 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was conducted to investigate the effects of dietary supplementation of L-glutamine and the synergistic effects between glutamic acid of wheat and glutamine on the performance, carcase characteristics and intestinal morphometry in guinea fowl chickens fed with corn–soybean meal–wheat-based diets. A total of 288 one-day-old guinea fowl chicks (Numida meleagris) were used in a completely randomised design with six treatments, six replicates and 8 male chickens per replicate. The treatments were as follows: (1) corn–soybean meal-based diet (control), (2) corn–soybean meal–wheat-based diet, (3) treatment 1 containing 0.5% L-glutamine, (4) treatment 1 containing 1% L-glutamine, (5) treatment 2 containing 0.5% L-glutamine and (6) treatment 2 containing 1% L-glutamine. The results showed that the body weight gain (BWG) was higher in glutamine-supplemented treatments and the best feed conversion ratio (FCR) was observed in treatment 2 with 0.5% glutamine supplementation (p < .05). The chicks fed by diets containing glutamine supplement showed a higher live weight and percentage of breast, thigh and wings compared to glutamine-free diets, whereas the highest percentage of liver and heart observed in treatment 1 and the highest percentage of gizzard in treatments 1 and 2 (p < .05). The length and the width of villi and crypt depth in all three parts of the small intestine (duodenum, jejunum and ileum) were higher in glutamine-based treatments compared to glutamine-free treatments (p < .05). Also, the addition of wheat to diet had no negative effects on the mentioned parameters; moreover, the 0.5% L-glutamine supplement in wheat–corn–soybean diets improved performance, carcase characteristics and intestinal morphometry.
Introduction
An increased demand for chicken meat throughout the world has shifted the attention to other species of poultry including turkeys, guinea fowls and quails. Guinea fowls are originally from Africa and they are from the pheasant family (Moutab et al. Citation2015). Breeding this chicken can produce highly nutritional meat which includes the amino acid profile that is appropriate for human needs, as it contains low cholesterol and many essential fatty acids. The meat of this bird is crisp and delicious and is a good source of vitamins and minerals including vitamin B6, iron and selenium (Ayeni Citation1980). Guinea fowls are also resistant to common diseases and require minimal labour and management (Jacob and Pescator Citation2013). Despite having many advantages, these birds have disadvantages as well. Guinea fowls have low meat production and high feed conversion rate (FCR), which results in low yield and high breeding cost (Nahashon et al. Citation2004). Better feeding strategies may help improve functional characteristics, growth and meat production, which would reduce the cost of breeding such valuable birds. Timely growth and maturation of the digestive tract in broiler chickens are key to gain optimal performance. Nutrition and the use of nutrients play a major role in the development and improvement of the function of this device and the development of small intestine, increasing the focus on the use of supplements and their positive effects (Bartell and Batal Citation2007). Research has shown that the use of amino acid supplements in broiler diets improves performance and carcase traits (Sigolo et al. Citation2017). Glutamine is one of the most abundant amino acids in the blood plasma that plays an important role in muscle structure, body tissue and body weight gain (BWG) (Lee et al. Citation2008). Glutamine can be a synthesised form combination of glutamic acid and ammonia by glutamine synthetase, especially in the muscle (Francis and Griffiths Citation2002). Glutamine’s role as an energy source for the intestinal cells has been widely accepted (Newsholme et al. Citation2003), as well for increasing mucin production (Tako et al. Citation2004) and modulating in gene expression (Kadam et al. Citation2008). It has been shown that glutamine modulation in the digestive system can improve absorption and subsequently increase performance and other traits (Wu Citation2010). Glutamine plays an important role in maintaining the integrity of the digestive system and the growth of beneficial microorganisms, it increases myosin synthesis to maintain the structure of the intestinal mucosa and strengthens the intestinal tract lining of the intestine against harmful bacterial attacks (Francis and Griffiths Citation2002; Mok and Hankard Citation2011). Glutamine decreases nitric oxide, urine toxicity and oxidation activity, increasing the production and function of hormones, including the growth hormone which contributes to improved animal performance (Suchner et al. Citation2000). Wheat is a good replacement for corn in the poultry diet. Gliadin and glutenin are the two wheat proteins that are found in wheat gluten, which has two major amino acids, glutamic acid and spastic acid, as well as proline. Wheat has high levels of gluten and glutamic acid, which can be converted to glutamine in the body, and may show synergistic effects with glutamine when added to the diet (Shewry et al. Citation2002). The L-glutamic acid is also considered an efficient source of non-specific nitrogen, capable of promoting the development and growth, improving the performance and decreasing mortality in broilers (Bezerra et al. Citation2013). Previously, most of the positive effects reported for glutamine were due to the addition of this supplement to human and mouse food; however, in recent years, researchers have shown more interest in the effects of adding glutamine to poultry diets. Further study is needed to establish the positive effects of glutamine supplement in the improved development of birds and to determine the optimal levels of use of this supplement.
In this research, we have tried to improve the performance, growth, meat production, carcase characteristics and morphometric parameters of guinea fowl by replacing part of the diet with wheat and adding glutamine supplements. Therefore, this study was conducted to evaluate the effects of L-glutamine supplementation in corn–soybean meal–wheat-based diets on performance, carcase characteristics and intestinal morphometry in Guinea fowl chickens.
Materials and methods
Birds
This study was conducted in East Azerbaijan Research Center for Agriculture and Natural Resources and all the test procedures were approved by standard committee of Research’s Science University (Approval date: 04/02/2016; No: 10030). A total of 288 Guinea fowl chickens (Numida meleagris), one day of age with weight mean of 25 ± 5 g, were allocated to a completely randomised design with six treatments each consisting of six replicates and eight male chickens per replicate for 90 days. Experimental conditions were similar for all birds: for the first week, there was continuous lighting; from the second week to the end of the fourth week, 20 hours of brightness and 4 hours of darkness; from the fifth week to the last week, 16 hours of lighting and 8 hours of darkness. The temperature was 32 ± 1° C in the first week and was decreased by 3 degrees per week to 21° C ± 1° C which was controlled 24 hours a day. All the chicks had free access to feed and water. Chickens were fed with corn–soybean meal–wheat-based diets containing 0.5 and 1% L-glutamine. Experimental treatments were as follows: (1) corn–soybean meal-based diets (control), (2) corn–soybean meal–wheat-based diets, (3) treatment 1 containing 0.5% L-glutamine, (4) treatment 1 containing 1% L-glutamine, (5) treatment 2 containing 0.5% L-glutamine and (6) treatment 2 containing 1% L-glutamine. Glutamine was purchased from Wellife South Korean Company. Diets were formulated according to the standards recommended by Shamsaie (Citation1994). The diet composition is presented in Table . The proximate analysis of diets was performed according to Association of Official Analytical Chemists (AOAC Citation2004).
Table 1. Ingredient and nutrient composition of experimental diets.
Analysis of amino acids
In this study, corn, wheat and soybean meal ingredients are diets. Thus, analysis of amino acid corn, wheat and soybean meal was important. Analysis of amino acids was performed using high-performance liquid chromatography (HPLC) as explained by Moral et al. (Citation2007) and the data are presented in Table . The amino acid composition was reported as percentage of protein content (i.e. in g/100 g of protein).
Table 2. Amino acid composition (g/100 g of protein) for used wheat, corn and soybean meal.
Performance
During the experiment, the amount of feed intake (FI) and BWG were measured every two weeks and the FCR was also calculated. The casualties were recorded daily and the amount of feed consumed based on the chicken day was calculated.
Characteristics of the carcase and small intestine morphometry
Investigation of carcase traits (relative weight of organs) and morphometry of the small intestine on the last day of the experiment after 4 hours of starvation, from each replication of a male bird, were selected on the basis of average weight of each unit, weighed and slaughtered. The net weights of carcase, thighs, breast, wings, liver, gizzard, heart and spleen were measured using a digital scale (accurately ±0.01 g) and then, by dividing these weights into the bird’s weight, their relative weight was calculated.
To measure the small intestinal morphology (length, width of villi and crypt depth), approximately 2 cm from the middle tissue of the duodenum, jejunum and ileum was removed and after washing and extraction of the contents with physiologic serum (0.9% salt), they were fixed in 10% formalin buffer solution and placed in a histolectric device for processing. After paraffin moulding, sections of 5 micrometres of each sample were prepared. Then, they were stained with haematoxylin and eosin and investigated by a light microscope connected to the computer (Iji et al. Citation2001).
Statistical analyses
All birds’ data were subjected to statistical analysis (SAS Institute Citation2000) using analysis of variance (ANOVA) appropriate for a completely randomised design. When significant effects were detected by ANOVA, treatment means were compared using Duncan’s multiple range test. Differences were considered significant at p < .05. All parameters were examined as follows according to EquationEquation 1(1)
(1) :
(1)
(1)
where Yij is the individual observation, μ is the overall mean, Ti is the effect of treatment and eij shows the random error.
Results and discussion
Table shows the results of the performance of the fowl chickens. The lowest FI was attributed to birds fed with control diet and most of them were for diets containing 1% glutamine supplementation compared to other birds (p < .05). As seen in the table, the lowest feed conversion rate (FCR) and the highest BWG were observed in treatment 2 containing 0.5% glutamine (treatment 5) and the highest FCR was observed in control and treatment 2 with 1% glutamine supplementation (treatment 6). The lowest amount of BWG is related to the control treatment. The BWG in treatments containing glutamine supplement had the highest increase compared to the glutamine-free treatments and treatment 2 diet that contained wheat had more BWG than treatment 1 (control and without wheat). These findings clearly show the beneficial effects of glutamine on performance and BWG. In corn–soybean meal diets, with increased levels of glutamine, FI, higher weight gain and lower FCRs were obtained. By comparing treatments 1 and 2, it can be seen that adding wheat to the diet also increases FI and BWG and reduces feed conversion factor (p < .05). The 0.5% glutamine in wheat–corn–soybean meal diet had favourable effect on performance in that it had lowest FI, highest BWG and the lowest FCR. This finding clearly shows that lower levels of glutamine (0.5%) in corn–soybean meal–wheat diets improve performance compared to corn–soybean meal-based diets. However, at higher levels of supplementation, 1% glutamine increases the body weight of the bird but with increasing FI and increases the FCR. Comparing the treatments 1 and 2, it can be concluded that the addition of wheat to the diet has no negative effects on the performance of the fowl chickens. In general, wheat has less energy than corn, but in terms of other nutrients such as protein and many amino acids such as lysine, methionine, glutamic acid, arginine, tryptophan and phenylalanine, wheat has higher levels (Meng et al. Citation2005). Studies of Shekari et al. (Citation2012) and Mohamadi and Aghazade (Citation2014) showed that the growth performance of broiler chickens fed with wheat-based diets was significantly higher than those fed with corn-based diets (p < .05). It was expected that the addition of higher amount of glutamine in a wheat-containing diet (treatment 6) would have more favourable effects on functional attributes than that of corn, but this was not observed in relation to productive performance. On the other hand, considering the effects of some anti-nutritional substances in wheat (Gutierrez del Alamo et al. Citation2008), productive performance was expected to be significantly lower in treatment 2 than treatment 1; however, this was not observed in this study. Since the entire corn has not been replaced with wheat, there may be less anti-nutritional effects. Another possible reason is that the negative effects of wheat anti-nutritional substances on the digestive tract may be influenced by the positive effects of glutamic acid in wheat, thus reducing such negative effects (Shewry et al. Citation2002).
Table 3. Effect of dietary supplementation of L-glutamine on performance in guinea fowl chickens.
However, the findings indicate improved performance in both diets by adding glutamine. In relationship with these findings, Jahide et al. (Citation2014) stated that adding 1% glutamine to the broiler chickens diet significantly (p < .05) improved their growth performance. Similar findings have been reported by other researchers on the role of glutamine as a supplement to improve the growth performance of birds (Yi et al. Citation2005). The positive role of glutamine in improving performance is in several aspects. Glutamine positively affects the growth and development of the digestive system, increasing the length of the intestinal villi and improves the digestion and absorption of nutrients, which improves performance and FCR (Jahide et al. Citation2014). Glutamine via hexoses amine biosynthesis increases gastric mucin production (Tako et al. Citation2004). It increases the expression of genes and the biological activity of enzymes secreted from intestinal mucosal cells (Kadam et al. Citation2008). It also improves performance and weight gain. Glutamine is a source of energy for the mucosa turnover, via the ATP produced from Krebs cycle, a source of nitrogen and carbon for synthesis of other amino acids and nitrogen compounds via the increased activity of protein kinase gene mTOR, and most importantly, it is a glutathione precursor (Bezerra et al. Citation2013). Yi et al. (Citation2005) showed that adding 1% glutamine to diet of chicken turkeys in the first week of breeding period improves BWG and FCR compared to control (corn and soybeans) and it increases the length of the intestinal villi. Dai et al. (Citation2011) and Priya et al. (Citation2010) also reported improved weight gain (BWG) in broilers as a result of glutamine supplements. The role of glutamine as an effective nutrient in the growth of beneficial intestinal microflora (Francis and Griffiths Citation2002; Mok and Hankard Citation2011) has also been reported to be important in wheat-based diets. Therefore, the addition of wheat to the diet has no negative effects on performance and diet supplementation with glutamine improves the performance of the fowl chickens.
Table shows the effects of glutamine supplementation in diet and wheat replacement on carcase characteristics. The net weight of the carcase was higher in treatments containing glutamine supplement versus treatments 1 and 2 and the percentage of breast in the diet of treatment 2 with 0.5% glutamine supplement (treatment 5) was the highest compared to the rest of the groups. The percentage of liver and heart in treatment 1 and gizzard in treatment 1 and treatment 2 were the highest among all treatments. Diet 2 that contained 1% glutamine supplement (treatment 6) had the highest percentage of wings compared to other treatments (p < .05). Spleen percentage was not affected by nutritional interventions (p > .05). This finding is well illustrated by the role of glutamine in improving these carcase traits (net carcase weight, thigh percentage, percentage of breast and wings). The results are consistent with Mendanha et al. (Citation2014), which found that using 1% glutamine in broilers diets for 1–42 days significantly improved growth and carcase quality. Salmanzadeh and Shahryar (Citation2013) reported that using 0.5% glutamine supplementation for 42 days in Japanese quail ration significantly increased weight, improved FCR and carcase characteristics. L-glutamine has a pivotal role in the transfer of nitrogen between tissues, especially that of muscle to the liver, kidney and intestines, and thus plays a role in the synthesis and degradation of protein and muscle in the body (Suchner et al. Citation2000). Glutamine plays a role in balancing and retaining muscle water, increasing muscle mass and weight (Watford and Wu Citation2005). According to Adjei et al. (Citation1994), glutamine helps to metabolise fats, helps to relieve amino acids and prevents muscle breakdown and degradation. Glutamine also plays a role in the synthesis of arginine in the body, which increases the size of the liver and pancreas. Increasing plasma glutamine concentrations increases the activity of the mTOR protein kinase gene. This protein promotes the growth, differentiation and survival of cells and by protein synthesis, and it prevents muscle breakdown and causes muscle and tissue growth (Wu Citation2010). Therefore, according to the above, positive effects of glutamine on the improvement of carcase characteristics, which was also observed in this experiment, have been concluded. However, the increase in the weight of liver and spleen due to glutamine affecting synthesis of arginine was not observed in this experiment. Comparing treatments 1 and 2, it can be concluded that the use of wheat increases the carcase weight and reduces liver and heart percentages (p < .05), and the variation in other carcase characteristics is not significant (p > .05). This clearly shows that adding wheat to the diet increases carcase weight. Glutamic acid and aspartic acid are the major amino acids in wheat; therefore, wheat is the source of glutamic acid and upon digestion it converts into glutamine in the body. However, the reason for reduction in the percentage of liver and heart in corn–soybean meal–wheat diets is unknown. This reduction may be related to anti-nutritional agents in wheat that increase the viscosity of the digestive system in birds and may be impeding absorption of some of the nutrients needed to improve liver and heart weight. According to Afshar and Moslehi (Citation2005), adding 5% wheat gluten to broiler diets for 42 days significantly increased the growth and weight of birds. Banfield et al. (Citation1999) and Garcia et al. (Citation2004) reported that the use of wheat in the diet of broilers increases gizzard and liver weight.
Table 4. Effect of dietary supplementation of L-glutamine on carcase characteristics in guinea fowl chickens (percentage of live weight).
Table shows the effects of glutamine supplementation in corn–soybean meal and corn–soybean meal–wheat diets on intestinal morphometry. The results show that the addition of glutamine in both diets with wheat and without wheat significantly (p < .05) affects three parts of the small intestine (duodenum, jejunum and ileum). In the first part of the intestine (duodenum) villi length and width, except for non-glutamine supplementation (treatments 1 and 2) was higher in all treatments containing glutamine supplement and the depth of the crypt in treatment 2 plus 1% glutamine supplement (treatment 6) was the highest compared to the rest of the treatments (Figure ). In the middle part of the intestine (jejunum), treatments containing 0.5% glutamine supplement in both diets (treatments 3 and 5) had the highest length and width of villi and crypt depths (Figure ). The length and width of the villi and the depth of the crypt in the end of the small intestine (ileum) in the diets of treatment 1 containing 0.5% (treatment 3) and 1% (treatment 4) glutamine supplementation and the treatment 2 containing 0.5% glutamine (treatment 5) are larger compared to other diets (Figure ). The results obtained according to Jahide et al. (Citation2014) indicate that the use of the 1% glutamine supplement in broilers diets increases the growth and development of the digestive tract and increases the length of the intestinal villi. The use of glutamine at 1% level in broiler improves gastrointestinal growth and development, increases villi and increases nutrients digestion and absorption (Bartell and Batal Citation2007). Mendanha et al. (Citation2014) and Menconi et al. (Citation2013) also reported that using 1% glutamine in a 42-day-old broiler diet of one day increased the growth of the digestive tract and increased crypt depth and intestinal villi length. Glutamine is a source of energy for cells with high reproductive rates, such as gastrointestinal cells. Glutamine is a source of nitrogen and carbon and it plays a role in the synthesis of protein and muscle, and glutamine also causes the growth and development of the digestive system (Suchner et al. Citation2000). Smirnov et al. (Citation2006) reported that the presence of glutamine in the intestine stimulates and enhances the goblet cells of the intestine. As a result, it increases the production of myosin and gastric mucosa, which acts as a protective layer of the digestive system against pathogens and causes health, improves digestive development including length, width of villi and crypt depth, and ultimately causes the animal to be healthy. Glutamine plays a role in the synthesis of polyamines, including putrescine, spermine and spermidine, that these polyamines are the necessary molecules for the proliferation, differentiation and growth of intestinal cells (Tako et al. Citation2004; Uni et al. Citation2005; Foye et al. Citation2007). The intestinal crypts are the location of the cells that are absorbing differentiation, the goblet cells, the entero-endocrine and the stem cells. Therefore, increasing the depth of the crypt seems to be due to the beneficial effects of glutamine on growth and differentiation of intestinal cells (Newsholme et al. Citation2003). Also, comparing the treatments 1 and 2, it can be concluded that there was no difference in the morphometry of the small intestine between the two treatments (p > .05). Further investigation is justified to establish that wheat has a negative or positive effect on the gastrointestinal tract due to its viscosity and anti-nutritional agents or glutamic acid and its conversion to glutamine, respectively.
Figure 1. Morphological images from three parts of the small intestine in guinea fowl chickens (comparison of treatment 1 with complementary treatments). (a) Treatment 1 duodenum, (b) treatment 6 duodenum, (c) treatment 1 jejunum, (d) treatment 5 jejunum, (e) treatment 1 ileum and (f) treatment 5 ileum.
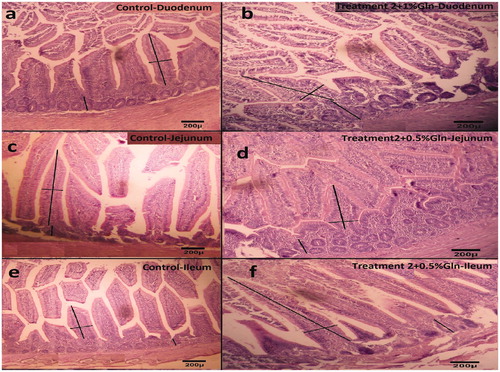
Table 5. Effect of dietary supplementation of L-glutamine on small intestinal morphology in guinea fowl chickens.
Generally, glutamine levels in corn–soybean meal–wheat-based diets responded to performance, carcase characteristics and intestinal morphology to some extent better than corn–soybean meal diets, although the diet of treatment 1 containing glutamine supplement also had a positive effect. This finding shows the positive interaction between the efficiency of wheat and glutamine on performance, carcase characteristics and morphology of broiler chicks, which is also applicable to guinea fowl. This positive interaction may be related to glutamic acid present in wheat and synthetic glutamine. However, the synergistic effect between glutamic acid in wheat and glutamine, which was the purpose of this study, seems to be well suited to the fowl chicken.
Conclusions
This study was performed to evaluate the synergistic effects between glutamic acid in wheat and synthetic glutamine on performance improvement, BWG, carcase characteristics and intestinal morphometry in fowl chickens. The results showed that the addition of glutamine to diets had a significant positive effect on performance, BWG, carcase characteristics and intestinal morphology in both the wheat and wheat-free diets. Also, the addition of wheat to diets did not differ from those based on corn–soybean meal; however, the interaction between glutamic acid in wheat and synthetic glutamine added to wheat diets had significantly better effect on performance, BWG, carcase characteristics and intestinal morphology in fowl chicken compared to non-wheat diets. The use of 0.5% glutamine supplement in wheat-based diet had the best effect on performance, carcase characteristics and morphometric function of the intestine. Therefore, in the high FCR, the replacement of part of the diet with wheat and glutamine supplement produces a more efficient diet that improves performance, digestive function and production. Another finding was the interaction between glutamic acid in wheat and synthetic glutamine, which was well shown in performance, carcases and intestinal morphology, and this interaction is valuable in fowl chickens with low performance. Further studies are needed to understand the mechanism of interaction of the components of wheat with glutamine on the aforementioned traits.
Disclosure statement
The authors certify that there is no conflict of interest whatsoever in this publication.
Additional information
Funding
References
- Adjei AA, Matsumoto Y, Oku T, Hiroi Y, Yamamoto S. 1994. Dietary arginine and glutamine combination improves survival in septic mice. Int J Food Nutr Sci. 14:1591–1599.
- Afshar M, Moslehi H. 2005. Investigation in the effect of using wheat gluten meal on broiler performance. Poult Sci. 87:23–34.
- AOAC. 2004. Official methods of analysis. 18th ed. Washington (DC): Association of Official Analytical Chemists.
- Ayeni J. 1980. The biology and utilisation of the helmet guinea fowl in Nigeria [PhD thesis]. Nigeria: University of Ibadan.
- Bartell SM, Batal AB. 2007. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers. Poult Sci. 86:1940–1947.
- Banfield MJ, Kwakkel RP, Groeneveld M, Doeschate RA, Forbes JM. 1999. Effects of whole wheat substitution in broiler diets and viscosity on a coccidial infection in broilers. Br Poult Sci. 40:58–59.
- Bezerra RM, Perazzo FG, Santos RA. 2013. Glutamic acid and protein reduction for broilers and commercial laying hens. Braz J Appl Technol Agric Sci. 6:101–109.
- Dai SF, Gao F, Zhang WH, Song SX, Xu XL, Zhou GH. 2011. Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. An Feed Sci. 168:51–60.
- Foye O, Ferket P, Uni Z. 2007. The effects of in ovo feeding of arginine and/or beta-hydroxybeta-methylbutyrate (HMB) on glycogen metabolism and growth in turkey poults. Poult Sci. 84:9–10.
- Francis JA, Griffiths RD. 2002. Glutamine: essential for immune nutrition in the critically ill. Br J Nutr. 87:3–8.
- Garcia RG, Mendes AA, Sartori JR, Paz ZC, Takahashi LA, Pelicia SE, Komiyama CM, Quinteiro RR. 2004. Digestibility of feeds containing sorghum with and without tannin for broiler chickens submitted to three room temperatures. Rev Bras Cienc Avic. 6:55–60.
- Gutierrez del Alamo A, Verstegen MW, Den Hartog LA, Perez de Ayala P, Villamide MJ. 2008. Effect of wheat cultivar and enzyme addition to broiler chicken diets on nutrient digestibility, performance, and apparent metabolizable energy content. Poult Sci. 87:759–767.
- Iji PA, Saki AA, Tivey DR. 2001. Intestinal development and body growth of broiler chicks on diets supplemented with non-starch polysaccharides. Anim Feed Sci. 89:175–188.
- Jacob J, Pescator T. 2013. Raising guinea fowl. Lexington (KY): University of Kentucky College of Agriculture, Food and Environment.
- Jahide F, Farhoomand P, Daneshyar M, Najafi G. 2014. The effects of dietary glutamine supplementation on growth performance and intestinal morphology of broiler chickens reared under hot conditions. Turk J Vet Anim Sci. 38:264–270.
- Kadam MM, Bhanja SK, Mandal AB, Thakur R, Vasan P, Bhattacharyya A, Tyagi JS. 2008. Effect of in ovo threonine supplementation on early growth, immunological responses and digestive enzyme activates in broiler chickens. Br Poult Sci. 49:736–741.
- Lee DN, Chang WF, Yu IT, Chiou WS, Weng CF. 2008. Effects of diets supplemented with recombinant epidermal growth factor and glutamine on gastrointestinal tract development of early-weaned piglets. Asian-Aust J Anim Sci. 4:582–589.
- Menconi AG, Kallapura X, Hernandez-Velasco J, Latorre1 M, Morgan N, Pumford R, Layton S, Urbano T, Caseres M, Pixley C, et al. 2013. Effect of glutamine supplementation associated with probiotics on Salmonella typhimurium and nitric oxide or glutamine with perinatal supplement on growth performance and intestinal morphology in broiler chickens. J Clin Microbiol. 2:120.
- Mendanha G, Mogyca S, Barcellos M. 2014. Performance and intestinal characteristics of broiler chicken fed diet with glutamine in the diet without anticoccidials agents. Anim Feed Res. 9:637–648.
- Meng X, Slominski BA, Nyachoti CM, Campbell LD, Guenter W. 2005. Degradation of cell wall polysaccharides by combinations of carbohydrase enzymes and their effect on nutrient utilization and broiler chicken performance. Poult Sci. 84:37–47.
- Mohamadi S, Aghazade A. 2014. The effect of feeding different carbohydrate sources on performance, carcass characteristics and gastrointestinal tract growth and development in broilers. J Anim Sci. 15:11–26.
- Mok E, Hankard R. 2011. Glutamine supplementation in sick children: is it beneficial. J Nutr Metabol. 2011:1–41.
- Moral L, Rharrabti Y, Martos V, Royo C. 2007. Environmentally induced changes in amino acid composition in the grain of durum wheat grown under different water and temperature regimes in Mediterranean environment. J Agr Food Chem. 55:8144–8151.
- Moutab J, Abbaszade P, Toni S. 2015. Study of the morphology and histology of the liver, spleen and pancreas in fowl. Vet J N. 106:76–83.
- Nahashon SN, Aggrey S, Adefope N, Amenyenu A, Wright D. 2004. Growth characteristics of the pearl gray guinea fowl as predicted by Richard’s Gompertz and logistic models. Poult Sci. 83:1798.
- Newsholme P, Procopio J, Lima MMR, Pithon-Curi TC, Curi R. 2003. Glutamine and glutamate-their central role in cell metabolism and function. Cell Biochem Funct. 21:1–9.
- Priya KP, Selvaraj K, Nanjappan S, Chandran J, Visha P. 2010. Oral supplementation of putrescine and L-glutamine on the growth performance, immunity, intestinal enzymes in the broiler chickens. Anim Sci. 6:250–254.
- Salmanzadeh M, Shahryar HA. 2013. Effects of dietary glutamine addition on growth performance, carcass characteristics and development of the gastrointestinal tract in Japanese quails. Revue Med Vet. 164:471–475.
- SAS Institute. 2000. SAS SQL procedure user guide, version 9. 1st ed. Cary (NC): SAS; p. 576.
- Shamsaie AH. 1994. Guinea–fowls (breeding-incubation-diseases). 1st ed. Tehran (IR): Animal Husbandry Research Institute Publishing; pp. 82–100.
- Shekari M, Shahir M, Abdi H, Ghezelje A. 2012. Effects of different levels of rapeseed meal on corn or wheat diets on performance of broilers. J Anim Sci Res. 22:145–131.
- Shewry R, Halford G, Belton S, Tatham S. 2002. The structure and properties of gluten: an elastic protein from wheat grain. Philos Trans R Soc Lond B Biol Sci. 357:133–142.
- Sigolo S, Zohrabi Z, Gallo A, Seidavi A, Prandini A. 2017. Effect of a low crude protein diet supplemented with different levels of threonine on growth performance, carcass traits, blood parameters, and immune responses of growing broilers. Poult Sci. 96:2751–2760.
- Smirnov A, Tako E, Ferket PR, Uni Z. 2006. Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult Sci. 85:669–673.
- Suchner UK, Kuhn S, Fürst P. 2000. The scientific basis of immunonutrition. Proc Nutr Soc. 59:553–563.
- Tako E, Ferket PR, Uni Z. 2004. Effects of in ovo feeding of carbohydrates and beta-hydroxy-beta-methylbutyrate on the development of chicken intestine. Poult Sci. 83:2023–2028.
- Uni Z, Ferket PR, Tako E, Kedar O. 2005. In ovo feeding improves energy status of late-term chicken embryos. Poult Sci. 84:764–770.
- Watford M, Wu G. 2005. Glutamine metabolism in uricotelic species: variation in skeletal muscle glutamine synthetase, glutaminase, glutamine levels and rates of protein synthesis. Trends Comp Biochem Physiol. 140:607–614.
- Wu G. 2010. Functional amino acids in growth, reproduction, and health. Adv Nutr. 1:31–37.
- Yi FG, Allee GL, Knight CD, Dibner JJ. 2005. Impact of glutamine and oasis hatchling supplement on growth performance, small intestinal morphology and immune response of broilers vaccinated and challenged with Eimeria maxima. Poult Sci. 84:283–293.
