Abstract
Two independent trials were carried out to assess the effect of two enrichment tools on the prevalence of skin and tail lesions recorded prior of slaughtering, lesions of the pars oesophagea (OGL) of the stomach, and on carcase and meat quality traits in Italian heavy pigs (body weight range: 25–160 kg). Eighty undocked barrows (Landrace × Large White) were used in two trials (20 pigs/experimental group, 5 pigs/pen). In Trial 1, the control group received a hanging metal chain (C1), while the other group received wood logs (WL) placed inside a metal rack. In Trial 2, the control group was provided with hanging chain (C2), while the pen of the other group was enriched with a vegetal edible block (EB) placed inside the metal rack. In both trials, no differences were observed in the prevalence and severity of skin, tail and gastric lesions (p > .05). In Trial 1, WL pigs presented lower backfat (p = .01), higher lean meat percentage (p = .03) and higher drip loss in the loin muscle (p = .02) than C1 pigs. Tail score and gastric lesions showed a moderate correlation (r = 0.42; p = .01) in Trial 1. Treatments had no effect on carcase or meat quality traits in Trial 2 (p > .05). In conclusion, the two enrichments provided did not affect body and gastric lesions, carcase and meat quality of Italian heavy pigs, if compared to the metal chains.
Introduction
Research has showed that raising pigs in enriched housing conditions reduces aggressiveness, body lesions, and tail biting (Studnitz et al. Citation2007). Furthermore, environmental enrichment may help reduce the prevalence of oesophago-gastric lesions (OGL) observed in pigs raised in barren rearing condition resulting in slow growth rate (Friendship Citation2004; Di Martino et al. Citation2013), poor welfare (Friendship Citation2004; EFSA Citation2007) and even sudden death (Melnichouk Citation2002; Robertson et al. Citation2002; Friendship Citation2004; Di Martino et al. Citation2013). Enriching the pen with manipulable material for pigs has been mandatory in the EU since 2013 (Directive Citation2008/120/EC). However, the effectiveness of this enrichment strategy on pig welfare and performance is still unclear (Hothersal et al. Citation2016). Currently, metal chains are widespread in pig intensive production in Europe, and are presently the most common enrichment at conventional Italian pig farms (Barbieri et al. Citation2014). However, chains are deemed insufficient to satisfy exploratory needs in the long-term (Bracke Citation2006) and to prevent abnormal behaviours, such as tail biting (EFSA Citation2007). Therefore, their use cannot be considered compliant with the EU recommendation 2016/336. Wooden and edible materials are rootable and chewable (Bracke Citation2006). Furthermore, edible substrates may be able to sustain pigs’ interest through the whole grow-to-finish period (van de Weerd and Day Citation2009).
Results on the effects of environmental enrichment at the farm on pork carcase and meat quality are conflicting, ranging from no effect (Hill et al. Citation1998), a lower ultimate pH (pHu) and greater drip loss (Foury et al. Citation2011; Rocha et al. Citation2016) to greater pHu and water-holding capacity values (Klont et al. Citation2001) in the loin muscle of pigs raised in enriched housing conditions compared to those housed in barren conditions. Beattie et al. (Citation2000) observed higher carcase weight and backfat, and lower cooking losses in pigs reared in an enriched compared to those reared in a barren environment. The validation of the effects of manipulable material in the pen on meat quality is particularly important in Italian heavy pigs considering that they are intended for the production of valuable protected designation of origin (PDO) meat products (such as Parma Ham), that are subjected to strict production rules and meat quality requirements (Consortium for Parma Ham Citation1992).
The objective of this study was to assess the impact of three manipulable tools (i.e. wood logs, a block of edible material inside a rack and metal chains) on preslaughter skin and tail lesions, oesophago gastric lesions and on carcase and meat quality traits in Italian heavy pigs.
Materials and methods
This study was carried out at the experimental farm of the Department of Veterinary Medical Sciences (DIMEVET) of the University of Bologna (Italy) and was authorised by the Institutional Ethics Committee (Authorization Prot. n. 2-IX/9—27 February 2012). To mimic farm conditions (Directive Citation2008/120 on swine protection), the experimental protocol did not include a negative control (i.e. without enrichment) group. A total of 80 barrows (Landrace × Large White) were used in two independent trials (40 pigs/trial, in terms of four pens of five pigs per treatment). Animals were reared on partially slatted floor and fed on a liquid diet. Experimental conditions are summarized in Table , while full description of the farm conditions and the enrichment devices is given in Nannoni et al. (Citation2018). For each trial, all animals were withdrawn of feed for 6 h before transport (last feed to slaughter of 18 h) and were transported for 1 h to the abattoir the day before slaughter. During transport and lairage, treatment groups were kept separated (no mixing). Two pens were merged to fill each of the truck and lairage compartments. Animals were kept in pens overnight and slaughtered the following morning. Skin and tail lesions were assessed at the farm according to the Welfare Quality® (Citation2009) protocol for growing and finishing pigs. However, due to the limited number of animals involved in the study, this protocol was slightly modified, in terms of assessment of both sides of each body region, to increase the number of assessed skin lesions. The average number of lesions between the two sides was calculated for each body region (ears, front, middle, hindquarters and legs) and used for classification according to the Welfare Quality® protocol (2009): each region was scored as ‘a’ (up to 4 lesions), ‘b’ (5 to 10 lesions) or ‘c’ (11–15 lesions) and regions with more than 15 lesions were recorded. Then each pig was scored using a 0-to-2 scale, where 0 corresponded to a pig having the full body classified as ‘a’, 1 to a pig having any body region with and individual score ‘b’ and/or a maximum of one region scored as ‘c’; and 2 to a pig having at least two body regions or more classified as ‘c’, or at least one body region with more than 15 lesions. Tail biting was assessed according to the following scale: 0 (intact tail), 1 (superficial biting) and 2 (fresh blood, swelling or infection, or tissue missing with crust formation). An antibiotic was sprayed as soon as a fresh tail lesion was observed. To trace each carcase on the slaughter line, a progressive number (slaughter order) was marked on each pig at stunning and the corresponding ear tag was recorded. Stomachs were collected and marked on the slaughter line, opened along the greater curvature, emptied of their content and carefully washed before proceeding with the assessment of the lesions on the pars oesophagea. Lesions scores were obtained according to Hessing et al. (Citation1992, in Amory et al. Citation2006) as follows: 0 (intact epithelium); 1 (small degree of hyperkeratosis occupying <50% of total surface); 2 (distinct hyperkeratosis at stage 1: > 50% of total surface but thickness <1 mm); 3 (distinct hyperkeratosis at stage 2: > 50% of total surface but >1 mm thickness); 4 (hyperkeratosis plus less than five erosions <2.5 cm in diameter); 5 (hyperkeratosis plus more than five erosions and/or erosions >2.5 cm in diameter); 6 (hyperkeratosis plus more than 10 erosions and/or erosions >5 cm in diameter, and/or ulcers, with or without bleeding or stenosis of the oesophagus towards the stomach). Scores were then regrouped in three classes of damage: absent or slight (0–1), medium (2–3) and severe (5–6). Some examples of the gastric lesions observed in this study are shown in Figure .
Figure 1. Examples of oesophago-gastric lesions (OGL) observed during the study (the numbers in the lower left corner of each picture indicate the score attributed to the lesion). 0 = intact epithelium; 1 = small degree of hyperkeratosis; 2 = distinct hyperkeratosis; 3 = distinct hyperkeratosis and erosion at an early stage; 4 = hyperkeratosis, mucus and one erosion; 5 = hyperkeratosis plus more than five erosions and/or erosions >2.5 cm in diameter; 6a = hyperkeratosis and ulcers; 6b = stenosis.
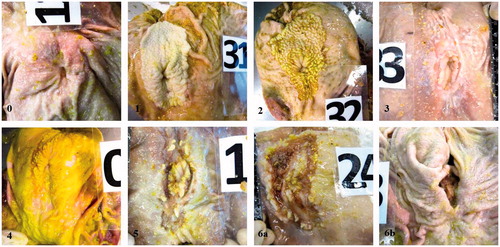
Table 1. Description of farm conditions and distribution of experimental groups.
Backfat and loin thickness were measured and lean meat percentage per each carcase was calculated by means of a Fat-o-Meater (F-o-M) probe (FOM-SFK, Copenhagen, Denmark) inserted in the P2 position, 6.5 cm from the edge of the dorsal mid-line at the level of the last rib. The weight of each carcase was recorded in order to calculate the dressing-out percentage. After carcase dissection, the weight of the main commercial cuts (thigh, loin and shoulder) was recorded and their yield (cut weight/carcase weight) was calculated. Muscle pH was measured using a pH-meter (model 250A; Orion Research, Boston, MA) in the Longissimus dorsi (LD) and in the Semimembranosus (SM) muscles at 45 min and in the LD muscle at 24 h post-mortem. Colour was measured in the LD and in the Biceps femoris (BF) muscles according to the CIE L* a* b* colour space (CIE Citation1976), using a Minolta Chromameter (CR-400, Konica Minolta Optics Inc., Tokyo, Japan) at 24 h post-mortem. A chop was taken from the LD muscle of each carcase (sampled at 7rd/8th thoracic vertebrae level) and stored at 4 °C until the analysis of drip and cooking loss, according to the method described by Honikel (Citation1998), and Warner–Bratzler shear force (WBSF). As for the latter analysis, six muscle cores, parallel to the longitudinal orientation of the muscle fibres, were taken from each cooked muscle chop and analysed for WBSF using an Instron Universal Testing Machine (model 1011, Instron Ltd., Buckinghamshire, England) fitted with a Warner–Bratzler device at a cross-head speed of 200 mm/min.
Hams were weighed at the beginning and at the end of dry-curing process (18 months) in order to calculate weight losses during the process. Data analysis was carried out for each trial separately using the software SAS Inst. Inc. (Cary, NC; release 8.0, 2014) considering the individual pig as the experimental unit for all parameters. Normal distribution of the number of skin lesions, and carcase and meat quality traits was tested using the UNIVARIATE procedure of SAS and all data were submitted to one-way ANOVA using the MIXED procedure, with enrichment as the main effect and pen as random effect. Chi-squared test was used to evaluate the distribution of skin, tail and gastric lesion scores across the severity classes. Spearman and Pearson correlations (PROC CORR) were calculated to assess the relationship between lesions and carcase quality parameters. Significance level was set at p < .05 for all tests.
Results and discussion
No health problems occurred during the experimental trials and no animal had to be removed. As shown in Table , in both trials, the exposure of pigs to manipulable tools neither had an effect on the proportion of skin and tail lesions nor on the overall number of lesions (p > .05, see the table for the exact p values). The prevalence of severe skin and tail lesions was low (below 5%, corresponding to maximum 1 affected animal per experimental group). Such a low prevalence in skin and tail lesions in undocked pigs in this study may be explained by other factors known to be positively correlated to a low incidence of skin and tail lesions, namely the stability of the groups (Van Staaveren et al. Citation2015), the low number of pigs per pen (Moinard et al. Citation2003; Scollo et al. Citation2016), the use of liquid meal (Moinard et al. Citation2003) and gentle-handling the pigs (Taylor et al. Citation2010).
Table 2. Severity classes of skin, tail and oesophago-gastric lesions in pigs exposed to hanging chains (C1 and C2) and wood logs (WL) and edible block (EB).
Overall, the prevalence of severe OGL observed in this study is similar to what reported by Di Martino et al. (Citation2013) on Italian heavy pigs (47% of stomachs affected by severe OGL) but also by Friendship (Citation2004) who reported 20% of severe erosion and 60% of pre-ulcerative parakeratosis as an average between different categories of pigs at the slaughter plants. However, similarly to skin and tail lesions, the manipulable tools used in this study had no effect of on OGL prevalence and severity (p > .05, as reported in Table ). Conversely, Di Martino et al. (Citation2013) found a reduced incidence of OGL in market-weight Italian heavy pigs provided with supplemental straw offered in a rack during the growing-finishing period. The type and the fibrous content of the enrichment material used by these authors could explain the different outcomes.
As shown in Table , in Trial 1 WL group presented higher lean meat percentage (p= .03), lower backfat thickness (p = .01) and higher drip loss (p = .02) when compared with C1. Klont et al. (Citation2001) observed an increased drip loss in pigs kept in a barren environment, while Beattie et al. (Citation2000) reported an increased cooking loss (but not drip loss) and reduced backfat thickness in pigs kept in a barren environment compared to pigs with a fully enriched environment (extra space, plus peat and straw in a rack). Similarly, in pigs slaughtered at 110 kg BW, Lebret et al. (Citation2006) observed lower backfat thickness, higher lean meat percentage and increased drip loss in pigs kept in a barren environment compared to pigs kept in an enriched environment (access to outdoor space). In Trial 2, except for lower (p = .03) loin thickness in EB carcases than C2 ones, the exposure to manipulable tools in the pen had no effect on carcase and meat quality traits (p > .05; Table ). Lastly, in both trials, thigh colour (BF muscle) and hams weight losses during dry-curing did not differ between the experimental groups (p > .05). Except for a positive, although rather weak, correlation between tail lesion score and OGL (r = 0.42; p = .01) observed in Trial 1, no significant correlations were found between lesions and carcase or meat quality parameters in this study. The relationship between tail biting and OGL might suggest concurrent causal events occurred during Trial 1 given that, according to EFSA (Citation2007), some risk factors identified for gastric ulcers (e.g. barren environment, lack of dietary fibre) are involved also in tail biting outbreaks.
Table 3. Carcass and meat quality traits in pigs exposed to hanging chains (C1 and C2) and wood logs (WL) and edible block (EB).
Conclusions
Results of both trials showed that, apart from minimal and of no commercial importance modifications in carcase and meat quality, the manipulable tools, i.e. wood logs or edible block, used in this study as environmental enrichments in alternative to the common hanging metal chains were not able to reduce prevalence and severity of body (including tails) and gastric lesions and did not affect carcase and meat quality traits of growing-finishing Italian heavy pigs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Amory JR, Mackenzie AM, Pearce GP. 2006. Factors in the housing environment of finisher pigs associated with the development of gastric ulcers. Vet Rec. 158:260–264.
- Barbieri S, Tremolada C, Cantafora A, Canali E, Gastaldo A, Borciani M, Iotti G. 2014. Use of manipulable material as environmental enrichment in the postweaning and fattening periods: what do Italian farmers do and think. Layman's Report AGER Filiera verde del suino. pp. 21–22. [accessed 2018 Mar 5]. http://agersuino.crpa.it/media/documents/agersuino_www/divulgazione/LRAger.pdf.
- Beattie VE, O’Connell N, Moss BW, O’Connell NE, Moss BW. 2000. Influence of environmental enrichment on the behaviour, performance and meat quality of domestic pigs. Livest Prod Sci. 65:71–79.
- Bracke MBM. 2006. Expert opinion regarding environmental enrichment materials for pigs. Anim Welf. 15:67–70.
- [CIE] Commission Internationale de l’Eclairage. 1976. Colorimetry. Publ. n. 15, Bureau Central de la CIE, Wien, Austria.
- Consortium for Parma Ham. 1992. Prosciutto di Parma (Parma Ham), Protected Designation of Origin. Specifications and Dossier. [accessed 2018 Mar 5]. http://www.prosciuttodiparma.com/pdf/en_UK/Specifications.pdf.
- Di Martino G, Capello K, Scollo A, Gottardo F, Stefani AL, Rampin F, Schiavon E, Marangon S, Bonfanti L. 2013. Continuous straw provision reduces prevalence of oesophago-gastric ulcer in pigs slaughtered at 170 kg (heavy pigs). Res Vet Sci. 95:1271–1273.
- [EFSA] European Food Safety Authority. 2007. Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to animal health and welfare in fattening pigs in relation to housing and husbandry. EFSA J. 5:564.
- EU Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs. Official J European Union L47:5–13.
- Foury A, Lebret B, Chevillon P, Vautier A, Terlouw C, Mormede P. 2011. Alternative rearing systems in pigs: consequences on stress indicators at slaughter and meat quality. Animal. 5:1620–1625.
- Friendship RM. 2004. Gastric ulceration in swine. J Swine Health Prod. 12:34–35.
- Hessing MJC, Geudeke MJ, Scheepens CJM, Tielen MJM, Schouten WGP, Wiepkema PR. 1992. Slijmvliesveranderingen in de pars oesophagea bij varkens: prevalentie en invloed van stress. Tijdschrift Voor Diergeneeskunde. 117:445–450.
- Hill JD, McGlone JJ, Fullwood SD, Miller MF. 1998. Environmental enrichment influences on pig behavior, performance and meat quality. Appl Anim Behav Sci. 57:51–68.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:447–457.
- Hothersal B, Whistance L, Zedlacher Z, Algers B, Andersson E, Bracke M, Courboulay V, Ferrari P, Leeb C, Mullan S, et al. 2016. Standardising the assessment of environmental enrichment and tail-docking legal requirements for finishing pigs in Europe. Anim Welf. 25:499–509.
- Klont RE, Hulsegge B, Gerritzen MA, Kurt E, De Jong IC, Kranen RW. 2001. Relationships between behavioral and meat quality characteristics of pigs raised under barren and enriched housing conditions. J Anim Sci. 79:2835–2843.
- Lebret B, Meunier-Salaü MC, Foury A, Mormède P, Dransfield E, Dourmad JY. 2006. Influence of rearing conditions on performance, behavioral, and physiological responses of pigs to preslaughter handling, carcass traits, and meat quality. J Anim Sci. 84:2436–2447.
- Melnichouk SI. 2002. Mortality associated with gastric ulceration in swine. Can Vet J. 43:223–225.
- Moinard C, Mendl M, Nicol CJ, Green LE. 2003. A case control study of on-farm risk factors for tail biting in pigs. Appl Anim Behav Sci. 81:333–355.
- Nannoni E, Sardi L, Vitali M, Trevisi E, Ferrari A, Ferri ME, Bacci ML, Govoni N, Barbieri S, Martelli G. 2018. Enrichment devices for undocked heavy pigs: Effects on animal welfare, blood parameters and production traits. (submitted to Italian Journal of Animal Science). https://doi.org/10.1080/1828051X.2018.1472531.
- Robertson ID, Accioly JM, Moore KM, Driesen SJ, Pethick DW, Hampson DJ. 2002. Risk factors for gastric ulcers in australian pigs at slaughter. Prev Vet Med. 53:293–303.
- Rocha LM, Velarde A, Dalmau A, Saucier L, Faucitano L. 2016. Can the monitoring of animal welfare parameters predict pork meat quality variation through the supply chain (from farm to slaughter)? J Anim Sci. 94:359–376.
- Scollo A, Contiero B, Gottardo F. 2016. Frequency of tail lesions and risk factors for tail biting in heavy pig production from weaning to 170 kg live weight. Vet J. 207:92–98.
- Studnitz M, Jensen MB, Pedersen LJ. 2007. Why do pigs root and in what will they root? A review on the exploratory behaviour of pigs in relation to environmental enrichment. Appl Anim Behav Sci. 107:183–197.
- Taylor NR, Main DCJ, Mendl M, Edwards SA. 2010. Tail-biting: A new perspective. Vet J. 186:137–147.
- Van de Weerd HA, Day JEL. 2009. A review of environmental enrichment for pigs housed in intensive housing systems. Appl Anim Behav Sci. 116:1–20.
- Van Staaveren N, Teixeira DL, Hanlon A, Boyle LA. 2015. The effect of mixing entire male pigs prior to transport to slaughter on behaviour, welfare and carcass lesions. PLoS One. 10:e0122841.
- Welfare Quality®. 2009. Welfare Quality® assessment protocol for pigs (sow and piglets, growing and finishing pigs). Welfare Quality® Consortium, Lelystad, Netherlands. [accessed 2018 Mar 5]. http://www.welfarequalitynetwork.net/network/45848/7/0/40.
