 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Studies into the polymorphism of the bovine osteopontin (OPN) gene have so far been focussed on the association between milk production and milk composition, lactation persistency and the rate of growth and weight gain in young cattle. Results obtained by some authors have shown different associations for various milk parameters. Even though osteopontin is an essential component of immunological response, the relationship between the polymorphism of the osteopontin gene and the incidence of diseases in cows has not been researched as yet. The purpose of this study was to analyse the c.8514 C > T polymorphism and its association with milk parameters such as: average daily yield, average protein and fat percentage and average somatic cell count in Holstein-Friesian cattle. The study also included an analysis of the impact of polymorphism on the incidence of the most frequent cattle diseases such as: clinical mastitis (CM), ovarian cysts (OC), clinical hypocalcaemia (CH) and pyometra (P). The study population of cows was found to have two alleles in the analysed locus of the osteopontin gene: C (0.529) and T (0.471), and three genotypes: CC, CT and TT (0.288, 0.482 and 0.230, respectively). Although the study showed no significant association between the polymorphism of the OPN gene and the incidence of diseases or milk parameters, it is an important contribution to research aimed at identifying the most essential SNPs which could be used for marker-assisted selection in dairy cattle herds.
The study population was found to have two alleles in the locus of the osteopontin gene (c.8514C>T ): C and T, and three genotypes: CC, CT and TT.
CT was the most frequent genotype of the osteopontin gene (c.8514C>T ), identified in about 48% of the study population of cows.
No significant differences were focused between the genotypes in the c.8514C>T locus and the milk parameters and the incidence of cattle diseases.
Highlights
Introduction
Osteopontin (OPN) was first described in 1979 as a protein involved in the transformation of epithelial cells (Senger et al. Citation1979). The term is derived from two Latin words: osteo – for bone and pontin – for bridge, which is related to the vital role of OPN in the body: bonding calcium and forming the skeletal structure (Oldberg et al. Citation1986). OPN is sometimes referred to as early T-lymphocyte activation protein 1 (ETA-1) (Patarca et al. Citation1989) or secreted phosphoprotein 1 (SPP-1) (Sodek et al. Citation2000). OPN is a chemokine-like glycoprotein that is expressed primarily on osteoclasts, osteoblasts and teeth, but also on the epithelial cells of breasts, kidneys and the skin, on neurons and vascular endothelial cells (O'Regan and Berman Citation2000; Weber Citation2001). In addition to its primary role associated with the skeletal system, OPN is also of relevance to the immune system (Iwata et al. Citation2004; Patarca et al. Citation1989). Osteopontin is capable of inducing chemotaxis and the migration of immune system cells to the inflammation site (Ashkar et al. Citation2000). It was shown that acute and chronic inflammations are associated with greater expression of OPN on T cells, macrophages, NK and dendritic cells, and an increase in the OPN level in bodily fluids (O'Regan et al. Citation1999; O'Regan and Berman, Citation2000). This protein is found in blood, milk, urine, bile and semen (Salehi et al. Citation2015). It was also determined that OPN is involved in processes such as angiogenesis, cell adhesion, apoptosis, inflammation regulation and wound healing (Denhardt et al. Citation2001), regulation of growth and foetal development, and maintenance of pregnancy to full term (Johnson et al. Citation2003; Leonard et al. Citation2005), and the development of bovine in-vitro embryos (Monaco et al. Citation2009).
Bovine osteopontin is encoded via a single gene composed of seven exons and located in chromosome 6 (Chakraborty et al. Citation2010). According to the Ensemble genome browser, this gene has a second transcript containing nine exons (http://www.ensembl.org). Previous studies relating to the polymorphism of the osteopontin gene analysed its effects on milk yield and milk composition (Leonard et al. Citation2005; Khatib et al. Citation2007; Boleckova et al. Citation2012; De Mello et al. Citation2012; Kowalewska-Łuczak and Kulig, Citation2013a, Citation2013b; Salehi et al. Citation2015; Manzoor et al. Citation2018), somatic cell count in milk (Alain et al. Citation2009; Kowalewska-Łuczak and Kulig Citation2013b) and lactation persistency (Bissonnette Citation2018). They were also focussed on the association with the rate of growth of young cattle (Pareek et al. Citation2008a) and the birth weight and weaning weight of calves, and the weight of one-year-old cows (Allan et al. Citation2007). The majority of these studies concerned the c.8514 C > T polymorphism in intron 4 of the OPN gene (Leonard et al. Citation2005; Khatib et al. Citation2007; Boleckova et al. Citation2012; De Mello et al. Citation2012; Kowalewska-Łuczak and Kulig Citation2013a, Citation2013b; Salehi et al. Citation2015; Pareek et al. Citation2008a). Some of these studies resulted in dissimilar associations (Table ). Therefore, the purpose of this study was to analyse one of the most frequently studied polymorphisms, the c.8514 C > T polymorphism, and its association with milk parameters such as: average daily milk yield, average protein and fat percentage and average somatic cell count in black-and-white Holstein-Friesian cattle. Given the large number of functions of osteopontin in the body and the close association of this protein with immune system cells, the aim of the study was also to assess the impact of this polymorphism on the incidence of the most frequent cattle diseases such as clinical mastitis (CM), ovarian cysts (OC), clinical hypocalcaemia (CH) and pyometra (P), although this type of studies have not been carried out in cows as yet.
Table 1. Effect of c.8514C > T osteopontin gene polymorphism on milk production and composition in different cattle breeds.
Materials and methods
The test material included milk samples collected from 243 black-and-white Holstein-Friesian cows (HO) from a factory cattle farm in Poland. The animals were kept in a free stall system and fed on total mixed ration. The data relating to milk parameters (daily milk yield, fat and protein percentage and SCC) were obtained based on monthly milk reports for the study herd.
Milk was collected into sterile 50-mL test tubes without anti-coagulant and centrifuged, and the precipitate of somatic cells was used to isolate DNA for further analysis. The isolation was performed as per the procedure developed by Pokorska et al. (Citation2016).
Genotypes within the analysed locus c.8514C > T (rs110930453) were determined using the PCR-RFLP method. The segment containing intron 4 of the osteopontin gene was amplified using primers designed with the help of the Primer3Plus software based on the reference sequence (GenBank Accession No. AC_000163.1). The primer sequences were as follows: F-5′taagattctatttgaagatgctgagaa 3′ and R-5′ctgtcccttaggctttacagtg 3′. PCR tests were carried out in the final volume of 20.0 µL, containing approximately 100 ng of template DNA, using the C1000 Thermal Cycler (Bio-Rad). The composition of the PCR reaction mixtures was as follows: 1× Taq buffer + KCl (Thermal Scientific, USA), 2.25 mM of MgCl2, 0.2 mM of each dNTP, 0.35 µM of both primers, 1% DMSO and 0.35 units of Taq polymerase (Thermal Scientific, USA). The cycling conditions were as follows: initial denaturation at 95 °C for 5 min, followed by 35 cycles: at 95 °C for 40 s, at 58 °C for 45 s, at 72 °C for 45 s, and final elongation at 72 °C for 5 min. A total of 10 μL of the resulting products were digested in 2 μL of 1× buffer (BioLabs, UK), 10 U of BseNI (BioLabs, UK) and 7 µL of ultra-pure water. The mixtures were incubated at 65 °C for 16 h. The digested products were distributed in 5% agarose gel electrophoresis (Nusive GTG) stained with ethidium bromide. Electrophoresis was performed using Mini-PROTEAN (Bio-Rad) at 100 V for 90 min in the presence of the pUC19 DNA/MspI marker (Thermo Scientific, USA).
The frequencies of genes and genotypes were determined by a direct count. The standard error for the allele frequency was calculated using the formula developed by Falconer and Mackay (Citation1996): , where n – the number of animals, p – the frequency of the C allele. The level of conformity with the Hardy-Weinberg principle was assessed using a free-access version of the calculator proposed by Rodriguez et al. (Citation2009).
Based on a mixed variance analysis, which allowed for the effect of genotype and the effect of bull, the effects of genotype in the c.8514C > T locus was analysed in terms of its impact on the following production parameters of cattle: average daily milk yield, average protein and fat percentage in milk, and average somatic cell count. To normalise the distribution, the somatic cell count (SCC) in the milk was log-transformed to the so-called somatic cell score (SCS) according to the following formula (Ali and Shook Citation1980):
The data were analysed as average values of the test milking results for the first three lactation periods, using the PROC GLM of SAS® 9.4 software which featured Scheffe’s test (SAS Institute, Cary, NC, USA), according to the following model:
where Yijk – milk parameters (average daily yield, average fat percentage, average protein percentage, average SCC (log-transformed to somatic cell score)); µ – overall mean; ai – effect of the ith OST genotype (CC, CT and TT), oj – effect of bull (the cows originated from 113 bulls), εijk – random error.
The impact of genotype on the incidence of cattle diseases such as CM, OC, CH, P was assessed using logistic regression.
All the animals were divided into two groups: “disease resistant” (class 1) and “disease susceptible” (class 2) according to the incidence of the individual diseases. Cows which were found to have had zero episodes or no more than a single episode of any of the diseases (CM, OC, CH or P, respectively) during the first three lactation periods were classified into the “disease resistant” group. The “disease susceptible” group featured cows with more frequent disease episodes (from 2 to 12 episodes recorded during the first three lactation periods). The PROC LOGISTIC of SAS procedure was implemented according to the following model:
where P = p(Y = 1); Y – occurrence of a disease, where 1 means 0 or 1 episode of the disease and 2 means at least 2 episodes of the disease during the first three lactation periods; b0 – intercept; b1 – regression coefficients of disease occurrence on fixed effects; G – genotype (CC, CT and TT).
Results
The individual genotypes in the analysed locus were determined based on agarose gel electrophoresis of RFLP bands (Figure ). The study population of cows was found to have two alleles in the analysed locus of the osteopontin gene: C and T, and three genotypes: CC, CT and TT. The frequency of the C allele was 0.529, and that of the T allele – 0.471. CT wasthe most frequent genotype, identified in 0.482 of the study population. The frequency of the other genotypes was 0.288 for the CC genotype and 0.230 for the TT genotype (Table ).
Figure 1. PCR-RFLP analysis of c.8514C > T osteopontin gene in cattle using BseNI endonuclease. Line 1 – pUC19 DNA/MspI marker, line 2 – CT genotype, line 3 – CC genotype, line 4 – CT, line 5 – CT, line 6 – TT, line 7 – TT, line 8 – CT, line 9 – CT and line 10 – CT.
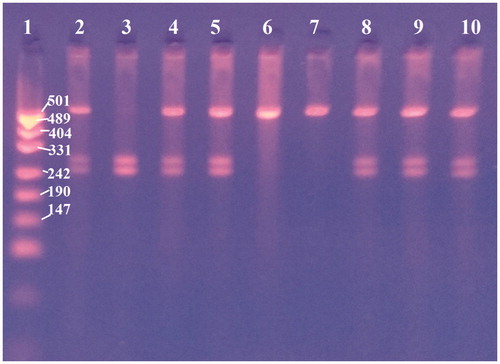
Table 2. Genetic information of population for c.8514 C > T polymorphism of osteopontin gene.
An analysis of conformity with the Hardy-Weinberg principle showed that the population of cows reached a genetic equilibrium with respect to the c.8514C > T locus, even though the herd was subject to selection according to yield, and artificial insemination procedures were performed on a regular basis (Table ). Table shows the effects of the SNP polymorphism in the c.8514C > T locus on milk parameters, taking into account the effect of bull. P-value ranged from 0.153 for somatic cell score to 0.970 for average daily milk. No significant associations were determined between the genotypes and the milk parameters analysed in the study, although it was noticed that animals with CT genotypes were characterised by a slightly lower content of somatic cells in milk than others. The logistic regression analysis showed no significant differences between the genotypes in terms of the incidence of the most frequent cattle diseases (Table ). In this case, P-value ranged from 0.166 for pyometra to 0.563 for clinical hypocalcaemia. It should be note here, that there were relatively few cases of these diseases in the studied herd of cattle. This was the first analysis covering osteopontin gene polymorphism and cattle diseases, so this studies should be continued on a larger animal populations.
Table 3. Effects of c.8514C > T SNP and bull on the milk traits.
Table 4. Evaluation of the genotype impact on the occurrence of cattle diseases using logistic regression.
Discussion
Studies on Holstein cattle showed that CT was a high-frequency genotype. This frequency varied between 0.486 according to Salehi et al. (Citation2015) and 0.607 according to Pareek et al. (Citation2008b). The CC genotype was identified in this cattle breed with a frequency of 0.143 (Pareek et al. Citation2008b) to 0.23 (Khatib et al. Citation2007), and the TT genotype – with a frequency of 0.24 (Pasandideh et al. Citation2015; Koopaee et al. Citation2016) to 0.347 (Salehi et al. Citation2015). The subpopulation featuring the CC genotype was a slightly larger group in the study population of Holstein cattle, which selected for the experiment described in this paper (0.288). On the other hand, the frequencies of the CT and TT genotypes were similar to those determined for this cattle breed by other authors (Table ).
It seems that the frequency of genotype is a characteristic of specific cattle breeds (Table ). In Jersey cattle, the CC genotype was found to be most frequent (0.578), and the TT genotype was least frequent (0.011) (Kowalewska-Łuczak and Kulig Citation2013a, Citation2013b). On the contrary, in Czech Fleckvieh cows or Girolando cattle, the TT genotype was most frequent, identified in more than half of the study population (0.680 and 0.525, respectively), and the CC genotype was least frequent (0.04 and 0.087, respectively) (Boleckova et al. Citation2012; De Mello et al. Citation2012). Butana and Kenana breeds were found to feature only the TT genotype within the analysed polymorphic locus (Rahmatalla et al. Citation2015). The CC genotype was found to be similarly rare in Polish Red cattle, and it was identified in only 4% of the population (Pareek et al. Citation2008b). In the Hereford and the Limousin breeds, the CT genotype was dominant (Pareek et al. Citation2008b).
Some attempts have been made to correlate the OPN polymorphism and milk parameters, given that this physiological fluid was found to contain the greatest concentration of this protein (Christensen and Sørensen Citation2016). It was also shown that the OPN gene product has an essential role in the modulation of the expression of milk protein genes (Sheehy et al. Citation2009).
This study was carried out with a view to analysing the differences between results obtained by various authors for the association between the c.8514 C > T polymorphism in the bovine osteopontin gene and milk parameters. Leonard et al. (Citation2005) was one of the first scientists to notice that the C allele was more frequent in cows producing milk with higher protein and fat percentage. These results were confirmed by Khatib et al. (Citation2007), who showed an additive effect between the polymorphism of the OPN gene, and the protein and fat percentage in milk, and the fat yield, and by Boleckova et al. (Citation2012), who reported an additive effect of the C allele on protein percentage and the breeding value of this parameter. However, Khatib et al. (Citation2007) showed that the dominance effects were not significant for any of the examined parameters. On the other hand, the study of Salehi et al. (Citation2015) showed that there was no association between the genotype in the c.8514 C > T locus and milk parameters such as milk yield, fat yield, fat percentage, protein yield or protein percentage. The study involving Jersey cattle also showed that the genotype in the analysed locus had no significant impact on milk parameters such as daily milk yield, protein or fat percentage (Kowalewska-Łuczak and Kulig Citation2013a, Citation2013b). Other studies showed that in the Jersey breed, individuals featuring the TT genotype had a much higher somatic cell count as compared to cows with the CC and CT genotypes, however, the herd included only three individuals with the TT genotype, and therefore no conclusions can be drawn as to the general association (Kowalewska-Łuczak and Kulig 2012). De Mello et al. (Citation2012), who studied the Girolando breed, a dairy mix comprised of Holstein and Gyr breeds, showed that the genotype of osteopontin had no significant association with milk performance, however, it was noted that higher milk yield was associated with animals with at least one copy of the T allele. The different results may be attributable to a number of reasons: starting from differences in the allele frequency, differences in the models and statistical methods used, and ending with differences in the normal breeding environment of animals (Berry et al. Citation2010). It would also be desirable to consider investigating other polymorphic loci within the osteopontin gene, which would allow a more distinctive identification of the relevant associations and a more effective use of the polymorphism of the gene associated with this protein, which is of such importance in the cattle selection process. Such studies have already been attempted. For example, the polymorphism of c.-1301G > A (rs109637038) in the promoter region of the OPN gene was studied in terms of effects on lactation persistency, and it was determined that this mutation was relevant to the persistency of the 1st, 2nd and 3rd lactation periods and lactation in general, which might suggest that this polymorphism is worth attention (Bissonnette Citation2018). The 37421762 (C > A) polymorphism in the 3’-untranslated region (3’-UTR) of the osteopontin gene in Riverine buffaloes was analysed in terms of impact on chemical parameters of milk, however, no significant association was found (Manzoor et al. Citation2017). A total of 10 polymorphisms were examined, 6 in exons and 4 in introns. It was shown that SNP of g.38329758 T > C in exon 4, which causes the valine-to-alanine substitution (V127A) in an amino acid chain, was correlated with high protein percentage in milk obtained from Riverine buffaloes (Manzoor et al. Citation2018). Alain et al. (Citation2009) analysed the impact of various SNPs on somatic cell count. It was shown inter alia that there is a significant association between the SPP1 c.-1251C > T and SPP1 c.-430 G > A polymorphisms in the 5′UTR region and the SPP1 c. *40A > C polymorphism in the 3′UTR region, and the log-transformed somatic cell count. They also determined the impact of the SPP1 c.-1301G > A polymorphism on milk yield and protein percentage in milk.
Previous studies have shown that osteopontin acts similarly to opsonin, and as such it bonds some bacterial variants, thus strengthening phagocytosis and improving resistance to infections (Schack et al. Citation2009). It was reported that osteopontin was also a regulator involved in neoplasm development, and it was correlated with over 30 types of human neoplasms (Weber Citation2011). Osteopontin present in milk is involved in the development and differentiation of the mammary gland (Christensen and Sørensen Citation2016). As a result, it seems reasonable to seek an association between polymorphic variants of this protein, and the disease susceptibility/resistance of cattle and milk composition. Although the study showed no significant associations between the polymorphism of the OPN gene and the incidence of diseases or milk parameters, it is an important contribution to research aimed at identifying the most essential SNPs which could be used for marker-assisted selection in dairy cattle herds. On one hand the selection should be aimed at improving milk yield and the percentage of individual milk components, while maintaining appropriate disease resistance, which is indispensable for effective dairy cattle breeding.
Conclusions
In this study, we analysed the most frequently examined polymorphism in bovine osteopontin gene and its potential relationship with milk parameters and the incidence of the most frequent diseases in Holstein-Friesian cattle. Our results showed that there was no significant association between nucleotide substitution in the c.8514 C > T locus and average daily milk yield, average protein and fat percentage in milk, average somatic cell count in milk and cattle diseases.
Some studies of other authors resulted in dissimilar associations between this polymorphism and analysed milk parameters, so it shows that this issue requires further analysis covering larger populations and different breeds of cattle.
In our knowledge, this study is the first which include an analysis of the impact of osteopontin gene polymorphism on the incidence of the most frequent cattle diseases such as: clinical mastitis, ovarian cysts, clinical hypocalcaemia and pyometra. This analysis also seems important due to the fact that osteopontin is an essential component of immunological response.
Our results are an significant contribution to research aimed at identifying the most essential SNPs which could be used for marker-assisted selection in dairy cattle herds, but such studies should be continue.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alain K, Karrow NA, Thibault C, St-Pierre J, Lessard M, Bissonnette N. 2009. Osteopontin: an early innate immune marker of Escherichia coli mastitis harbors genetic polymorphisms with possible links with resistance to mastitis. BMC Genomics. 10:444.
- Allan MF, Thallman RM, Cushman RA, Echternkamp SE, White SN, Kuehn LA, Casas E, Smith TP. 2007. Association of a single nucleotide polymorphism in SPP1 with growth traits and twinning in a cattle population selected for twinning rate. J Anim Sci. 85:341–347.
- Ali AKA, Shook GE. 1980. An optimum transformation for somatic cell concentration in milk. J Dairy Sci. 63:487–490.
- Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. 2000. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 287:860–864.
- Berry DP, Howard D, Boyle PO, Water S, Kearney JF, McCabe M. 2010. Associations between the K232A poly-morphism in the diacylglycerol-O-transferase 1 (DGAT1) gene and performance in Irish Holstein-Friesian dairy cattle. Irish J Agr Food Res. 49:1–9.
- Bissonnette N. 2018. Short communication: genetic association of variations in the osteopontin gene (SPP1) with lactation persistency in dairy cattle. J Dairy Sci. 101:456–461.
- Boleckova J, Matejickova J, Stipkova M, Kyselova J, Barton L. 2012. The association of five polymorphisms with milk production traits in Czech Fleckvieh cattle. Czech J Anim Sci. 57:45–53.
- Chakraborty D, Sharma A, Tantia MS, Singh A, Yathish HM. 2010. Role of secreted phosphoprotein 1 (spp1) gene in Bovines – a review. Agric Rev. 31:184–193.
- Christensen B, Sørensen ES. 2016. Structure, function and nutritional potential of milk osteopontin. Int Dairy J. 57:1–6.
- De Mello F, Cobuci JA, Martins MF, Silva MVGB, Neto JB. 2012. Association of the polymorphism g.8514C > T in the osteopontin gene (SPP1) with milk yield in the dairy cattle breed Girolando. Anim Genet. 43:647–648.
- Denhardt DT, Noda M, O`Regan AW, Pavlin D, Jeffrey S, Berman JS. 2001. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 107:1055–1061.
- Falconer DS, Mackay TFC. 1996. Introduction to quantitative genetics. Harlow, UK: Addison Wesley Longman Ltd.
- Iwata M, Awaya N, Graf L, Kahl C, Torok-Storb B. 2004. Human marrow stromal cells activate monocytes to secret osteopontin, which down-regulates Notch1 gene expression in CD34+ cells. Blood. 103:4496–4502.
- Johnson GA, Burghardt RC, Bazer FW, Spencer TE. 2003. Osteopontin: roles in implantation and placentation. Biol Reprod. 69:1458–1471.
- Khatib H, Zaitoun I, Wiebelhaus-Finger J, Chang YM, Rosa GJM. 2007. The association of bovine PPARGC1A and OPN genes with milk composition in two independent holstein cattle populations. J Dairy Sci. 90:2966–2970.
- Koopaee KH, Pasandideh M, Dadpasand M, Koshkoiyeh EA, Abadi MMR. 2016. Joint analysis of the DGAT1, OPN and PPARGC1A genes effects on variation of milk production and composition in Holstein cattle population. Iran J Appl Anim Sci. 6:797–803.
- Kowalewska-Łuczak I, Kulig H. 2013a. Genetic polymorphisms of FAM13A1, OPN, LAP3, and HCAP-G genes in Jersey cattle. Turk J Vet Anim Sci. 37:631–635.
- Kowalewska-Łuczak I, Kulig H. 2013b. Polymorphism of the FAM13A, ABCG2, OPN, LAP3, HCAP-G, PPARGC1A genes and somatic cell count of Jersey cows-preliminary study. Res Vet Sci. 94:252–255.
- Leonard S, Khatib H, Schutzkus V, Chang YM, Maltecca C. 2005. Effects of the osteopontin gene variants on milk production traits in dairy cattle. J Dairy Sci. 88:4083–4086.
- Manzoor S, Nadeem A, Babar ME, Shehzad W, Hashmi AS, Imran M, Hussain T, Wajid A, Javed M. 2017. Short communication: molecular effects of polymorphism in the 3’UTR of osteopontin gene in Riverine buffalo. Pakistan J Zool. 49:1527–1529.
- Manzoor S, Nadeem A, Maryam J, Hashmi AS, Imran M, Babar ME. 2018. Osteopontin gene polymorphism association with milk traits and its expression analysis in milk of riverine buffalo. Trop Anim Health Prod. 50:275–281.
- Monaco E, Gasparrini B, Boccia L, De Rosa A, Attanasio L, Zicarelli L, Killian G. 2009. Effect of osteopontin (OPN) on in vitro embryo development in cattle. Theriogenology. 71:450–457.
- Oldberg A, Franzén A, Heinegård D. 1986. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci USA. 83:8819–8823.
- O'Regan AW, Chupp GL, Lowry JA, Goetschkes M, Mulligan N, Berman JS. 1999. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro . J Immunol. 162:1024–1031.
- O'Regan A, Berman JS. 2000. Osteopontin: a key cytokine in cell-mediated and granulomatous inflammation. Int J Exp Pathol. 81:373–390.
- Pareek CS, Czarnik U, Pierzchała M, Zwierzchowski L. 2008a. An association between the C > T single nucleotide polymorphism within intron IV of osteopontin encoding gene (SPP1) and body weight of growing Polish Holstein-Friesian cattle. Anim Sci Pap Rep. 26:251–257.
- Pareek CS, Zięba M, Michno J, Czarnik U, Zwierzchowski L. 2008b. Study of SNP C > T polymorphism within the candidate genes for dairy and beef traits in a panel of selected cattle breeds. J Agrobiol. 25:121–124.
- Pasandideh M, Mohammadabadi MR, Esmailizadeh AK, Tarang A. 2015. Association of bovine PPARGC1A and OPN genes with milk production and composition in Holstein cattle. Czech J Anim Sci. 60:97–104.
- Patarca R, Freeman GJ, Singh RP, Wei FY, Durfee T, Blattner F, Regnier DC, Kozak CA, Mock BA, Morse HC 3rd, et al. 1989. Structural and functional studies of the early T lymphocyte activation (Eta-1) gene. Definition of a novel T-cell dependent response associated with genetic resistance to bacterial infection. J Exp Med. 170:145–162.
- Pokorska J, Kułaj D, Dusza M, Żychlińska-Buczek J, Makulska J. 2016. New rapid method of DNA isolation from milk somatic cells. Anim Biotechnol. 27:113–117.
- Rahmatalla SA, ReiBmann M, Mueller U, Brockmann GA. 2015. Identification of genetic variants influencing milk production traits in Sudanese dairy cattle. Res J Anim Sci. 9:12–22.
- Rodriguez S, Gaunt TR, Day INM. 2009. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 169:505–514.
- Salehi A, Nasiri K, Aminafshar M, Sayaadnejad MB, Sobhani R. 2015. The association of bovine osteopontin (OPN) gene with milk production traits in Iranian Holstein bulls. Iran J Biotechnol. 13:43–48.
- Schack L, Lange A, Kelsen J, Agnholt J, Christensen B, Petersen TE, Sørensen ES. 2009. Considerable variation in the concentration of osteopontin in human milk, bovine milk, and infant formulas. J Dairy Sci. 92:5378–5385.
- Senger DR, Wirth DF, Hynes RO. 1979. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 16:885–893.
- Sheehy PA, Riley LG, Raadsma HW, Williamson P, Wynn PC. 2009. A functional genomics approach to evaluate candidate genes located in a QTL interval for milk production traits on BTA6. Anim Genet. 40:492–498.
- Sodek J, Ganss B, McKee MD. 2000. Osteopontin. Crit Rev Oral Biol Med. 11:279–303.
- Weber GF. 2001. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 1552:61–85.
- Weber GF. 2011. The cancer biomarker osteopontin: combination with other markers. Cancer Genom Proteom. 8:263–288. http://www.ensembl.org.
