 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to assess the effect of rearing system (intensive vs. free-range under vineyard) on the qualitative characteristics of goose meat. In particular, the integration of a goose raising system with an organic grape production was evaluated. Six hundred Romagnola geese of both sexes were divided into two groups at 21 d of age: Vineyard group (V) – 480 geese (120 geese/ha) pasturing in 4 ha of vineyard, and Control Group (C) – 120 geese of the same genotype in the experimental farm of Perugia, without any access to the pasture. Live weight, feed consumption and other performance were registered weekly on 30 selected and marked animals/group. At 160 days of age, 15 geese/group were slaughtered and characteristics of carcase, breast and drumstick (physical, chemical, oxidative status, fatty acids profile) were evaluated. The chemical analysis of diet and pasture and the ingestion of crude protein, digestible energy and bioactive compounds were also estimated. Vineyard geese, showed lower productive performance than the C ones (live and carcase weight), however, due to pasture availability, the intake of bioactive compounds (vitamin E, retinol, n-3 long-chain fatty acids) was higher and positively affected the antioxidant content of breast and drumstick. The higher kinetic activity of V geese reduced the fat amount of carcase and meat, whereas increased the development of drumstick muscle (higher meat/bone ratio) and worsened the oxidative status of meat. Concluding, the free-range vineyard geese resulted in a positive payoff on the geese meat quality viewpoint.
Pasture enhance the amount of VIT E in goose muscle.
Pasture reduce the amount of lipid in goose carcass and meat.
Pasture enhance the amount of n-3 PUFA in goose meat.
Highlights
Introduction
Agroforestry is defined as the integration of woody vegetation (shrubs or trees) with agriculture and/or livestock production (Mosquera-Losada et al. Citation2009; Nair Citation2012). Such system is designed for improving the ecological-economic advantages respect to the conventional one (Clark and Gage Citation1996Citation; Sha et al. Citation2015).
This integration is particularly sound when the production system requires significant amount of land use (extensive livestock system). Accordingly, the significant growth in outdoor poultry production leading to increased demands of land resources (Brownlow et al. Citation2000).
Therefore, combining livestock with orchards, rather than grazing native pastures, results in less land use and provides further benefits as the protection exerted by trees and bushes on animals from predators and extreme temperatures (Dal Bosco et al. Citation2016), low need for chemical fertilisation, weeding and pest control (Paolotti et al. Citation2016).
In the context of agroforestry, goose is a very interesting species due to its unique ability to use high-fibre feeds. Accordingly, geese have been used as natural weeders with positive effect on the environment due to the removal of chemical weeding and provision of organic matter and natural fertilisers to the soil through droppings (Clauer and Skinner Citation2007).
Providing geese with access to pasture can also result in substantial savings of grain feed (Buckland and Guy Citation2002); in addition, geese do not require expensive housing system and fencing (Liu and Zhou Citation2013).
Meat characteristic of free-range and organically reared chickens has been widely investigated and previous studies have shown that pasture positively influences the quality of poultry (Dal Bosco et al. Citation2011, Citation2016), by increasing the content of bioactive substance (tocopherols, carotenoids and α-linolenic acid). However, reports concerning the effect of the rearing systems on the carcase and meat quality of geese are few (Liu and Zhou Citation2013) and the performance of Italian breeds is practically unknown.
Thus, the aim of the current study is to determine the effect of this rearing system on pasture and antioxidant intake, performance, carcass and meat quality of Romagnola geese.
Materials and methods
Description of systems
The case-study farm is located in Cannara (Umbria, Italy). This farm (30 ha) is dedicated to organic grape production and has recently experienced the introduction of geese in 4 ha of the vineyard. According to organic rules (Council Regulation: EC 834/2007 and 889/2008), grape was only sprayed with copper-based fungicides.
The trial was carried out from March to August 2016. At 21 days of age, the geese were divided into two homogeneous groups:
Vineyard Group (V): 120 Romagnola geese of both sexes per hectare (480 geese in total) were used; this number of animals approximately produces the concentration of nutrients required by the vineyard, that are, in terms of N, P and K, about to 69, 80 and 44 kg/ha, respectively.
Control Group (C): 120 geese of the same genotype were reared in the experimental farm of Perugia University without any access to the pasture.
The main differences between control and experimental group were represented by feed and housing.
The density in the house was the same (5 geese/m2) but in V, early in the morning (7:00 am), the house was opened, and geese came out to the pasture. The climatic classification of the area according to Kottek et al. (Citation2006) is Cfa (warm temperate climate, fully humid, with hot summer), where the minimum and maximum temperatures ranged from 15 to 35 °C and 45 to 90% relative humidity. In the C the geese remained in the same house, with temperature ranging from 20 to 35 °C and relative humidity from 65 to 70%.
In both groups, the diet was represented by a commercial diet (Table ) and the free-range geese also ate the freely available pasture. Two diets have been used: a starter diet (from 1 to 28 days) and a grower one (from 29 to 160 days).
Table 1. Ingredients (%) of commercial diets (starter and grower).
The housing system of V geese system provided only overnight shelter (wood and welded mesh), while the control geese were reared in a warehouse for all days of their life. Birds were raised until the slaughter age (160 d) according to EU Regulation 834/07, EU Regulation 889/2008 and Italian directives (Gazzetta Ufficiale Citation1992) on animal welfare for experimental and other scientific purposes.
At the beginning of the trial 30 geese/group, out of the 120 geese/group considered, have been marked with a colour spray on the back, in order to evaluate the productive performance on the same birds. During the trial, productive performances were recorded weekly (body weights and daily gain; n = 30/group, as well as the feed intake of each pen; n = 4). The average feed consumption of the group was used to calculate the feed/gain ratio. The number of culled and dead birds was also recorded.
To estimate the forage intake, the modified method of Lantinga et al. (Citation2004) was applied. At the start of the rearing cycles, five metallic frames (exclusion pens, 0.50 × 0.50 m) were positioned in each replication (n = 4).
Forage intake (GI) was estimated using the following equation:
where GMs: herbage mass present at the entrance of the birds in each pen; GMe: forage that remained at the end of the trial; and GMu: undisturbed forage mass from the exclusion. Crude protein (CP) and digestible energy (DE) was estimated with the equation of Lodhi et al. (Citation1976).
At 160 d, 15 geese per group were slaughtered, 12 h after the feed withdrawal in a mobile abattoir within 1 h from catching (Castellini et al. Citation2016). Geese were electrically stunned, bleeded, plucked eviscerated (non-edible viscera: intestines, proventriculus, gall bladder, spleen, oesophagus and full crop) and stored for 24 h at +4 °C. Head, neck, legs, edible viscera (heart, liver, gizzard) and fat (perivisceral, perineal and abdominal) were removed in order to obtain the ready-to-cook carcase (WPSA Citation1984). Carcasses were immediately transferred to the laboratory of the Department in order to determine quality traits as carcase and physical-chemical characteristics, fatty acid and oxidative status of meat.
Carcass dissection, sampling and analytical determinations
On 15 samples of Pectoralis major and Biceps femoris muscle per groups, moisture, ash and total nitrogen content was assessed by using the AOAC methods (950.46B, 920.153 and 928.08, respectively; 1995). Total protein content was calculated by Kjeldahl nitrogen using a 6.25 conversion factor. The fat content was gravimetrically determined using ether solvent extraction (method 960.30).
Ultimate pH (pHu) was measured with a Knick digital pHmeter (Broadly James Corp., Santa Ana, CA) after homogenisation of 1 g of raw muscle for 30 s in 10 mL of 5 M iodoacetate (Korkeala et al. Citation1986). The water-holding capacity was estimated by placing 1 g of whole muscle on tissue paper inside a tube and centrifuging for 4 min at 1500 × g. The water amount remaining after centrifugation was quantified by drying the samples at 70 °C overnight.
Water-holding capacity was calculated as follows: (weight after centrifugation – weight after drying)/initial weight ×100 (Castellini et al. Citation2002).
The cooking loss (CL) was measured on samples of about 20 g placed in open aluminium pans and cooked in an electric oven (pre-heated to 200 °C) for 15 min to an internal temperature of 80 °C. The CL was estimated as the percentage of the weight of the cooked samples (cooled for 30 min to about 15 °C and dried on the surface with a paper towel), respect to the weight of the raw samples (Cyril et al. Citation1996). Shear force was evaluated on cores (1.25 cm Ø; 2 cm length) obtained from the mid-portions of the roasted samples by cutting them perpendicularly to the direction of the fibre, using an Instron (model 1011; Instron, Norwood, MA), equipped with a Warner-Bratzler meat shear apparatus. The colour parameters [brightness (L*), redness (a*) and yellowness (b*)] were measured using a tristimulus analyser (Minolta Chromameter CR-200; Minolta, Tokyo, Japan), with the Cielab colour system (CIELAB Citation1976).
The lipids were extracted and esterified as described by Branciari et al. (Citation2017). Fatty acids were quantified as methyl esters (FAME with a gas chromatograph (Agilent Technologies 6890N Network GC System) equipped with a flame ionisation detector (FID) and an automatic sampler (Agilent Technologies 7683 Series Injector). A CP-Select CB for a FAME fused silica capillary column (100 m × 0.25 mm i.d., film thickness 0.39 µm, J&W, Agilent technologies, Palo Alto, CA, US) was used to separate and analyse the methyl esters. The injector temperature was 270 °C, while the detector temperature was 300 °C. The sample (1 µL) was injected into a split/splitless system (split ratio 1:25). The carrier gas was helium, at a flow rate of 1.6 mL/min. The oven temperature programme was as follows: temperature raised from 60 °C to 150 °C at a rate of 30 °C/min, the temperature was then held for 3 min and then raised to 185 °C at a rate of 0.5 °C, after 1 min the temperature was raised to 220 °C at a rate of 1.5 °C/min and held for 12 min. The identification of individual fatty acid methyl esters was done by comparison with a standard mixture by Sigma (PUFA No. 1, Marine Source, 37 FAMEs, methyl cis-7,10,13,16,19-docosapentaenoate Supelco, Bellefonte, PA). The percentage of each FA was calculated by using the peak area of the samples corrected with the respective correction factors (AOAC Citation2012). The average amount of each fatty acid was used to calculate the sum of the total saturated (SFA), total monounsaturated (MUFA) and total polyunsaturated (PUFA) fatty acids. Furthermore, the mg/100 g tissue of α-linolenic acid (ALA) and linolenic acid (LA) was calculated as reported by Joseph and Ackman (Citation1992), to estimate their daily intake. The following equation was applied:
where AX is the ALA or LA area, AIS is the internal standard area, CRFx is the theoretical correction factor for ALA and LA, CNFx is the conversion factor from FAME to the corresponding fatty acid, WIS is the weight of the internal standard added to the lipids, Ws is the weight of the derivatised lipids and WL is the percentage of sample lipid.
Tocopherols (α-tocopherol and its isoform β+ɣ and δ), α-tocotrienol and retinol meat content were quantified by HPLC according to Hewavitharana et al. (Citation2004). In particular, 5 mL of distilled water and 4 mL of ethanol were added to 2 g of meat sample and then vortexed for 10 s. After mixing, 4 mL of hexane containing BHT (200 mg/L) was added and the mixture was carefully shaken and centrifuged. An aliquot of supernatant (3 mL) was dried under a stream of nitrogen and then redissolved in 300 μL of acetonitrile. Fifty microlitres were injected into the HPLC system (Jasco, pump model PU-1580, equipped with an autosampler system, model AS 950-10, Tokyo, Japan) on a Ultrasphere ODS column (250 × 4.6 mm internal diameter, 5 µm particles size; CPS analytic, Milan, Italy). Tocopherols and tocotrienols were identify using a FD detector (model Jasco, FP-1520) set at excitation and emission wavelength of 295 nm 328 nm, respectively, and were quantified using external calibration curves prepared with increasing amounts of pure standards in ethanol. Retinol was detected by UV (JASCO UV 2075 Plus) at 325 nm, and quantified using an external calibration curve as described for tocopherols.
The meat lipid oxidation was evaluated according to Ke et al. (Citation1984) by a spectrophotometer set at 532 nm (Shimadzu Corporation UV-2550, Kyoto, Japan), which measured the absorbance of TBARS. Oxidation products were quantified as μg of malondialdehyde (MDA) per g of muscle.
Statistical analysis
The data were statistically analysed with a linear model comprising the fixed effect of rearing system (StataCorp Citation2015). Multiple comparisons were performed using Bonferroni’s range test and significance was set at p < .05.
Results and discussion
The chemical composition of diet and pasture mainly differed in the dry matter (Table ; 88.5 vs.18.5%, respectively), crude fibre (4.8 vs.18.6% DM, respectively) and in the content of ALA, tocopherols and carotenoids (1.6 vs.10.5 mg/g DM, 40.5 vs. 190.7 and 8.5 vs. 65.1 μg/g DM, respectively).
Table 2. Chemical composition and bioactive compounds content of diet (mean of starter and grower-finisher diets) and pasture.
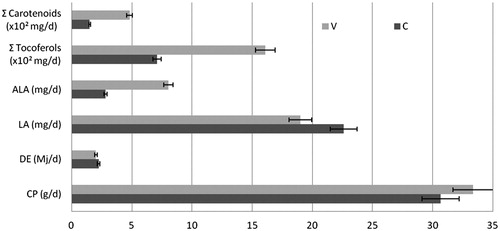
The V group ranged in all the available space (about 1 ha/group), as revealed by the difference between vegetation in the pasture and in the exclusion pens. Despite the higher intake of dry matter (solid feed + pasture) by vineyard-geese, the estimated intake of crude proteins (CP) and the digestible energy (DE) were similar in both groups (Figure ). On the contrary, the main bioactive compounds (fatty acids and antioxidants) were very different: the ALA, carotenoids and tocopherols contents were about 1.9, 1.3, and 2.3 times higher in V group, whereas the LA was 15% lower (Figure ).
Figure 1. Estimated intake of crude protein (CP, g/d), digestible energy (DE, Mj/d) and the main bioactive molecules (mg/d) by geese. DE and CP were estimated by Lodhi et al. (Citation1976). V: Vineyard group; C: Control group; ALA: α-Linolenic acid; LA: Linoleic acid.
Geese are naturally grazing animals and while doing this, they eat small invertebrates and/or earthworms that supplemented the ingested pasture (Schader et al. Citation2015). This pasture aptitude permitted to eliminate the weeding (about two times per year) and the fertilisation of vineyard.
Indeed, vineyard-geese had a lower live and carcass weight than the control group mainly due to the higher locomotor activity, with a lower solid feed consumption due to the pasture intake, which was about 68 g/d DM in the V group. Accordingly, the feed/gain ratio was lower in vineyard-reared geese (Table ) and mortality rate was similar.
Table 3. Performance and carcase characteristics of Romagnola geese.
The estimation of forage intake in birds reared free-range is difficult and only few studies have been carried out mainly with laying hens. Hughes and Dun (Citation1983), estimated that layers consume about 30–40 g DM/d of herbage, but also from worms and insects, plus 100 g of concentrate feed.
In our previous investigation (Dal Bosco et al. Citation2016), we showed that the forage intake was affected by the motor activity of chickens and both were influenced by outdoor enrichment and season. Chickens reared under olive trees had always higher herbage ingestion, exploiting the available area up to almost 50 m from the hut. In these rearing conditions, the maximum forage intake reached about 43 g DM/d.
The present results confirmed the great attitude of geese to exploit the pasture even in a not particularly favourable season like summer, reaching almost 70 g DM/d of herbage intake.
As in other avian species (Gatellier et al. Citation2004; Sossidou et al. Citation2015; Dal Bosco et al. Citation2016) the free-range system, connected with the pasture aptitude of animals, did not affect the intake of major nutrients (e.g. proteins, energy) but improved the amount of bioactive compounds, as observed in this trial.
Accordingly, the greater need for body maintenance due to kinetic activity of the vineyard-geese, connected with the availability of almost the same amount of DE and CP of control birds reduces the growth rate and the fat content (both in the carcass and in the meat, Table ), contemporarily improving the development of drumstick muscle and consequently the muscle/bone ratio (Dal Bosco et al. Citation2014).
In agreement, the higher movement also influenced other meat traits (e.g. pHu was 6.15 vs.6.36, Table ), which were probably affected by the higher amount of glycogen stored in the muscles of the more active animals (Dal Bosco et al. Citation2010) mainly in the muscles more engaged in the movement (drumstick), whereas the pHu of breast meat was not affected. Furthermore, as expected, the shear force value of C birds was lower than the free-range ones in both muscles.
Table 4. Physical-chemical characteristics of breast and drumstick muscle of Romagnola geese (n = 15/group).
The meat lipids content was the chemicals more affected by rearing system with lower percentage in V both in breast (2.29 vs. 4.20%) and drumstick (3.04 vs. 4.93%) muscle.
The oxidative status and antioxidant profile of muscles (α-tocopherol and its isoform β+ɣ and δ; α-tocotrienol and retinol) were deeply affected by rearing system (Table ). Control geese, despite a lower content of antioxidants in both muscles, showed a higher oxidative stability (TBARS values: 0.10 vs. 0.18 µg MDA/g and 0.07 vs. 0.16, respectively, in breast and drumstick meat).
Table 5. Oxidative status and main antioxidants of breast and drumstick muscle of Romagnola geese (n = 15/group).
Indeed, the antioxidants intake of V geese was higher than control as confirmed by the higher amount of vitamin E (α-tocotrienol, α- and δ-tocopherol, retinol) in both the muscles analysed. A similar trend was also observed in free-range chickens (Castellini et al. Citation2006, Citation2008).
However, these bioactive substances, taken by the pasture, are not sufficient to counteract the high oxidation processes determined by the movement (Mattioli et al. Citation2017). Indeed, the oxidative stability of V geese meat was lower than the C ones.
Generally, there was a positive correlation between antioxidant amount and TBARS; this discrepancy is probably related to the high locomotory activity of free-range geese, which increased the oxidative burst of muscle tissues. Vineyard geese had access to a free-range area, which encouraged the exploration and the movement. Probably, the kinetic activity of geese increased the oxygen demand and consequently raised the level of ROS in blood plasma and tissues with effect on PUFA stability (Dal Bosco et al. Citation2011; Mattioli et al. Citation2017).
Other papers found similar tendency (Nilzen et al. Citation2001; Castellini et al. Citation2002, Citation2006; Dal Bosco et al. Citation2016) confirming that the balance between kinetic behaviour and antioxidant intake is very unstable and subject to changes due to genetic strain, botanic essences of pasture, temperature and other environmental inputs (Cartoni Mancinelli et al. Citation2017).
The difference in the diets subministered could also be responsible for change in the colour of the meat, especially for its yellowness, as diet richer in yellow pigments (i.e. zeaxanthin) could lead to higher b* value of the meat (Toyomizu et al. Citation2001).
The major changes in the fatty acids content of breast concerned the PUFA profile with a higher n-6 and lower n-3 (mainly due to the Long Chain Fatty Acids – LCPn-3; Table ) in C than vineyard-geese. Accordingly, the n-6/n-3 ratio of control geese was about three times higher (17.91 vs. 6.06, respectively). Minor changes regarded C16:0, C18:0 and C18:1n-7.
Table 6. Main fatty acid of breast and drumstick muscles of Romagnola geese (n = 15/group).
The same trend was recorded in the drumstick muscle, with a lower difference between groups. The meat of vineyard geese showed a lower content of fat (p < .05) and higher value of n-3 PUFA compared with the control ones.
The locomotor activity positively influenced the foraging behaviour, and geese reared on pasture ingested a large amount of grass rich in ALA that is the precursor of LCPn-3. Furthermore, the pasture provide higher quantity of antioxidants which positively affected the susceptibility to the oxidation of the long-chain fatty acids (Woodand Enser 1997).
It should be noted that the n-6/n-3 ratio was better in the vineyard-meat due to the higher content of n-3 fatty acids (ALA, eicosapentaenoic acid, docosapentaenoic acids and docosaexaenoic acids), such result is interesting considering that the human diets in Western countries is strongly unbalanced in terms of n-6 and n-3 fatty acids intake (20–10:1 compared to the 4:1 recommended, FAO/MHO Citation2010). In agreement with the FAO recommendation (2010), the values found in vineyard-gees meat were closed to 4 that is considered the optimal value (6.06 and 3.06, respectively, in breast and drumstick meat).
The results of this trial are in agreement with Liu and Zhou (Citation2013), which observed that geese at pasture showed lower subcutaneous and abdominal fat respect to Control group. Geese with access to pasture had lower pH at 24 h post-mortem and higher ALA and eicosapentaenoic acid content and reduced n-6:n-3 ratio. The only data in countertendency was related to TBARS that in this trial was higher in V geese, whereas in the above-mentioned study was higher in the Control group; probably, this discrepancy is because the control group of the Chinese paper was performed in large pens and thus the movement of the two groups was probably similar while in our experiment the control group is reared without any external outdoor.
Conclusions
The agroforestry system, consisting in geese reared in vineyard-free-range, resulted in positive payoffs on the meat quality viewpoint. Although, the space availability, negatively affected the growth rate of vineyard-geese (lower live and carcase weight) and the lipid oxidative status of meat (higher TBARS value) compared to a convention reared systems, several advantages may be underlined:
the pasture availability promote the intake of some bioactive compounds, which positively affected the geese meat quality (higher tocopherols, retinol and LCPn-3 content);
the higher kinetic activity promote the development of the drumstick muscle (higher meat/bone ratio) and reduce the depot fat and the lipid content of meat. Furthermore, such integrated systems (vineyard plus animals) did not affect the grape production, also reducing the land use (two simultaneous production in the same land) and the environmental impact (Patrizi et al. Citation2017).
Acknowledgements
Authors deeply thank the winemaker farm Di Filippo for providing data.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AOAC. 1995. Official methods of analysis. 15th ed. Washington, DC: Association of Official Analytical Chemists.
- AOAC. 2012. Official methods of analysis. 18th ed. Arlington (VA): Association of Official Analytical Chemists.
- Branciari R, Ranucci D, Urbani E, Valiani A, Trabalza-Marinucci M, Dal Bosco A, Franceschini R. 2017. Freshwater fish burgers made from four different fish species as a valuable strategy appreciated by consumers for introducing EPA and DHA into a human diet. J Aquatic Food Product Techn. 26:686–694.
- Brownlow MJC, Carruthers SP, Dorward PT. 2000. Alternatives to grazing livestock. In: Hislop M, Claridge J, editors. Agroforestry in the UK. Forestry Commission Bulletin 122. Edinburgh, UK: Forestry Commission; p. 58–70.
- Buckland R, Guy G. 2002. FAO animal production and health paper 154: Goose production. Rome, Italy: Food and Agriculture Organization of the United Nations; p. 158.
- Cartoni Mancinelli A, Guarino Amato M, Meo Zili D, Dal Bosco A, Mattioli S, Castellini C. 2017. Adaptive response of chicken strains to the organic and free range rearing systems. Dairy Vet Sci J. 4:555644. doi:10.19080/JDVS.2017.04.555644
- Castellini C, Berri C, Le Bihan-Duval E, Martino G. 2008. Qualitative attributes and consumer perception of organic and free-range poultry meat. World's Poult Sci J. 64:500–512.
- Castellini C, Mattioli S, Piottoli L, Cartoni Mancinelli A, Ranucci D, Branciari R, Guarino Amato M, Dal Bosco A. 2016. Effect of transport length on in vivo oxidative status and breast meat characteristics in outdoor-reared chicken genotypes. It J Anim Sci. 15:191–199.
- Castellini C, Mugnai C, Dal Bosco A. 2002. Meat quality of three chicken genotypes reared according to the organic system. It J Food Sci. 14:411–412.
- Castellini C, Mugnai C, Pedrazzoli M, Dal Bosco A. 2006. Productive performance and carcass traits of Leghorn chickens and their crosses reared according to the organic farming system. Atti XII European Poultry Conference; Verona. Italy; p. 10–14.
- CIELAB. 1976. Colour System. Commission International de l’Eclairage. CIE, Publication 36 PARIS.
- Clark MS, Gage SH. 1996. Effects of free-range chickens and geese on insect pests and weeds in an agroecosystem. Am J Altern Agric. 11:39–47.
- Clauer PJ, Skinner JL. 2007. Raising waterfowl (A3311). Wisconsin: https://learningstore.uwex.edu/Assets/pdfs/A3311.pdf.
- Council Regulation (EC). 2007. No 834/07, 2007: on organic production and labeling of organic products and repealing Regulation (EEC) No 2092/. Off J Eur Union, L. 189:1–23.
- Cyril HW, Castellini C, Dal Bosco A. 1996. Comparison of three cooking methods of rabbit meat. It J Food Sci. 8:337–340.
- Dal Bosco A, Mugnai C, Amato MG, Piottoli L, Cartoni A, Castellini C. 2014. Effect of slaughtering age in different commercial chicken genotypes reared according to the organic system: 1. Welfare, carcass and meat traits. Ital J Anim Sci. 13:3308.
- Dal Bosco A, Mugnai C, Castellini C. 2011. Performance and meat quality of pure Ancona and Cornish × Ancona chickens organically reared. Arch Geflugelkd. 1:7–12.
- Dal Bosco A, Mugnai C, Mattioli S, Rosati A, Ruggeri S, Ranucci D, Castellini C. 2016. Transfer of bioactive compounds from pasture to meat in organic free-range chickens. Poult Sci. 95:2464–2471.
- Dal Bosco A, Mugnai C, Sirri F, Zamparini C, Castellini C. 2010. Assessment of a global positioning system to evaluate activities of organic chickens at pasture. J App Poultry Res. 19:213–218.
- FAO/MHO. 2010. Fats and fatty acids in human nutrition. Food Nutr Paper. 91:1–161.
- Gatellier P, Mercier Y, Renerre M. 2004. Effect of diet finishing mode (pasture or mixed diet) on antioxidant status of Charolais bovine meat. Meat Sci. 67:385–394.
- Gazzetta Ufficiale. 1992. Attuazione della Direttiva 86/609/CEE in materia di protezione degli animali utilizzati ai fini sperimentali o ad altri fini scientifici, D.L. January 27, 1992, n. 116. In: Supplemento ordinario alla Gazzetta Ufficiale, n. 40, 1992; 1–12.
- Hewavitharana AK, Lanari MC, Becu C. 2004. Simultaneous determination of vitamin E homologs in chicken meat by liquid chromatography with fluorescence detection. J Chrom A. 1025:313–317.
- Hughes BA, Dun P. 1983. Production and behaviour of laying domestic fowls in outside pens. Appl Anim Ethol. 11:201–206.
- Joseph JD, Ackman RG. 1992. Capillary column gas chromatography method for analysis of encapsulated fish oil and fish oil ethyl esters: Collaborative study. J AOAC Int. 75:488–506.
- Ke PJ, Cervantes E, Robles-Martinez C. 1984. Determination of thiobarbituric acid reactive substances (TBARS) in fish tissue by an improved distillation–spectrophotometric method. J Sci Food Agric. 35:1248–1254.
- Korkeala H, Mäki-Petäys O, Alanko T, Sorvettula O. 1986. Determination of pH in meat. Meat Sci. 18:121–132.
- Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. 2006. World map of the Köppen–Geiger climate classification updated. Meteorologische Zeitschrift. 15:259–263.
- Lantinga EA, Neuteboom JH, Meijs JAC. 2004. Sward methods. In: Penning PD, editor. Herbage intake handbook. 2nd ed. Reading, UK: The British Grassland Society; p. 23–52.
- Liu HW, Zhou DW. 2013. Influence of pasture intake on meat quality, lipid oxidation, and fatty acid composition of geese. J Anim Sci. 91:774–771.
- Lodhi G, Singh D, Ichhponani JS. 1976. Variation in nutrient content of feeding stuffs rich in protein and reassessment of the chemical method for metabolizable energy estimation for poultry. J Agric Sci. 86:293–303.
- Mattioli S, Dal Bosco A, Ruggeri S, Martino M, Moscati L, Pesca C, Castellini C. 2017. Adaptive response to exercise of fast-growing and slow-growing chicken strains: blood oxidative status and non-enzymatic antioxidant defense. Poult Sci. 96:4096–4102.
- Mosquera-Losada MR, McAdam JH, Romero-Franco R, Santiago-Freijanes JJ, Rigueiro-Rodróguez A. 2009. Definitions and components of agroforestry practices in Europe. In Agroforestry in Europe. Dordrecht: Springer; p. 3–19.
- Nair PKR. 2012. Climate change mitigation: a low-hanging fruit of agroforestry. In Agroforestry – the future of global land use. The Netherlands: Springer; p. 31–67.
- Nilzen V, Babol J, Dutta PC, Lundeheim N, Enfält AC, Lundström K. 2001. Free range rearing of pigs with access to pasture grazing – effect on fatty acid composition and lipid oxidation products. Meat Sci. 58:267–275.
- Paolotti L, Boggia A, Castellini C, Rocchi L, Rosati A. 2016. Combining livestock and tree crops to improve sustainability in agriculture: a case study using the LCA approach. J Clean Prod. 131:351–363.
- Patrizi N, Niccolucci V, Castellini C, Pulselli FM, Bastianoni S. 2017. Sustainability of agro-livestock integration: implications and results of energy evaluation. Sci Total Environ. 622:1543–1552.
- Schader C, Muller A, El-HageScialabba N, Hecht J, Isensee A, Erb KH, Smith P, Makkar H, Klocke PS, Leiber F, et al. 2015. Impacts of feeding less food-competing feedstuffs to livestock on global food system sustainability. J R Soc Interface. 12:91–99.
- Sha Z, Guan F, Wang J, Zhang Y, Liu H, Wang C. 2015. Evaluation of raising geese in cornfields based on energy analysis: a case study in southeastern Tibet China. Ecol Eng. 84:485–491.
- Sossidou EN, Dal Bosco A, Castellini C, Grashorn MA. 2015. Effects of pasture management on poultry welfare and meat quality in organic. Worlds Poult Sci J. 71:375–384.
- StataCorp. 2015. Statistical software, release 14. College Station (TX): StataCorp.
- Toyomizu M, Sato K, Taroda H, Kato T, Akiba Y. 2001. Effects of dietary Spirulina on meat colour in muscle of broiler chickens. Brit Poultry Sci. 42:197–202.
- Wood JD, Enser M. 1997. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Brit J Nut. 78:S49–S60.
- WPSA [World’s Poultry Science Association]. 1984. Method of dissection of broiler carcasses and description of parts. Fris Jensen J, Frederiksberg C-D, editors. Chapter 5. Cambridge (UK): Papworth Everard.
