recording disease occurrence, vaccinations, tests, medications, and other relevant information to control and prevent diseases (Shanahan et al. Citation2009; Voulodimos et al. Citation2010). The electronic identifier can be a ruminal bolus, an ear tag, an electronic mark on the pastern or an injectable transponder. The device must be a read-only passive transponder using half duplex (HDX) or full duplex (FDX-B) technology (Council Regulation (EC) Citation2006, Citation2010).
In Italy, electronic identification in sheep is essential especially in breeders, since lambs intended for slaughter before the age of 12 months are identified in a simplified manner by a traditional ear tag applied to one ear (Decree of the Health Ministry Citation2004). Moreover, the use of any type of passive injectable transponder (PIT) has not yet been authorised because of a lack of comprehensive data on their performance and their ability to guarantee food safety, despite that the EU legislation includes the PIT as a possible mean of electronic identification (Council Regulation (EC) Citation2008).
The choice of injection site of PIT is critical for ensuring proper identification of the animal, and the injection site must not cause harm to the animal and must minimise the chance of device breakage or loss and ensure that the PIT remains in the same place for the entire lifespan. Finally, after death or slaughter, recovery of the PIT should be easy and rapid to avoid any possible persistence in the food processing chain. Different PIT locations have been tested in sheep under experimental conditions (Hogewerf et al. Citation2007). However, no official guidelines on the type of PIT to be used or on the preferred sites of injection have been established.
The armpit and retro-auricular regions present good readability in both adult sheep (Marie et al. Citation1995; Caja et al. Citation1998) and other animal species (Merks and Lambooy Citation1990; Lambooij et al. Citation1999; Conill et al. Citation2000; Caja et al. Citation2014). In addition, both sites have been suggested to be suitable because they are associated with moderate migration of the PIT, high ease of injection and low behavioural reactivity of the animal during PIT inoculation (Caja et al. Citation1998).
The objective of this study was to evaluate the performance of PITs at two different injection sites (retro-auricular and armpit) in adult ewes and determine the ease of injection, the behavioural reaction of the animal, the readability of the device, the frequency of loss and recovery of the PIT at slaughter over a period of one year.
Materials and methods
Animals
The experiment was conducted on a flock of 3000 Biellese sheep, which is a meat breed in which the ewes exhibit an average weight of 72 kg and height of 82 cm at the withers (EFABIS Citation2015). The herd was reared under nomadic farming conditions with continuous displacement using alpine and pre-alpine summer pastures, marginal forage resources in other seasons and alternating periods of indoor and outdoor housing during winter. GPS coordinates of the study area were WGS84/Latitude–Longitude, 45.135386 and 11.966152.
The animals involved in the study were one-year-old females that were maintained in the flock with the other sheep during the study period and were slaughtered at two years of age. All of the selected animals had similar weights and heights to avoid the effects of different body structures and fattening statuses on the outcome of the test.
Transponders and injection procedures
Half duplex, glass-encapsulated, read-only PITs (Rumitag™ Datamars SA, Bedano-Lugano, Switzerland; authorised by JRC-ISPRA no. JRC/117/04 and JRC/118/04) were used. The PITs (3.85 ± 0.05 mm × 31.2 ± 0.6 mm, 0.8 g) had identification codes embedded by the supplier. The self-sterilizing stainless steel injectors (Planet ID, Rumitag™ Datamars SA, Bedano-Lugano, Switzerland) that were employed had interchangeable needles, and the application needle was immersed in a 10% iodine solution prior to each injection and changed every five applications.
The retro-auricular region (R) is the area caudal to the concavity of the ear; the PIT was injected at this site by placing the injector behind the ear directed downwards in a dorso-ventral orientation, as close as possible to the auricular cartilage (scapha), as described by Conill et al. (Citation2002). The armpit region (A) is the space between the chest wall and the caudal muscles of the arm (triceps brachii); the PIT was injected at this site after stretching the skin between the foreleg and chest, with the injector placed in a cranio-caudal orientation, as described by Caja et al. (Citation1998). Both injection sites were on the right side of the animal to facilitate subsequent evaluations of the PITs. Two people were needed for each intervention: a farm operator restrained the animal while a veterinarian performed the injection. The animals injected at site A were restrained by a single experienced operator sitting in a supine position, with the animal positioned between his legs with its back towards the operator; the animals inoculated at the R site were restrained in a standing position with the head held firmly by the operator.
Shearing was not required, and the skin was disinfected with a solution of 10% iodine (Betadine, Mundipharma, Basel, Switzerland) at both injection sites. Insertion of the PIT was performed by the same veterinarian over 5 days during a four-week period.
During the application of the PIT, time required for injection, behavioural reaction of the animal and ease of injection were evaluated. After injection, the PIT was read under static conditions using a handheld transceiver (GesReader Ges2S, Rumitag™ Datamars SA, Bedano-Lugano, Switzerland) with a stick antenna, and its presence at the correct injection site was confirmed via palpation.
PIT evaluation at the farm and recovery at the slaughterhouse
The presence and integrity of the PITs were assessed through palpation and based on readings performed 15 days post-injection (p.i.). Inflammation was evaluated at the injection site and in the surrounding area and was described in terms of the presence or absence of redness and/or swelling or residual scars.
At this time, the animals were marked with a supplementary ear tag (Flexoplus LL/LL, G&G, Bergamo, Italy), with different colours used for each experimental group to make them visually recognisable within the flock. On the farm, the presence and integrity of the PIT were assessed under the same conditions at three and twelve months p.i. and prior to loading for slaughter.
The slaughterhouse was a small operation (150–200 sheep slaughtered weekly); the animals with a PIT were slaughtered at the end of the day and grouped according to the location of the PIT (A or R). The PIT was recovered by the veterinary or slaughterhouse staff after adequate training. Prior to stunning, the presence of the PIT was confirmed by reading the device. After the head was cut off at the beginning of the slaughter line, the group R carcases were searched with the reader to confirm the absence of the PIT, and the head was set aside in a container for recovery of the PIT. The PIT readings were performed on the carcases in group A for easy retrieval during the process of skinning, which was conducted manually. The animals showing no readings were slaughtered and examined subsequently to check the absence of a broken PIT in the carcase. All the 40 carcases in which the PIT was not retrieved were destroyed to prevent adulteration with foreign bodies.
Haematological tests
At 15 days p.i., a blood sample was taken from the jugular vein using K3 EDTA test tubes (Vacutest Kima, Arzergrande, PD, Italy) and VACUETTE needles (Greiner Bio-One GmbH, Kremsmünster, Austria), model 1.25 × 38 mm.
The samples were stored at a temperature of 4 °C, sent to the laboratory within 6 hours, and immediately processed. The analysis of blood samples was performed with a Cell Dyn 3500 automated analyser (Abbott Diagnostics, Abbott Park, IL), which can quantify the total number of white blood cells and determine differential white blood cell counts using two different channels: optical and impedimetric.
Statistical analyses
Sample size
A sample size of 83 animals for each group (A and R) was defined supposing an expected success rate in the R group of 82% (Conill et al. Citation2002), a desired increase in success for the A group of at least 14% (Hogewerf et al. Citation2007), an error α = .05, a power 1 − β = .9 (Proc Power twosamplefreq, 9.3 SAS software). To take into account possible death or loss of animals, 92 and 93 animals were randomly selected and injected at sites A and R, respectively.
At the end of the study, the proportions of PITs injected at the A and R sites recovered at the slaughterhouse and the corresponding 95% confidence interval were calculated. A Z test was conducted to verify whether a significant difference existed between the percentages of recovery at the two sites.
Analysis of relationships among measured variables
To determine whether the injection site was associated with the animals' behavioural reaction (absent = quiet and still; light = feeble resistance; moderate = head or limb shaking; and marked = struggling to escape from restraints), ease of injection (easy = one; moderate = two; and difficult = three or more attempts), time required for injection (short <50 s; intermediate 50–60 s; and long >60 s), inflammation (yes/no), presence of the PIT at 15 days, 3 months and 12 months p.i. and recovery at the slaughterhouse, the chi-square test for categorical variables was performed. The same test was used to determine whether the behavioural reaction, injection duration, ease of injection and inflammation affected the 3- and 12-month readings and recovery at the slaughterhouse, independent of the injection site. The presence of a possible association was quantified using Cramer’s V index (Thrusfield Citation2007), and index values were interpreted as described in the Introduction to Research Methods (I-CHASS Citation2013). For statistically significant associations among variables with two modalities, a one-tailed Z test for proportion was conducted to assess the direction of the difference.
In addition, three multivariable logistic regressions (Hosmer and Lemeshow Citation2000) were performed to estimate (1) the probability of PIT reading at 3 months p.i, (2) the probability of PIT reading at 12 months p.i and (3) the probability to recovery the PIT at slaughter (outcomes). Each model assessed the effect of all the above-mentioned categorical variables on each probability. The Hosmer–Lemeshow (Hosmer and Lemesbow Citation1980) was conducted to evaluate the fit of the proposed models.
Analysis of blood parameters
Descriptive statistics were provided for the haematic parameters and injection sites, and box plots were used to display the distribution of these quantitative values. The Shapiro–Francia test (Shapiro and Francia Citation1972) was employed to verify the normality of the data for each blood parameter. When the data were normally distributed, a parametric analysis was performed; otherwise, a non-parametric analysis was conducted.
For normally distributed data, the homoscedasticity of the variances was verified using the F test. For comparisons with equal variances, a simple Student's t-test for two independent samples was used; otherwise, Student’s t-test with the Satterthwaite correction for the degrees of freedom was performed (Thrusfield Citation2007). For non-normally distributed data, the homoscedasticity of the variances was compared using Levene’s test (Olkin Citation1960). For comparisons with equal variances, the non-parametric Wilcoxon test for two independent samples was employed (Wilcoxon Citation1945; Mann and Whitney Citation1947); otherwise, the Kolmogorov–Smirnov test was used (Smirnov Citation1939).
Software
SAS 9.3 software (SAS Institute Inc., Cary, NC) and STATA 12.0 software (StataCorp LP, College Station, TX) were used for the statistical analysis. A p value < .10 (p) was considered significant.
Ethical approval
This work was approved under decision No. CE.IZSVE.11/2011, by the Istituto Zooprofilattico Sperimentale delle Venezie Animal Research Ethics Committee, which adhered to the animal use guidelines of the Italian Ministry of Health on Animal Care. The necessary measures to reduce the number of animals without compromising the statistical significance of the sample were applied, and animal welfare standards were followed during animal identification and slaughter (Council Regulation (EC) Citation2009).
Results
Identification operations
The data collected during the PIT injections are shown in Table . All PITs were successfully injected and were functioning after injection.
Table 1. Results of PIT injection in retro-auricular (R) and armpit (A) region in term of duration, ease of injection and animal reaction.
The behavioural reaction of the animal to the injection and the ease and duration of the PIT injection were not independent of the site of injection (p < .05; Table ). Specifically, a reaction was virtually absent in the sheep injected at site A (animal still and quiet), whereas the animals injected at site R showed mild to strong behavioural reactions. The injections were somewhat easier at site A than at the R site, and the injection duration was significantly shorter in the A group than in the R group (Table ).
Checks at the farm
Fifteen-day check
No significant differences in the proportion of PITs found through palpation or in readable PITs were observed between groups A and R (p > .10; Table ).
Table 2. Results of PIT performances at the farm in term of palpation, reading, inflammation presence per injection site and observation times.
The presence of inflammation was not related to the site of injection (p = .504). In the animals with inflammation (five animals in the A group and one animal in the R group), the PIT was not read or palpated.
Three-month and twelve-month checks
Prior to the end of the 3rd month p.i., three animals in the R group and two animals in the A group died due to causes unrelated to the experiment; in all of these animals, the PIT had been readable at the previous check and was recovered at the site of injection. Therefore, 90 animals remained in both the A and R groups.
Moreover, prior to the check at 12 months, five sheep in the A group and three in the R group died, and another four animals in the R group died in a mountain pasture, and it was not possible to recover their bodies. In all of these animals, the PIT had been readable at 15 days and 3 months p.i. As a result, 83 and 85 animals remained in the R and A groups, respectively. At 12 months p.i. in seven animals of the A group and four animals of the R group, the PITs were no longer readable and were not recovered at the slaughterhouse.
Statistical analysis showed that no significant differences in the readability of the PITs were observed between groups A and R at 3 and 12 months p.i. (p = .649 and p = .477, respectively; Table ). However, successful readings in both checks were negatively associated with the presence of inflammation (V = −.3538, p = .000; V = −.2624, p = .004, respectively) and with the duration of the injection (V = −.4795, p = .000, V = −.3590, p = .000, respectively). Additionally, the ability to palpate the PIT at 3 months p.i. was associated with the injection site (V = .1623, p = .029; Table ), being significantly higher in the A than in the R group (p = .0147). Finally, readability at 12 months p.i. depended on the ability to palpate and read the PIT at 3 months p.i. (V = .398, p = .000; V = .755, p = .000, respectively).
Multivariable logistic regression indicated that both readings at 3 and 12 months p.i. were greatly reduced in the presence of inflammation and with longer times of injection (p = .000) (Model A and model B, Table ). Nevertheless, focussing on the PIT reading probability at 12 months p.i., the significance of the interaction between the presence of inflammation and the injection site (p = .026) indicated that in the presence of inflammation, the probability was reduced only if the site of injection was the armpit. On the other hand, in the absence of inflammation, the probability was similar for the two injection sites (Model B, Table ).
Table 3. Results of multivariable logistic regression to estimate the probability to read the PIT at 3 months (Model A), at 12 months (Model B) and the probability to recover the PIT at the slaughterhouse (Model C).
The Hosmer and Lemeshow test showed that both models were well calibrated (p = .2704, p= .6360, respectively) and correctly classified more than 85% of the observations (Table ).
PIT recovery at slaughter
A total of 168 animals were delivered to the slaughterhouse, although only 136 had a readable PIT at 12 months p.i. and before slaughter; 128 PITs were recovered, including 62 in R and 66 in A (75% IC95[65–84] and 78% IC95[69–86], respectively; Table ). Not all of the readable PITs were recovered, and no significant difference existed between PITs recovered in R versus A. Two PITs in the R group were found in the neck, whereas the remaining PITs were detected in the area of injection. Six PITs in the A group were embedded in the musculature of the armpit at varying degrees of depth, and eight PITs were attached to the skin, including one wrapped in a fibrotic capsule (this was the only animal showing an inflammatory reaction and a readable PIT in the A group). The remaining PITs in the A group adhered to the surface of the carcase in the armpit.
Table 4. Number of animals and number of readable and recovered PIT per injection site (R and A), at the slaughterhouse.
As previously observed at 3 and 12 months p.i., recovery of the PIT at the slaughterhouse was independent of the animal’s behavioural reaction and the ease and site of injection (p > .10). On the contrary, it was negatively associated with the duration of the injection (V = −.2947, p = .000) and the presence of inflammation (V = −.2138, p = .013) and positively associated with palpation at 3 months p.i. (V = .4198, p = .000) and PIT readability at 3 (V = .653, p = .000) and 12 months p.i. (V = .8677, p = .000).
Multivariable logistic regression showed that the probability of recovering the PIT decreased when the duration of the injection increased (p = .000) and with the failure of the palpation at 3 months (p = .000). In this last model, the operator who searched for and recovered the PIT at slaughter (veterinary or slaughterhouse staff; Table ) and the interaction with the injection site were also added as variables. The probability of recovering the PIT from site R did not differ between the slaughterhouse and veterinary staff, whereas it was significantly higher among the veterinary staff when the PIT was located at site A (p = .048).
Table 5. Number of PITs recovered per operator and injection site (R and A) at the slaughterhouse.
The Hosmer and Lemeshow test showed that the model was well calibrated (p = .1447) and correctly classified more than 84.52% of the observations (Table , model C).
Blood counts
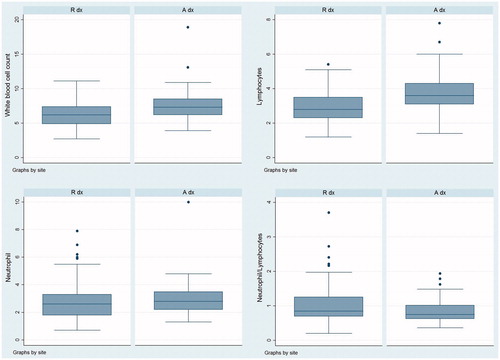
In 176 animals, the white blood cell (WBC) count fell between the benchmarks (4.0–12.0 k/μL) reported in the literature for sheep (Kramer Citation2000); six animals exhibited WBC counts below normal values (<4.0 k/μL) and two animals exhibit WBC counts above normal values (>12.0 k/μL). Although the absolute number of leukocytes did not increase sufficiently to indicate an inflammatory state, the distribution of white blood cell values was significantly higher in the A group than in the R group (p = .000; Figure ). Similarly, the mean neutrophil and lymphocyte counts were within the reference parameters (Figure ). The mean proportion of neutrophils (NEU %) was significantly higher in the sheep with the PIT at site R than at site A (p = .046; NEU% = 42.01 vs. NEU% = 39.55, respectively). Additionally, the mean proportion of lymphocytes (LIM%) was significantly higher in the sheep with the PIT at site A than at site R (p = .0033; LIM% = 50.02 vs. LYM% = 46.16, respectively). Moreover, the ratio of neutrophils to lymphocytes (N/L) differed significantly with the site of injection, with a tendency for higher values to be observed in the sheep with the PIT at site R than at site A (p = .0026; N/L = 1.03 vs. N/L = 0.84, respectively) (Figure ).
Figure 1. Box plot of the distribution values of white blood cell count (k/μl), Lymphocytes (k/μl), Neutrophils (k/μl) and the ratio of neutrophils to lymphocytes per injection site (R and A). The y-axis represents the haematic values. Each box shows the degree of dispersion in the data. The line inside the box is the median value and the points above the box are the outliers.
Discussion
In comparing the performances of the PITs in the two sites, the injection site influenced the behavioural reaction of the animal, the ease and duration of the injection.
In particular, the behavioural reaction induced by the injection process was much greater in animals inoculated at site R than those injected at site A, and the injection was easier at site A. These findings are consistent with the results described in adult sheep by Caja et al. (Citation1998) and in lambs by Conill et al. (Citation2002). Hogewerf et al. (Citation2007) found that PIT injection at the A site in lambs could be classified as easy to moderate and that PIT application was simpler, cheaper and produced less of a behavioural reaction in the animal than the application of an ear tag or a bolus.
The time required for the injection of the PIT was more frequently classified as short in the A group than in the R group, which is in accord with the results obtained in calves by Conill et al. (Citation2000). A short injection duration is indicative of rapid location of the site and positioning of the injector; thus, the experience of the operators and appropriate animal containment could improve PIT readability.
The injections were more difficult and required longer times in the R group. This finding can be explained by the greater behavioural reactivity of the animals to being restrained at their head. Moreover, the animals are usually sheared using restraint in a similar manner as they were injected in site A, so the restraint of the head for the injection in site R is unusual to the sheep and more likely to elicit the unfavourable behavioural reaction as noted.
At the first check at 15 days p.i., the proportion of non-readable PITs was higher than the values reported in previous studies on sheep (Ribó Citation1996; Caja et al. Citation1998; Conill et al. Citation2002). Only pigs have previously shown losses as high as 13.3% (Barbieri et al. Citation2012).
The loss of a PIT is expected to occur within the first several days p.i., when the point of entry has not yet healed (Carné et al. Citation2009), and backward movement of the PIT may be facilitated by the movements of the animal (Conill et al. Citation2002).
No apparent health problems were detected in animals following the injection of the PIT, thus confirming the findings reported by other authors (Caja et al. Citation1998; Conill et al. Citation2002; Hogewerf et al. Citation2007). At both sites, the presence of local inflammation affected the reading of the PIT because the inflammatory process facilitates PIT loss (Conill et al. Citation2000).
Regarding the haematic parameters, although the absolute number of leukocytes did not increase sufficiently to indicate an inflammatory state, the N/L ratio differed depending on the site of injection. The N/L ratio is a useful indicator of inflammation and/or stress conditions; specifically, in stressful situations, the activation of the hypothalamic–pituitary–adrenal axis leads to changes in blood cells (Claman Citation1972; Butcher and Lord Citation2004; Lee et al. Citation2013), including a decrease of lymphocytes, an increase in neutrophil life expectancy and a consequent increase in the N/L ratio. In this study, the higher N/L ratio observed in the R versus the A group could suggest a major inflammatory/stress state that could be related to both the injection duration and the animal’s behavioural reaction.
The proportion of PITs recovered at slaughter was low and it did not differ between the two groups: 75% in the R group and 78% in the A group, corresponding to 90% and 99% of the readable PITs before slaughter, respectively.
The difficult recovery of the PIT in the R group can be explained by its positioning along the cutting line of the head, which increased the likelihood of the PIT being destroyed or accidentally removed during the slaughter operation. With regard to the A group, the skinning process conducted by different operators with different strengths and abilities may have resulted in the decreased PIT recovery.
Migration of the PIT was not a major problem at either site, which is consistent with the literature. In fact, 12% of the PITs recovered from the A group were attached to the skin, similar to previously published results (Nehring et al. Citation1994; Conill et al. Citation2002), while no relevant level of PIT migration was identified in the R group.
Six of the 62 PITs injected at site A were recovered by cutting into the muscles in the area, which caused slight damage to the carcase and required a longer time for recovery of the PIT. Similar damage has been described in association with the same type of PIT in cattle, although at a higher rate, of 31.9% (Conill et al. Citation2000).
Furthermore, the differences associated with different operators during recovery indicated that PIT retrieval from the armpit requires a higher level of attention and care, which is more likely to be provided by a veterinarian than slaughterhouse staff.
With regard to the possible use of PITs for identifying carcases along the slaughter line, neither of the two sites provided a valid method of identification, as the PITs were removed from site A by skinning and from site R via removal of the head.
Conclusions
The two sites (A and R) considered for PIT inoculation displayed the same readability of PITs during the life of the animals and the same level of recovery at slaughter. The armpit should be considered the preferred site because injection was easier, faster and caused fewer behavioural reactions in inoculated animals in comparison with the retro-auricular region.
The PIT losses during the rearing period at both injection sites were too high to be suitable for long-term animal identification. PIT readability did not reach the 98% target set by the International Committee for Animal Recording (ICAR Citation2005, Citation2009). The retrieval of a PIT from the armpit or the retro-auricular region at the abattoir may raise economic and food safety concerns: the losses at slaughter associated with both the A and R sites were too high compared with the recommended 99% recovery of identification devices (ICAR Citation2005, Citation2009).
Moreover, the difficulty of PIT recovery at slaughter can lead to low acceptance of PITs by slaughterhouse operators because it lengthens slaughter operations and increases the workload compared with the removal of an electronic ear tag or bolus. In addition, from the point of view of costs, in Italy, the injection of a PIT can be performed only by a veterinarian, which increases the cost of animal identification for the farmer.
This trial did not provide new data and useful evidences in order to review the ban on PITs use in sheep identification applied in Italy. Further investigations should be done to evaluate the use of other type of PITs in term of size and material.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Ait-Saidi A, Caja G, Salama AAK, Carné S. 2014. Implementing electronic identification for performance recording in sheep: I. manual versus semiautomatic and automatic recording systems in dairy and meat farms. J Dairy Sci. 97:7505–7514.
- Barbieri S, Minero M, Barattiero D, Cantàfora AFA, Crimella MC. 2012. Recognised-by-law versus other identification systems in pigs: piglets discomfort evaluation and performance testing. Ital J Anim Sci. 11:e35.
- Butcher SK, Lord JM. 2004. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 3:151–160.
- Caja G, Carné S, Salama AAK, Ait-Saidi A, Rojas-Olivares MA, Rovai M, Capote J, Castro N, Argüello A, Ayadi M, et al. 2014. State-of-the-art of electronic identification techniques and applications in goats. Small Ruminant Res. 121:42–50.
- Caja G, Ribó O, Nehring R. 1998. Evaluation of migratory distance of passive transponders injected in different body sites of adult sheep for electronic identification. Livest Prod Sci. 55:279–289.
- Carné S, Caja G, Ghirardi JJ, Salama AAK. 2009. Long-term performance of visual and electronic identification devices in dairy goats. J Dairy Sci. 92:1500–1511.
- Claman HN. 1972. Corticosteroids and Lymphoid Cells. N Engl J Med. 287:388–397.
- Conill C, Caja G, Nehring R, Ribó O. 2000. Effects of injection position and transponder size on the performances of passive injectable transponders used for the electronic identification of cattle. J Anim Sci. 78:3001–3009.
- Conill C, Caja G, Nehring R, Ribó O. 2002. The use of passive injectable transponders in fattening lambs from birth to slaughter: effects of injection position, age, and breed. J Anim Sci. 80:919–925.
- Commission Regulation (EC). 2008. Commission Regulation (EC) No 933/2008 of 23 September 2008 amending the Annex to Council Regulation (EC) No 21/2004 as regards the means of identification of animals and the content of the movement documents.
- Council Regulation (EC). 2006. Commission Decision of 15 December 2006 implementing Council Regulation (EC) No 21/2004 as regards guidelines and procedures for the electronic identification of ovine and caprine animals. Official Journal of the European Commission.
- Council Regulation (EC). 2009. Council Regulation (EC) No. 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Official Journal of the European Union.
- Council Regulation (EC). 2010. Commission Decision of 12 May 2010 amending Decision 2006/968/EC implementing Council Regulation (EC) No 21/2004 as regards guidelines and procedures for the electronic identification of ovine and caprine animals. Official Journal of the European Commission.
- Decree of the Health Ministry. 2004. Decree of the Health Ministry of 28 July 2005 implementing Council Regulation (EC) No 21/2004 as regards a system of identification and registration of the ovine and caprine animals in Italy.
- EFABIS. 2015. EFABIS 2015. European Farm Animal Biodiversity Information System. Single Breed Reports.
- Eradus WJ, Jansen MB. 1999. Animal identification and monitoring. Comput Electron Agric. 24:91–98.
- Hogewerf PH, Ipema AH, Binnendijk GP, Lambooj E, Schuiling HJ. 2007. Using injectable transponders for sheep identification.
- Hosmer DW, Lemesbow S. 1980. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Methods. 9:1043–1069.
- Hosmer DW, Lemeshow S. 2000. Applied Logistic Regression. 2nd ed. New York: Wiley.
- ICAR. 2005. International Committee for Animal Recording. Practices Guidelines approved by the General Assembly held in Sousse, Tunisia, June 2004. In: International Agreement of recording practices. ICAR Publ. Roma, Italy.
- ICAR. 2009. International Committee for Animal Recording. International Agreement of recording practices. Guidelines approved by the General Assembly held in Niagara Falls, USA, June 2008. In: International Agreement of recording practices. ICAR Publ. Roma, Italy.
- I-CHASS. 2013. Institute for Computing in the Humanities, Arts and Social Sciences. Introduction to Research Methods.
- Kramer J. 2000. Normal hematology of cattle, sheep and goats. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s veterinary hematology. 5th ed. Philadelphia (USA): Lippincott, W. and Wilkins Press; p. 1075–1084.
- Lambooij E, van’t Klooster CE, Rossing W, Smits AC, Pieterse C. 1999. Electronic identification with passive transponders in veal calves. Comput Electron Agric. 24:81–90.
- Lee J-I, Shin J-S, Lee J-E, Kim MS, Park CG, Kim SJ. 2013. Changes of N/L ratio and cortisol levels associated with experimental training in untrained rhesus macaques. J Med Primatol. 42:10–14.
- Mann HB, Whitney DR. 1947. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 18:50–60.
- Marie C, Caja G, Barillet F, Ribó O. 1995. Electronic identification in sheep; initial results and considerations for application and testing transponders. Publ Assoc Anim Prod. 75:197–197.
- Merks JWM, Lambooy E. 1990. Injectable electronic identification systems in pig production. Pig News Inf. 11:35–36.
- Nehring R, Ribó O, Caja G. 1994. Study of electronic implantable transponders retrieval in sheep carcasses at the abattoir. Electronic Identification of Farm Animals Using Implantable Transponders. FEOGA Research Project (Contract CCAM93–342), Final Report, 1.
- Olkin I. 1960. Contributions to probability and statistics: essays in honor of Harold Hotelling.
- Ribó O. 1996. Identificación electrónica en ganado ovino y caprino: Factores que afectan a la implantación de transponders y eficacia de lectura en condiciones de campo. Spain: Facultat de Veterinaria, Universitat Autónoma de Barcelona.
- Shanahan C, Kernan B, Ayalew G, McDonnell K, Butler F, Ward S. 2009. A framework for beef traceability from farm to slaughter using global standards: an Irish perspective. Comput Electron Agric. 66:62–69.
- Shapiro SS, Francia RS. 1972. An approximate analysis of variance test for normality. J Am Stat Assoc. 67:215–216.
- Smirnov NV. 1939. Estimate of deviation between empirical distribution functions in two independent samples. Bull Moscow Univ. 2:3–16.
- Stanford K, Stitt J, Kellar JA, McAllister TA. 2001. Traceability in cattle and small ruminants in Canada. Rev. - Off. Int. Epizoot. 20:510–522.
- Thrusfield M. 2007. Demonstrating association. In: Veterinary epidemiology. Oxford: Blackwell Science Ltd, a Blackwell Publishing company; p. 247–265.
- Trevarthen A, Michael K. 2008. The RFID-enabled Dairy Farm: Towards Total Farm Management. In: Proceedings of the 7th International Conference on Mobile Business, Barcelona, Spain. p. 241–250.
- Voulodimos AS, Patrikakis CZ, Sideridis AB, Ntafis VA, Xylouri EM. 2010. A complete management system based on animal identification using RFID technology. Comput Electron Agric. 70:380–388.
- Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometrics Bull. 1:80–83.
- World Organisation for Animal Health. 2014. General principles on identification and traceability of live animals, Article 4.1.1. In: Terrestrial animal health Code. Paris: World Organisation for Animal Health (OIE).
