Abstract
The progressive decrease of mean SCC in dairy herds worldwide is affecting SCC accuracy as a subclinical mastitis marker. This evidence supports studies aiming to apply differential cell count (DSCC) as a tool to identify mastitis. Two of the major obstacles to apply DSCC were the unavailability of high-throughput milk analysers and the cost of these analyses. Recently availability of high-throughput milk analysers, able to perform a partial DSCC on milk, allowed designing a study aiming to identify subclinical mastitis in individual milk samples. This paper reports the result of this first Italian study performed under field conditions. The study considered 4386 milk test records from four dairy herds with different size, management and milking management. DSCC data were analysed by ROC procedure. This procedure allows identifying the threshold giving the highest accuracy and the highest combined value for sensitivity and specificity, among all the possible thresholds. Among the different ways used to classify milk samples, the analysis applied to days in milk (three classes) showed the highest mean values for sensitivity plus specificity, and the value for accuracy was very close to the highest one observed. At the time of submission, this is the first paper available on peer-reviewed scientific journals reporting the evaluation of DSCC as a marker for subclinical mastitis on individual milk samples collected during routine milk test. The results will help the improvement of mastitis diagnosis and will help dairy farmers to increase the levels of herd management and efficiency.
At the time of submission, this is the first paper available on peer-reviewed scientific journals on the evaluation of DSCC as a marker for subclinical mastitis on individual milk samples.
The analysis of data showed as DSCC has not consistent performances, confirming the presence of confounding factors such as parity and days in milk.
The thresholds calculated on samples classified by days in milk (three classes) showed to have the overall best test performances with an accuracy of 82.3%.
Highlights
Introduction
Individual somatic cell count (SCC) represents probably the most practical and sustainable method to monitor udder health in dairy cow, despite it has not the same accuracy as microbiological analysis (Sargeant et al. Citation2001; Ferronatto et al. Citation2018). However, the progressive decrease of mean SCC in dairy herds worldwide is further affecting in SCC accuracy as a subclinical mastitis marker. Currently, a level of 200,000 cells/mL is considered the threshold to identify subclinical mastitis (Piccinini et al. Citation2005; Zecconi et al. Citation2018), but udder inflammation was observed even below 100,000 cells/mL (Zecconi et al. Citation2006; Merle et al. Citation2007; Piccinini et al. Citation2007; Baumert et al. Citation2009; Schwarz et al. Citation2011), and nearly half of contagious pathogens have a SCC below 100,000 cells/ml (Zecconi et al. Citation2003; Zecconi Citation2010).
This evidence supports studies aiming to apply differential cell count (DSCC) as a tool to identify mastitis, alone or in combination with SCC. Two of the major obstacles to apply this diagnostic procedure were the unavailability of high-throughput milk analysers, as well as the cost for this analysis. Indeed, microscopy is the conventional method to perform DSCC, but it is time consuming and it has poor repeatability. Flow cytometry is more efficient, but still unsuitable for analysing more than few dozens of samples per day, and the cost of the analysis and its accuracy are still critical points that prevent its application outside the research field (Koess and Hamann Citation2008).
The technical problems are not the only ones that need to be solved. Indeed, several studies performed on mastitis diagnosis with DSCC gave controversial results (Dosogne et al. Citation2003; Sarikaya et al. Citation2005; Baumert et al. Citation2009; Schwarz et al. Citation2011; Pilla et al. Citation2013). These differences can be related to the different diagnostic methods applied, to the different milk samples selected and to the different fractions of milk considered. Nevertheless, a consensus on the distribution of cellular types in milk from healthy or diseased cows is still not achieved. Consequently, broadly accepted DSCC procedure suitable to identify infected/diseased cows is still not available.
This scenario changed very recently with the availability of high-throughput milk analysers able to perform a partial DSCC on milk (Damm et al. Citation2017) which is fully integrated in the instruments currently used to analyse individual milk samples within milk test recording programmes. The availability of this instrument represents an important improvement in the field and supports studies aiming to define DSCC thresholds to identify subclinical mastitis and infected cows.
The first instrument of this type was installed in Italy at the end of August 2017 at the laboratories of Lombardy Regional Breeder Association (ARAL). After the requested period of training and instrument testing (i.e. calibration, reproducibility, repeatability, etc.), the equipment was available for practical use at the end of September. A study was designed aiming to define DSCC thresholds useful to identify subclinical mastitis by using individual milk samples and the effects of potential confounding factors. This paper reports the result of this first Italian study performed under field conditions.
Materials and methods
Milk sample collection and analysis
Milk tests were performed by certified methods, currently applied by Italian Breeders Association (www.aia.it) at the laboratories of ARAL on Fossomatic 7C (Foss, Hilleroed, DK). This instrument measures the milk content of several parameters (i.e. fat, protein, lactose, casein, SCC). Moreover, it applies Foss DSCC Method Cell Staining (international patent PCT/EP2010/065615; Damm et al. Citation2017). The method allows us to identify within a milk sample the macrophages (MAC) and the combination of polimorphonuclear neutrophils (PMN) and lymphocytes (LYM). The two latter ones are reported as the sum of the proportions of both cellular types, being undifferentiated by the instrument. Diagnostic characteristics and method performances were as described by Damm et al. (Citation2017).
Data collection
The study considered cow milk test records (MTR) from four dairy herds located in Lombardy Region. Herds were selected based on their different size and management characteristics, as described in Table .
Table 1. Characteristics of the herds considered.
Statistical analysis
The data were collected in a database including herdID, cowID, parity (PAR), days in milk (DIM), milk yield, SCC and DSCC expressed as proportion (%) of PMN + LYM on the sum of cells identified (PMN + LYM + MAC).
To define the most accurate thresholds, data were analysed by calculation of ROC curves on Xlstat 2018.3 (Addinsoft, Paris, France). This statistical procedure allows to represent the evolution of the proportion of true positive cases (also called sensitivity) as a function of the proportion of false positives cases (corresponding to 1 minus specificity), and to evaluate a binary classifier test to diagnose a disease. In our case disease was defined as a milk sample with >200,000 cells/mL (Piccinini et al. Citation2005; Zecconi et al. Citation2018). Table describes the parameters calculated to evaluate DSCC diagnostic test and the abbreviations used in the text.
Table 2. Definitions and abbreviations of the epidemiological measures reported in the text, tables and figures.
The prevalence of subclinical mastitis was calculated as number of samples >200,000 cells/mL divided by the overall number of samples within any specific class considered (i.e. DIM, parity, etc.).
Ethical statement
All the experimental procedures are in compliance with the art. 2 of EU regulation 2010/63/UE about the protection of experimental animals.
Results
The study considered 4386 milk test records from four dairy herds. The overall prevalence of subclinical mastitis was 22.5% (985 samples).
DSCC data were analysed by ROC procedure to assess the accuracy of this new diagnostic technology in identifying subclinical mastitis. This procedure allows identifying the threshold giving the highest accuracy and the higher combined value for SENS and SPEC, among all the possible thresholds within the full range of PMN + LYM values observed.
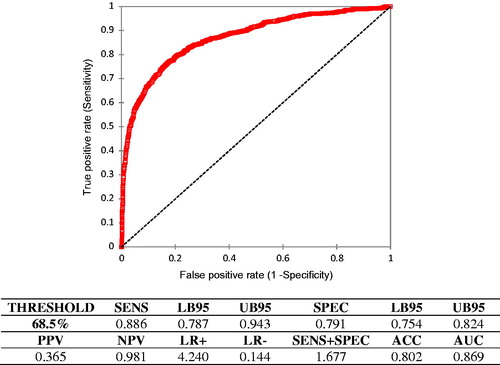
The overall data (Figure ) show as the threshold with the best performances is 68.5%. At this value, the test has an ACC of 0.816, with SENS of 0.763, SPEC of 0.831 and AUC of 0.869 (statistically significant, α = 0.05).
Figure 1. ROC curve calculated on all the milk test record (MTR) considered (4386) without classification. Abbreviations refer to parameters described in Table and are related to the identification of subclinical mastitis by differential cell counts (DSCC)
To assess the potential effects of confounding factor such as DIM and PAR, data were also analysed by classes; DIM were classified as <100 d, 101–200 d and >200 d, while PAR was classified as 1, 2 and >3 parturitions.
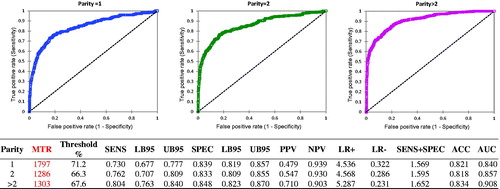
The results of ROC analysis on PAR classes is reported in Figure . Primiparous cows (40.6%) had a 16.9% prevalence of subclinical mastitis, while it was 20.9% in secondiparous cows (28.0%) and 31.7% in pluriparous cows (31.4%).
Figure 2. ROC curves calculated classifying the samples based on parity. Abbreviations refer to parameters described in Table and are related to the identification of subclinical mastitis by differential cell counts (DSCC)
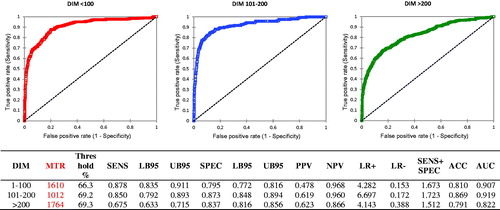
The results of ROC analysis on DIM classes (Figure ) showed as 37.1% cows in the first period of lactation (1–100 d) had a 17.6% prevalence of subclinical mastitis, it was 19.5% in cows with 101–200 d (22.6%), and 28.5%. in the last part of lactation (40.3%).
Figure 3. ROC curves calculated classifying the samples based on days in milk. Abbreviations refer to parameters described in Table and are related to the identification of subclinical mastitis by differential cell counts (DSCC)
In both analyses, the results showed significantly different thresholds when compared with the one calculated for the overall samples and significant differences among the thresholds calculated within each factor (DIM or PAR).
Based on these results, we verified also the diagnostic accuracy of DSCC based on a simplified classification of DIM and PAR. Only two classes were considered for both DIM (1–100 d and >100 d) and PAR (1, >1 parturition), then they were combined to define four classes (primiparous cows up to 100 DIM, primiparous cows with >100 DIM, pluriparous cows up to 100 DIM, and pluriparous cows with >100 DIM). The results (Figure ) showed, as expected, four different thresholds that statistically differ among them.
Figure 4. ROC curves calculated classifying the samples based on parity (PAR) and days in milk (DIM) (P100: PAR =1 DIM ≤100; P200: PAR >1 DIM >100; PL100: PAR >1 DIM ≤100; PL200: PAR >1 DIM >200). Abbreviations refer to parameters described in Table and are related to the identification of subclinical mastitis by DSCC. PAR: parity; DIM: days in milk; DSSC: differential cell count.
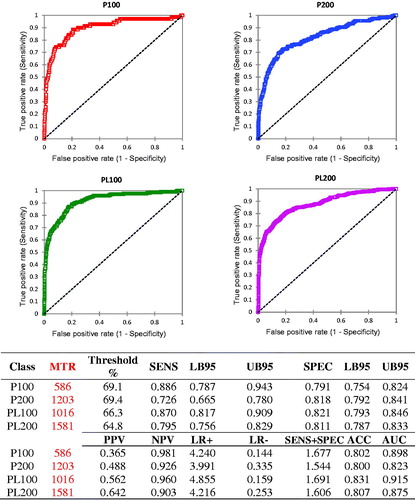
Table reports the summary of all the epidemiological parameters calculated in this study to compare them in order to identify the most accurate and useful set of DSCC thresholds applicable to subclinical mastitis diagnosis. Data showed as the highest values for each specific parameters are scattered among the different ways we classified samples. However, ROC analysis applied to DIM (three classes, Figure ) showed the highest mean AUC and the highest mean of the sum of SENS + SPEC, and the value for ACC was very close to the highest value observed.
Table 3. Summary of the parameters calculated to evaluate test performances based on the different methods of sample classification.
Discussion
The importance of DSCC for mastitis diagnosis is confirmed by the increasing number of papers on this topic observed in the last 10–15 years. Most of these studies were not performed under field conditions, because the tools available until 2017 did not allow applying DSCC to a large number of samples and the analyses were relatively expensive. The recent availability of a high-throughput milk analyser allows to overcome these latter problems and to apply DSCC to milk samples routinely collected by ARAL and analysed under field conditions.
At the time of submission, this is the first paper on a peer-reviewed scientific journal available reporting the evaluation of DSCC as a marker for subclinical mastitis on individual milk samples collected during routine milk test.
The analysis of data showed as DSCC has not consistent performances among the different methods of sample classification, confirming the presence of confounding factors (DIM and PAR), as suggested (Dosogne et al. Citation2003). However, when all the test performance data were compared, the thresholds calculated on samples classified by DIM (three classes) showed to have the overall best test performances. The evidence that the DIM-based thresholds have the highest value for the combination of SENS + SPEC, the highest AUC and only 0.001 less than the highest ACC observed supports the use of this approach. Indeed, it allows us to have the highest probability of precisely identify subclinical mastitis, the highest proportion of true positives and true negatives and nearly the highest accuracy in defining udder health. Therefore, values of 66.3%, 69.2% and 69.3% can be applied to samples collected from cows having, respectively, ≤100 DIM, 101–200 DIM and >200 DIM. These threshold values will give the highest probability to identify correctly udder health status.
Conclusions
The availability of this new high-throughput milk analyser opens the way to a series of new studies aiming to improve the performances of standardised and automatised milk analyses in detecting subclinical mastitis and inflammations. Therefore, these results should be considered as a first contribution to improve the diagnostic systems available under field conditions for dairy herds. This will improve the quality and usefulness of information supplied to dairy farmers, and it will help to increase the levels of herd management and efficiency.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Baumert A, Bruckmaier RM, Wellnitz O. 2009. Cell population, viability, and some key immunomodulatory molecules in different milk somatic cell samples in dairy cows. J Dairy Res. 76:356–364.
- Damm M, Holm C, Blaabjerg M, Bro MN, Schwarz D. 2017. Differential somatic cell count-A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J Dairy Sci. 100:4926–4940.
- Dosogne H, Vangroenweghe F, Mehrzad J, Massart-Leen AM, Burvenich C. 2003. Differential leukocyte count method for bovine low somatic cell count milk. J Dairy Sci. 86:828–834.
- Ferronatto JA, Ferronatto TC, Schneider M, Pessoa LF, Blagitz MG, Heinemann MB, Della Libera AMMP, Souza FN. 2018. Diagnosing mastitis in early lactation: use of Somaticell®, California mastitis test and somatic cell count. Ital J Anim Sci. 17:723–729.
- Koess C, Hamann J. 2008. Detection of mastitis in the bovine mammary gland by flow cytometry at early stages. J Dairy Res. 75:225–232.
- Merle R, Schroder A, Hamann J. 2007. Cell function in the bovine mammary gland: a preliminary study on interdependence of healthy and infected udder quarters. J Dairy Res. 74:174–179.
- Piccinini R, Binda E, Belotti M, Casirani G, Zecconi A. 2005. Comparison of blood and milk non-specific immune parameters in heifers after calving in relation to udder health. Vet Res. 36:747–757.
- Piccinini R, Binda E, Belotti M, Dapra V, Zecconi A. 2007. Evaluation of milk components during whole lactation in healthy quarters. J Dairy Res. 74:226–232.
- Pilla R, Malvisi M, Snel GGM, Schwarz D, Konig S, Czerny CP, Piccinini R. 2013. Differential cell count as an alternative method to diagnose dairy cow mastitis. J Dairy Sci. 96:1653–1660.
- Sargeant JM, Leslie KE, Shirley JE, Pulkrabek BJ, Lim GH. 2001. Sensitivity and specificity of somatic cell count and California Mastitis Test for identifying intramammary infection in early lactation. J Dairy Sci. 84:2018–2024.
- Sarikaya H, Schlamberger G, Bruckmaier RM. 2005. Leukocyte populations and cytokine mRNA expression in quarter milk fractions of dairy cows at different SCC levels. J Anim Sci. 83:297–298.
- Schwarz D, Diesterbeck US, Konig S, Brugemann K, Schlez K, Zschock M, Wolter W, Czerny CP. 2011. Flow cytometric differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J Dairy Sci. 94:5033–5044.
- Zecconi A. 2010. Staphylococcus aureus Mastitis: what we need to know to control them. Isr J Vet Med. 65:93–99.
- Zecconi A, Cesaris L, Liandris E, Daprà V, Piccinini R. 2006. Role of several Staphylococcus aureus virulence factors on the inflammatory response in bovine mammary gland. Microb Pathogen. 40:177–183.
- Zecconi A, Piccinini R, Fox KL. 2003. Epidemiologic study of intramammary infections with Staphylococcus aureus during a control program in nine commercial dairy herds. JAVMA. 223:684–688.
- Zecconi A, Sesana G, Vairani D, Cipolla M, Rizzi N, Zanini L. 2018. Somatic cell count as a decision tool for selective dry cow therapy in Italy. Ital J Anim Sci. accepted, in press, DOI:10.1080/1828051X.2018.1532328
