Abstract
In the last years, great importance has been given to the beneficial effects of polyphenols. Among the most relevant health promoting effects, there is the capacity to reduce the amount of free radicals and stimulate the immune response. In this study, polyphenols extracted from olive mill wastewater (OMWW), were fed to adult ‘Casertana’ pigs during the finishing period. No significant differences in the length of the jejunum-ileum villi and the depth of the colon crypts were detected between control and polyphenols fed pigs. Instead, intra-epithelial and lamina propria leukocytes were more abundant in pigs fed polyphenols (p < .05). Cyclooxygenase-2 immunoreactivity in the gastrointestinal tract, employed as marker of inflammation, was more intense in the control group. Superoxide anion production in primary cell cultures of both blood leukocytes and alveolar macrophages was lower in pigs fed polyphenols (p < .05). Taken together these data indicate that, according to our in vitro studies, OMWW polyphenols seem to be potent antioxidants, while the interpretation of the in vivo experiments is more problematic and further studies are necessary on the interactions between bioactive feed compounds and intestinal status. Such studies can contribute to a better understanding of both positive and negative interactions in vivo and to the identification of new functional feeds.
The effects of polyphenols extracted from olive mill wastewater (OMWW) have been studied in pigs.
Gut morphology, inflammation and immune response were investigated.
OMWW polyphenols act as potent antioxidants.
HIGHLIGHTS
Introduction
The European Commission, at the beginning of 2006, regulated the use of antibiotics in feed for livestock by prohibiting their use or limiting their quantities. This has stimulated the interest of research towards alternative solutions, such as bioactive molecules of plant origin, the so-called phytochemicals. Several studies in the last decades have shown that the therapeutic effects of these molecules are not exclusively linked to their antibacterial activity, but also to an immunomodulatory and anti-inflammatory action (Zhang and Tsao Citation2016). This double pharmacological action helps to improve the wellbeing of the animal and at the same time improve the quality of the product that is consumed (Lipiński et al. 2017).
The three main groups of phytochemicals commonly known and which are increasingly under attention, are alkaloids, terpenoids and phenolic compounds. Polyphenols are a large class of chemical compounds abundant in nature and extremely diverse. The term polyphenols includes more than 8000 molecules characterised by the presence of aromatic rings bearing one or more hydroxyl moieties, which play a pivotal role in mediating the antioxidant properties (Rice-Evans Citation1995; Rice-Evans et al. Citation1996; Alov et al. Citation2015). Polyphenols are normally produced by plants for their antibiotic and antifungal properties (Haslam Citation1988; Cheynier et al. Citation2015; Pagliarulo et al. Citation2018). Several studies showed that a diet rich in phenolic compounds has numerous beneficial and therapeutic effects in various acute and chronic diseases (Visioli and Galli Citation1998; Daglia Citation2012; Covas et al. Citation2015; Sureda and Tejada Citation2015; Wang et al. Citation2015).
The use of feed additives for the purpose of manipulating gut functions and the microbial habitat of domestic animals has been recognised as a valuable tool for improving growth performance and feed efficiency (Viveros et al. Citation2011). In piglets the effects of different polyphenol rich pomaces on the morphology of the intestinal tract have been reported (Sehm et al. Citation2007). In particular, both the red-wine and apple pomaces increased the size of the colon crypts suggesting a better nutrient absorption and reduced the activation of the gut associated lymphoid tissue (GALT) resulting in a presumable immuno-preventive effect. It has been suggested that dietary polyphenol-rich plant products improved the gain:feed ratio in growing pigs by altering the microbial composition and the anti-inflammatory effect in the intestine (Fiesel et al. Citation2014).
However, it must be mentioned that the health-promoting properties of polyphenols have been recently questioned and the either toxic or beneficial effects of these substances may depend on several aspects, such as type, concentration, absorption and metabolic transformation, doses and/or the experimental cell type (Surai Citation2014).
The use of phytochemicals or natural plant compounds as potential alternatives to improve the productivity of animals is certainly an idea of great public and private interest, but has main limitations, such as the lack of availability of these compounds, the cost of raw materials and the process of production. Agricultural by-products are a rich source of bioactive molecules, including polyphenols, and therefore have a remarkable potential that can be employed in the feed sector to produce new value added products such as feed supplements (Nazzaro et al. Citation2012). In the Mediterranean area these wastes are mainly related to cereals, grapes and olives transformations. In particular, the olive oil industry shows a large production of milling waste waters (4.7 millions tons per year). These wastes still contain more than 30000 tons of polyphenols which could be recovered as high added value chemicals. Growing interest has been focussed on the anti-inflammatory effects of phenolic components present in extra virgin olive oil. However, no experimental studies have been published on the effects of polyphenols extracted from olive mill wastewater in pigs. Thus, the objective of the present research was to investigate gut morphology, inflammation, and immune response of adult pigs (Casertana strain) fed with polyphenols extracted from olive mill wastewater added to the standard diet, by in vivo and in vitro analysis. The ‘Casertana’ pig used for the experimentation is an ancient autochthon genetic type; it represents an experimental model suitable for semi-wild controlled breeding technique, very common in the inland of the Campania Region (South of Italy). This genetic line is considered a genetic patrimony to be safeguarded and valorised not only because it is at risk of extinction but also because its meat has unique qualitative properties.
Material and methods
Experimental design
Animals used in this study were treated in accordance with the European Commission recommendation 2007/526/EC and 2010/63/UE on revised guidelines for the accommodation and care of animals used for experimentation and other scientific purposes.
This study was carried out on 12-month old pigs (genetic type autochthonous Casertana) housed at a local farm (Mastrofrancesco Farm, Morcone, Benevento, Italy) under a semi-wild system. The experimental investigations were carried out on pigs derived from four contemporary births that took place in the same animal husbandry. Males were castrated between the 8th and the 12th day after birth. Pigs employed in this study were 12 months old. The weight of the pigs at the beginning of the experiment was 140 ± 6 kg. Animals were distributed randomly (with a sex ratio of 1:1) in the experimental groups as suggested in the Guidelines for the Design and Statistical Analysis of Experiments Using Laboratory Animals (Festing and Altman Citation2002). The experiment took place during the finishing period of 4 months. The two experimental groups were confined to adjacent homogeneous land areas fenced with electrified wires, in which the pigs had functional areas equipped for watering, feeding and resting. The animals were fed ad libitum and without restriction of water; the feed intake of the administered feed for each animal was about 2.2 kg/day. The feed intake was determined from unconsumed feed collected daily (when present) before the next feed administration. The amount of consumed feed was utilised to calculate the dry matter intake of the ingested diet. Pigs in both groups were fed with a standard diet for the finishing stage ().
Table 1. Composition of the standard diet.
In the treated group the animals received polyphenols extracted from olive mill wastewaters (OMWW) added to the standard diet in form of capsules. The amount of polyphenols was 0.03 g/kg of feed per pig per day. We choose this dosage considering the antioxidant activity of our extract and comparing this with that reported in literature (Corino et al. Citation2007; Rossi et al. Citation2009; Pastorelli et al. Citation2012) considering the same type of breeding and the amount already given in the diet. In addition, both groups were given the pasture (acorns and natural grasses) in a percentage of 25% of the total feed intake. Pigs were slaughtered at 16 months of age. At the slaughter time, the animals of both groups showed a weight of 165 ± 6 kg, with a dead weight of 142 ± 8 kg and a slaughter yield of about 86%. No significant statistical differences were recorded. No medical therapy was performed during the experimental period.
Polyphenolic extraction and detection
The polyphenolic extract was obtained by membrane separation techniques of OMWW (a sequence of two ultrafiltraction processes followed by a final nanofiltration step) according to Cassano et al. (Citation2011, Citation2013). The system, built by the company Torchiani Impianti srl (Brescia, Italy), consists of a section of ceramic microfiltration and a section of ultrafiltration, nanofiltration and reverse osmosis on polymer-based membranes. The olive mill waste water coming out of the crusher is stored in an equalisation tank and subjected to a pre-treatment consisting of a centrifugal clarification, aimed at reducing the content of suspended solids in the wastewater in order to increase the permeability of the membranes. The clarified wastewater is then subjected to successive passages of membrane tangential filtration (microfiltration, ultrafiltration, and nanofiltration), with a progressively lower cut-off, in order to separate the organic fractions with different molecular weight and different biological properties. The amount of total polyphenols was determined by Folin Ciocalteu method and the identification and quantification of main compounds was obtained by high-performance liquid chromatography (HPLC)-ultravoilet (UV). Phenolic compounds analysis was performed by LC-4000 series integrated HPLC systems (JASCO, Tokyo, Japan) consisting of a column oven (model CO-2060 plus), a UV/vis photodiode array detector (model MD-2018 plus), an intelligent fluorescence detector (model PF-2020 plus), a liquid chromatography pump (model PU-2089 plus), an autosampler (AS-2059 plus) and a ChromNAV software programme (JASCO). A C18 Luna column 5-μm particle size, 25 cm ×3.00 mm I.D. (Phenomenex, Torrance, CA, U.S.A.) was used. All solvents were filtered through a 0.45-μm filter disk (Millipore Co., Bedford, MA, U.S.A.). A mobile phase composed by water–formic acid (99.75:0.25, v/v; Solvent A) and methanol–formic acid (99.75:0.25, v/v; Solvent B) was used. The following gradient elution was applied: from 0 to 45 min, 2%–55% B. The flow-rate was 1.0 mL/min. The injection volumes was 20 μL. All the analyses were carried out at room temperature. Polyphenols extracted were identified by comparing the retention times of the detected peaks with those of commercial standards and with literature data (Servili et al. Citation1999; Mulinacci et al. Citation2001; Obied et al. Citation2005). The quantification of each compound was performed using eight-point regression curves obtained using commercial standards. As reported in , the main phenolic compounds were hydroxytyrosol, verbascoside, tyrosol, caffeic acid along with their derivatives and flavonoids. The antioxidant activity of polyphenolic extract was calculated by trolox equivalent antioxidant capacity method, according to Miller et al. (Citation1993), modified by Re et al. (Citation1999) and shown in .
Table 2. Composition and antioxidant activity of the polyphenol extract from olive mill wastewater.
Sampling
Pigs were slaughtered 120 days after the treatment commencement, at a local slaughterhouses (Santa Croce del Sannio and Vitulano, Benevento, Italy). Internal organ sampling started soon after death, through a horizontal incision along the abdominal midline. Blood was collected at slaughter during the bleeding, alveolar macrophages and gastrointestinal tract were also collected from all animals. Tissue samples of 1 cm2 were taken of the central part of the following gut compartments: gastric fundus, duodenum, jejunum, ileum, caecum, and colon. Samples were washed three times in 0.9% NaCl solution and fixed in 10% buffered formaldehyde. To extract alveolar macrophages, lungs were washed with 200 mL of sterile phosphate buffered saline (PBS). The pulmonary regions (caudal lobes) were gently massaged and then the liquid was slowly collected in sterile tubes on ice. Blood was collected in chilled tubes containing ethylenediaminetetraacetic acid (EDTA) and placed on ice until further use.
Histomorphometry and immunohistochemistry
After formalin fixing, all gastrointestinal samples were dehydrated in an ascending series of alcohol, embedded in paraffin wax, and serially cut in transversal sections of 7 µm by microtome.
In order to obtain quantitative data, 20 consecutive sections were obtained from each gastrointestinal tract, in triplicate. Five non-adjacent sections from each series of 20 sections were stained with hematoxylin–eosin for histomorphometric analysis. The measurements of jejunum and ileum villi height and colon crypt depth were performed on well-orientated crypt-villus preparations. The villi and crypt measurements were performed according to Sehm et al. (Citation2007). Intra-epithelial and lamina propria leukocytes were counted by cell counts. In details, three different cell counts (in three different area of 0.004 cm2) were performed on each histological section, previously labelled by hematoxylin–eosin staining.
Cyclooxygenase-2 (COX-2) expression in the gastrointestinal tract was performed by immunohistochemical analysis as reported in Varricchio et al. (Citation2012) with minor modifications. Goat polyclonal antibodies raised against Cox2 (C-20) (sc-1745, Santa Cruz Biotechnology, Inc., Dallas, TX, U.S.A.) diluted 1:250, were applied on the histological sections overnight at 4 °C. The antibody specificity was declared by the manufacturer and tested in our laboratory by Western blotting. The other components of the immunological reaction were contained in the Vectastain Elite ABC kit (PK-6105 goat) from Vector Laboratories (Burlingame, CA, U.S.A.). The final staining was performed using 10 mg of 3-3′ diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO, U.S.A.) in 15 mL 0.5 M Tris buffer (pH 7.6), containing 0.03% hydrogen peroxide. Negative controls were obtained substituting the primary antisera with PBS or normal serum in the specific step, or alternatively, by absorbing each primary antiserum with an excess of the relative peptide (100 μg of peptide/mL of diluted antiserum).
Peripheral blood leukocyte formula and isolation
To determine the peripheral blood leukocytes formula, peripheral blood samples were smeared on slides soon after withdrawn. MGG-Quick (04 – 090805, Bio-Optica, Milan, Italy) rapid staining was carried out. Leukocyte isolation from peripheral blood was carried out in 15 mL tubes, where 3 mL of whole blood in EDTA were added to 3 mL of Histopaque (density 1083-Sigma, 10831). Subsequently, the samples were centrifuged at 400g for 30 min at 25 °C with speed deceleration rate 0. After centrifugation, the blood was layered on a discontinuous gradient of Histopaque. The blood components were distributed as follows: plasma and platelets were localised in the highest portion of the tube, lymphocytes and monocytes between plasma and Histopaque 62%, neutrophils in the interface between Histopaque 62% and 75% and most of the red blood cells on the bottom of the tube. Cells (blood leukocytes enriched fraction) were picked up from the interface and resuspended in PBS in tubes of 15 mL. Tubes were centrifuged at 250g for 10 min at 25 °C. The pellet was recovered and washed with 1× PBS (250g at 25 °C for 10 min). The cells were counted by an automatically counting cells apparatus Casy Innovative (Roche Applied Science, Indianapolis, IN, U.S.A.) and cultured with complete medium (RPMI 1640, 2 mM l-glutamine, antibiotic/antimycotic 25 mM HEPES, 100 mg/mL gentamycin) and 5% of serum of pig (Thermo Fisher Scientific, Waltham, MA, U.S.A.) at 37 °C overnight. A drop of leukocytes containing medium was smeared on a slide and stained with Diff-Quick rapid staining for detection of purity.
Alveolar macrophages isolation
Alveolar macrophages (AM) were collected and extracted from pig lungs according to Brockmeier et al. (Citation2008). A drop of fluid taken from the lungs was smeared on a slide and labelled with Diff-Quick staining to evaluate the composition of the cell population extracted. The lung fluid was centrifuged at 450g for 15 min at 25 °C and alveolar macrophages were isolated by plastic adherence. Alveolar macrophages were grown in complete medium (RPMI 1640, 2 mM l-glutamine, antibiotic/antimycotic 25 mM HEPES, 100 mg/mL gentamycin) and 5% of serum of pig (Invitrogen, Carlsbad, CA, U.S.A.). After 2 hr-incubation, non-adherent cells were washed away. Adherent cells were collected and incubated at 37 °C until assayed for superoxide anion.
Superoxide anion assay
Superoxide production was determined as the reduction of nitroblue tetrazolium (NBT) according to Choi et al. (Citation2007) with some modifications (Mariano et al. Citation2013). Both cultured pig AM (at a density of 60,000) and leukocytes (at a density of 120,000) were seeded in the 96-well microplates, in triplicate. The cells were incubated with 100 µL PBS (Lonza, Basel, Switzerland) containing NBT (1 mg/mL, Sigma) and zymosan A (2000 µg/mL, Sigma) for 90 min. The control samples were incubated only with NBT. Following incubation, peripheral leukocytes and macrophages were washed twice in PBS, to remove all residual NBT solution, leaving only a cell pellet containing formazan. To quantify the formazan product, the intracellular formazan was dissolved in 120 µL 2 M KOH and 140 µL dimethylsulphoxide (DMSO, Sigma), and the resulting colour reaction was measured with a microplate reader (Model 680 Biorad) at 620 nm.
Microscopical observations
Both cytological (peripheral blood smear, leukocytes isolated from peripheral blood and alveolar macrophages) and histological slides were observed using a microscope Nikon E600 (Nikon Instruments Inc., Melville, NY, U.S.A.) and photographed with the programme Lucia Measurament. Villi and crypt measurements were carried out using ImageJ programme (ImageJ, NIH, Maryland, U.S.A.).
Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA) at a significance level of .05, following confirmation of normality and homogeneity of variance. Significant differences were detected by ANOVA. Data were subjected to Tukey’s test. In all models diet and animal husbandry were fixed effects while gender and littermates were random effects. All values were reported as mean ± SEM, and all analyses were carried out using GraphPad Prism version 5.0 (GraphPad, San Diego, CA, U.S.A.).
Results
Histomorphometry and immunohistochemistry of pig gastrointestinal tract
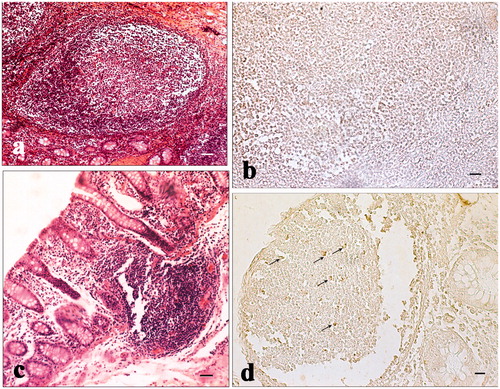
Jejunum and ileum villi length and colon crypt depth of control and treated pigs are reported in Figure . The results show no significant difference between pigs fed the standard finishing diet (control) and those fed the standard finishing diet with polyphenols extracted from olive mill wastewater (treated). A significant increase in the number of lamina propria (Figure ) and intraepithelial leukocytes (Figure ) present in the gastrointestinal tracts (stomach, duodenum, jejunum, ileum, caecum, and colon) of treated animals in comparison with the controls was registered. COX-2 immunopositive cells were identified mainly in the gastrointestinal tracts of the control group. In particular, immunopositivity was detected in the infiltrate leukocytes and in the intra-epithelial leukocytes of caecum (Figure ), and colon (Figure ). Immunopositivity was also present in the effector sites of GALT represented by ileum solitary follicles and Peyer’s patches (Figure ). Immunopositive cells were absent in the caecum and ileum tracts, and in the solitary follicles of treated animals (Figures ). Weak COX-2 immunopositivity was present in the colon tract (Figure ). Negative controls did not show any positivity (Figures ).
Figure 1. Effect of olive mill wastewater supplemented to a finishing diet on jejunum and ileum villi lengths and colon crypt depth. Bars represented as mean ± SEM.
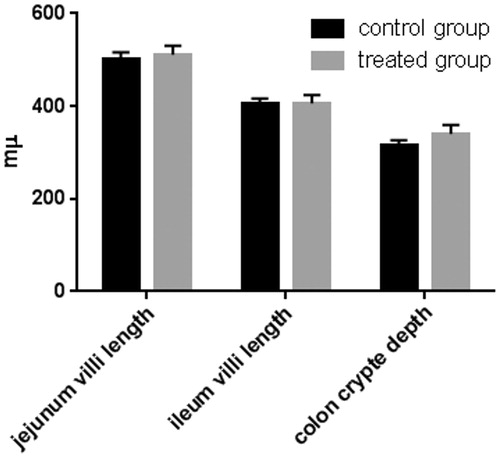
Figure 2. Effect of olive mill wastewater supplemented to a finishing diet on the count of leukocyte infiltrate in the stomach, duodenum, jejunum, ileum, caecum, and colon. Bars represented as mean ± SEM. *Annotate differences between control and experimental group at p < .05.
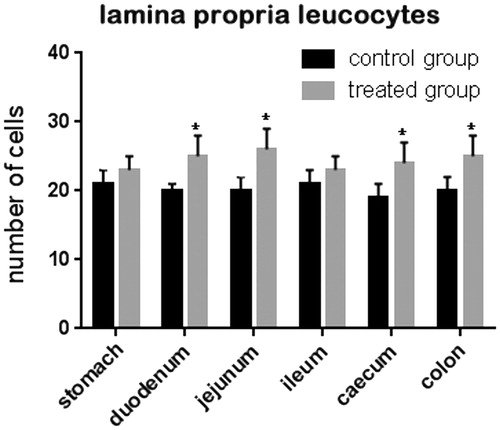
Figure 3. Effect of olive mill wastewater supplemented to a finishing diet on the count of intra-epithelial leukocytes (IEL) in the stomach, duodenum, jejunum, ileum, caecum, and colon. Bars represented as mean ± SEM. *Annotate differences between control and experimental group at p < .05.
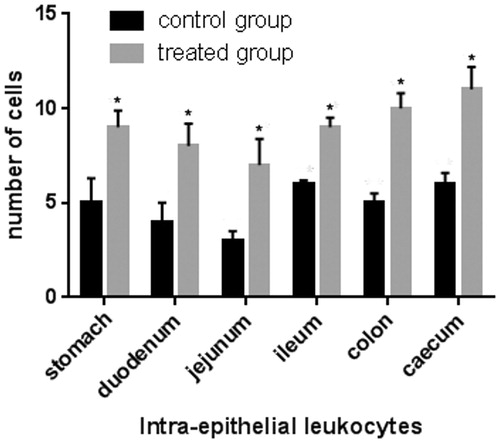
Figure 4. Immunohistochemical analysis of cyclooxygenase-2 (COX-2) in the caecum: (a) immunopositive infiltrated leukocytes in the mucosal layer of the caecum of a control pig; (b) negative control of the caecum of a control pig; (c) absence of immunopositivity in the mucosal layer of the caecum of a pig of the experimental group; and (d) immunopositive intra-epithelial leukocytes (IEL) in the epithelium of the caecum of a pig of the experimental group. Scale bars: 20 µm (a–d).
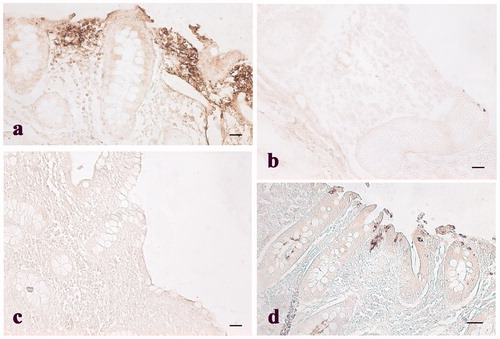
Figure 5. Immunohistochemical analysis of cyclooxygenase-2 (COX-2) in the colon: (a) Immunopositive stained leukocytes in the colon mucosal layer of control pig; (b) weak immunopositivities staining in the colon mucosal layer of a pig of the experimental group; and (c) negative control obtained from colon tissue of a control pig. Scale bars: 20 µm.
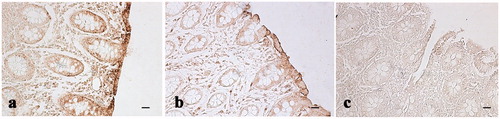
Figure 6. Immunohistochemical analysis of cyclooxygenase-2 (COX-2) in the ileum and Peyer’s patches: (a) hematoxylin–eosin staining of the ileum solitary follicle of a pig of the experimental group; (b) absence of immunopositivities staining in the same ileum follicle solitary showed in the previous picture (histological section consecutive to the previous one); (c) hematoxylin–eosin staining of a Peyer’s patch of the caecum of a control pig; and (d) various immunopositive cells (arrows) located in the same Peyer’s patch showed in the previous picture (histological section consecutive to the previous one). Scale bars: 20 µm (a and d); 10 µm (b); and 50 µm (c).
Peripheral blood leukocyte and alveolar macrophage isolation
The analysis of peripheral leukocyte formula performed on blood samples collected from both control and treated animals showed a typical leukocyte formula for pig (Figure ), in agreement with reference values (Table ). In the blood leukocyte enriched fraction the most representative cells were lymphocytes and monocytes (Figure ). The most abundant cells present in the alveolar macrophage fraction isolated from pig lungs were monocytes/macrophages, while very few lymphocytes and erythrocytes were observed (Figure ).
Figure 7. MGG-Quick rapid staining of leukocytes present in the peripheral blood smears: (a) neutrophilic granulocytes with lobed nucleus, (b) large lymphocyte, (c) monocyte, (d) basophilic granulocytes, and (e) eosinophilic granulocytes. Scale bars: 10 µm (a–e).
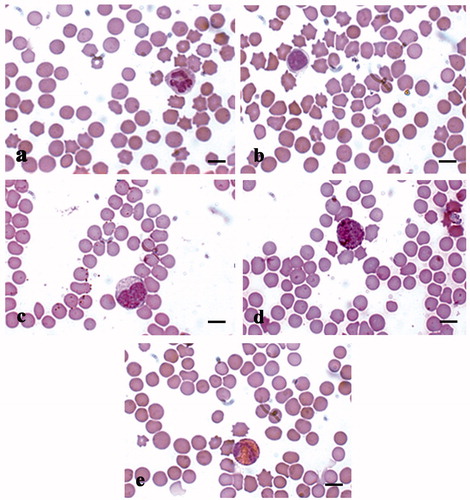
Figure 8. MGG-Quick rapid staining of leukocytes isolated from peripheral blood: (a–c) lymphocytes (thin arrows), monocytes (thick arrows), and rare red blood cells (*). Scale bars: 10 µm (a–c).
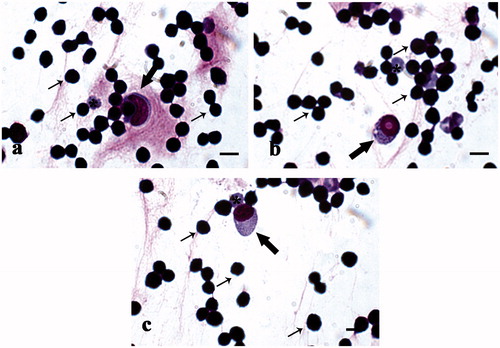
Figure 9. MGG-Quick rapid staining of fluid extract from lugs: (a) lower magnification and (b) higher magnification of extracted cell population; in detail alveolar macrophages (arrows). Scale bars: 50 µm (a) and 10 µm (b).
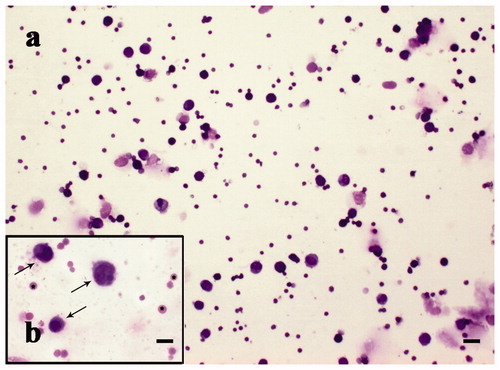
Table 3. Leukocyte formula of peripheral blood samples collected from control and experimental group.
Superoxide anion assay
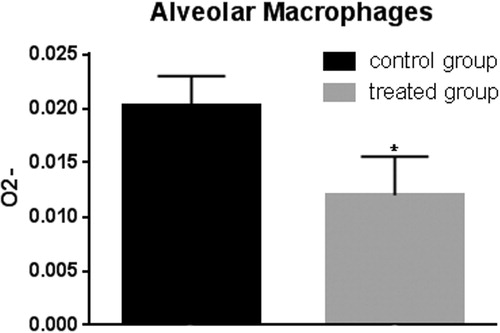
The polyphenols extracted from the olive mill wastewater were tested for anti-oxidant effects on pig blood leukocyte enriched fraction and alveolar macrophage fraction by superoxide anion assay. Peripheral blood leukocytes of pigs belonging to the treated group showed lower levels of superoxide anion compared to control animals after in vitro stimulation with zymosan (Figure ). Alveolar macrophages extracted from pig lungs of the control group showed higher superoxide anion levels with respect to treated animals (Figure ).
Figure 10. Superoxide anion assay in the peripheral blood leukocytes. The anion superoxide levels were lower in pigs belonging to the experimental group compared with control animals with and without zymosan stimulation. Bars represented as mean ± SEM of three separate experiments. *Annotate differences between control and the experimental group at p < .05.
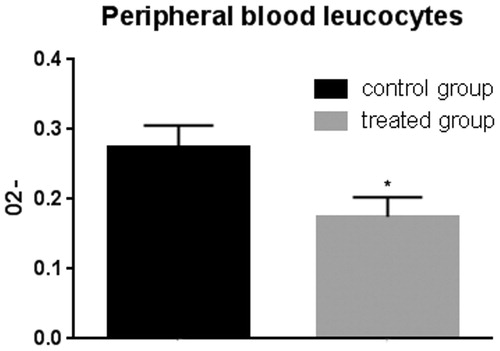
Figure 11. Superoxide anion assay in the alveolar macrophages. The anion superoxide levels were lower in pigs belonging to the experimental group compared with control animals after zymosan stimulation. Bars represented as mean ± SEM of three separate experiments. *Annotate differences between control and the experimental group at p < .05.
Discussion
To date enough evidence exists to support anti-inflammatory and immune stimulant activity of polyphenols (Guo et al. Citation2009; Hur et al. Citation2012; Joseph et al. Citation2015). Only few reports have documented the effect of dietary polyphenols or related phenolic compounds on the intestinal growth and function in pigs (Sehm et al. Citation2007; Fiesel et al. Citation2014), although there are no references in the literature in relation to feeding olive oil by-products. Thus, in this study, we present data on the inclusion of polyphenols extracted from olive mill wastewater to the diet of adult ‘Casertana’ pigs, during the finishing period.
The phenolic components of olive mill waste water are the object of various studies. A wide complexity of polyphenols is present in OMWW, among which hydroxytyrosol and secoiridoids derivatives are the most concentrated (De Marco et al. Citation2007; Bertin et al. Citation2011). Our study reveals that the composition of polyphenols extracted from olive mill wastewater shows the predominance of tyrosol, hydroxytyrosol and derivatives, verbascoside, and caffeic acid. These results are consistent with those found by Suárez et al. (Citation2010) and Ramos et al. (Citation2013). Tyrosol, hydroxytyrosol, caffeic acid and verbascoside have been identified in various studies on OMWW and their antioxidant and antimicrobial activities highlighted (Obied et al. Citation2007).
In agreement with the widely demonstrated radical-scavenging and anti-inflammatory activities of olives and olive by-product polyphenols (Cicerale et al. Citation2010), our results show a decrease in free radicals or reactive oxygen species production and a decrease in COX-2 (a marker of inflammation) expression in the experimental group of pigs fed with polyphenols integrated into the diet. The ability of polyphenols to act as antioxidants or free radical scavengers, as well as their capacity to inhibit some enzymes involved in free radical generation, such as various cytochrome P450 isoforms, lipoxygenases, COX and xanthine oxidase, is due to the hydroxyl groups that are good hydrogen donors (Pereira et al. Citation2009). Polyphenols can exert their anti-inflammatory properties at multiple levels, through the modulation of mitogen-activated protein kinase and nuclear factor-κB signalling pathways, the inhibition of inflammatory cytokines and chemokines, the suppression of the activity of cytokine inducible-nitric oxide synthase and COX (Plummer et al. Citation1999; Luceri et al. Citation2002; Hou et al. Citation2007). COX-2 is an inducible enzyme that converts the arachidonic acid into prostaglandins and generally is induced at the site of inflammation in response to inflammatory stimuli including pro-inflammatory cytokines such as interleukin-1α/β, interferon-γ, and tumor necrosis factor-α produced by inflammatory cells, and tumour promoters such as tetradecanoyl phorbol acetate and Ras (Wang and Dubois Citation2010). In this study, COX-2 immunoreactivity was detected in mucosal infiltrated leukocytes, Peyer’s patches and solitary follicles of caecum-colon tracts of pigs fed with the basal diet, while in pigs fed with polyphenol enriched diet COX-2 immunoreactivity was quite faint. The low level of expression of COX-2 in immunoreactive cells in the intestine of treated pigs could suggests a protective role of polyphenols, modulating and reducing the inflammatory response. This is in agreement with Willenberg et al. (Citation2015), reporting polyphenol-induced reduction of COX-2 expression both in vitro (cancer cell line HCA-7 and primary monocytes) and in vivo (C57BL/6N mice).
In the present study, the inclusion of polyphenols extracted from olive mill wastewater did not modify the intestinal morphology in comparison to pigs fed the standard diet. The mucosa status and its microscopic structure can be good indicators of the intestinal tract response to the active substances in feeds (Ferguson et al. Citation2007; Schrenk Citation2009). The lack of intestinal response in this study, may be attributed to different causes. On one hand, the length of time necessary to modify the intestinal histological structure may be longer than the duration of our study. On the other hand, we cannot rule out that the presence of polyphenols may not be so beneficial as expected on the basis of the evidence of polyphenols as health promoters. Indeed, mixed results have been obtained in in vivo studies. Fiesel et al. (Citation2014), report the absence of alteration of villus height and crypt depth in the small intestine of weaned pigs fed grape seed and grape marc meal extract, while Sehm et al. (Citation2007) report that in piglets, red-wine pomace had an inhibitory effect on the jejunum villi growth, while apple and red-wine pomace showed stimulating effect on crypt colon size. Regarding other measures of intestinal integrity, Sell et al. (Citation1985) observed a slight reduction in the crypt depth of the duodenal tissue in rats, chicks, and laying hens fed the high tannin sorghum.
In this study, the inclusion of polyphenols in the diet caused the increase in the amount of lamina propria and intraepithelial leukocytes, indicating that a massive leukocyte migration took place in the experimental group. During the intestinal inflammation, leukocytes are recruited in the site of infection or inflammation where, through a complex interplay, contribute to the recruitment of other immune cells and facilitate mucosal healing by releasing mediators necessary for the inflammation response. Although such responses are clearly beneficial, excessive recruitment and accumulation of leukocytes in the intestine under pathological conditions may be detrimental, depending on the circumstances (Fournier and Parkos Citation2012). It has been commonly accepted that leukocytes directly contribute to disease pathology when excessive recruitment and activation lead to release of toxic products and massive transepithelial migration, eventually resulting in morphological alterations of villi and crypts and extensive mucosal injury (Fournier and Parkos Citation2012). It is apparent that leukocytes can act as double-edged sword in that contribute to intestinal homeostasis through the elimination of unwanted pathogens and participate in harmful inflammatory processes and exacerbate the inflammation owing to the release of toxic granule contents and pro-inflammatory molecules. Thus, the presence of numerous leukocytes in the basal lamina suggests that the OMWW polyphenols may not have a clear cut health promoting activity in adult Casertana pigs.
In this study, the results of the superoxide anion assay that measures the free radical production showed higher levels in the control pigs compared to pigs fed with polyphenol integrated diet, both in alveolar macrophages and in peripheral blood leukocytes. The lower levels of superoxide anion in treated pigs may indicate a protective effect of polyphenols against oxidative burst triggered by zimosan stimulation. Our results are in agreement with previous researches in murine monocyte/macrophage cell line J774, where different doses of hydroxytyrosol were able to prevents macrophage activation (Maiuri et al. Citation2005). Polyphenols exert a modulatory effect on inflammatory response also in leukocyte cultures (Derochette et al. Citation2013; Burzynska-Pedziwiatr et al. Citation2014). In fact, they are able to down-regulate the inflammatory response, preserving tissues from free radicals and inflammatory cascade (Denev et al. Citation2014). These results lead us to hypothesise a protective effect exerted by polyphenols on alveolar macrophage and peripheral blood leukocyte oxidative burst.
Conclusions
In conclusion, our results suggest that OMWW polyphenols are potent antioxidants through in vitro studies, while the demonstration in vivo is more problematic and further studies are necessary to clarify the interactions between bioactive feed compounds and intestinal status. Such studies can contribute to a better understanding of both positive and negative interactions in vivo and to the identification of new functional feeds.
Aknowledgments
We would like to thank the Olive Industry of Dr. Biagio Mataluni (Montesarchio, Benevento, Italy) for having kindly provided polyphenols from OMWW.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alov P, Tsakovska I, Pajeva I. 2015. Computational studies of free radical-scavenging properties of phenolic compounds. Curr Top Med Chem. 15:85–104.
- Bertin L, Ferri F, Scoma A, Marchetti L, Fava F. 2011. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem Eng J. 171:1287–1293.
- Brockmeier SL, Loving CL, Nicholson TL, Palmer MV. 2008. Coinfection of pigs with porcine respiratory coronavirus and Bordetella bronchiseptica. Vet Microbiol. 128:36–47.
- Burzynska-Pedziwiatr I, Bukowiecka-Matusiak M, Wojcik M, Machala W, Bienkiewicz M, Spolnik G, Danikiewicz W, Wozniak LA. 2014. Dual stimulus-dependent effect of Oenothera paradoxa extract on the respiratory burst in human leukocytes: suppressing for Escherichia coli and phorbol myristate acetate and stimulating for formyl-methionyl-leucyl-phenylalanine. Oxid Med Cell Long. 2014:764367.
- Cassano A, Conidi C, Drioli E. 2011. Comparison of the performance of UF membranes in olive mill wastewaters treatment. Water Res. 45:3197–3204.
- Cassano A, Conidi C, Giorno L, Drioli E. 2013. Fractionation of olive mill wastewaters by membrane separation techniques. J Hazard Mater. 248-249:185–193.
- Cheynier V, Tomas-Barberan FA, Yoshida K. 2015. Polyphenols: from plants to a variety of food and nonfood uses. J Agric Food Chem. 63:7589–7594.
- Choi HS, Kim JW, Cha YN, Kim C. 2007. A quantitative nitroblue tetrazolium assay for determing intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 27:31–44.
- Cicerale S, Lucas L, Keast R. 2010. Biological activities of phenolic compounds present in virgin olive oil. Int J Mol Sci. 11:458–479.
- Corino C, Rossi R, Musella M, Cannata S, Pastorelli G. 2007. Growth performance and oxidative status in piglets supplemented with verbascoside and teupolioside. Ital J Animal Sci. 6:292–294.
- Covas MI, de la Torre R, Fitó M. 2015. Virgin olive oil: a key food for cardiovascular risk protection. Br J Nutr. 2:19–28.
- Daglia M. 2012. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 23:174–181.
- De Marco E, Savarese M, Paduano A, Sacchi R. 2007. Characterization and fractionation of phenolic compounds extracted from olive oil mill wastewaters. Food Chem. 104:858–867.
- Denev P, Kratchanova M, Ciz M, Lojek A, Vasicek O, Nedelcheva P, Blazheva D, Toshkova R, Gardeva E, Yossifova L, et al. 2014. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 157:37–44.
- Derochette S, Franck T, Mouithys-Mickalad A, Deby-Dupont G, Neven P, Serteyn D. 2013. Intra- and extracellular antioxidant capacities of the new water-soluble form of curcumin (NDS27) on stimulated neutrophils and HL-60 cells. Chem Biol Interact. 201:49–57.
- Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. 2007. Genes, diet and inflammatory bowel disease. Mutat Res. 622:70–83.
- Festing MFW, Altman DG. 2002. Guidelines for the design and statistical analysis of experiments using laboratory animals. Ilar J. 43:4.
- Fiesel A, Gessner DK, Most E, Eder K. 2014. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res. 10:196–206.
- Fournier BM, Parkos CA. 2012. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 5:354–366.
- Guo W, Kong E, Meydani M. 2009. Dietary polyphenols, inflammation, and cancer. Nutr Cancer. 61:807–810.
- Haslam E. 1988. Plant polyphenols (syn. Vegetable tannins) and chemical defense-A reappraisal. J Chem Ecol. 14:1789–1805.
- Hou DX, Masuzaki S, Hashimoto F, Uto T, Tanigawa S, Fujii M, Sakata Y. 2007. Green tea proanthocyanidins inhibit cyclooxygenase-2 expression in LPS-activated mouse macrophages: molecular mechanisms and structure-activity relationship. Arch Biochem Biophys. 460:67–74.
- Hur SJ, Kang SH, Jung HS, Kim SC, Jeon HS, Kim IH, Lee JD. 2012. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr Res. 32:801–816.
- Joseph SV, Edirisinghe I, Burton-Freeman BM. 2015. Fruit polyphenols: a review of anti-inflammatory effects in humans. Crit Rev Food Sci Nutr. 56:419–444
- Lipinski K, Mazur M, Antoszkiewicz Z, Purwin C. 2017. Polyphenols in monogastric nutrition – a review. Ann Anim Sci. 17:41–58.
- Luceri C, Caderni G, Sanna A, Dolara P. 2002. Red wine and black tea polyphenols modulate the expression of cycloxygenase-2, inducible nitric oxide synthase and glutathione-related enzymes in azoxymethane-induced f344 rat colon tumors. J Nutr. 132:1376–1379.
- Maiuri MC, De Stefano D, Di Meglio P, Irace C, Savarese M, Sacchi R, Cinelli MP, Carnuccio R. 2005. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedebergs Arch Pharmacol. 371:457–465.
- Mariano G, Stilo R, Terrazzano G, Coccia E, Vito P, Varricchio E, Paolucci M. 2013. Effects of recombinant trout leptin in superoxide production and NF-κB/MAPK phosphorylation in blood leukocytes. Peptides. 48:59–69.
- Miller NJ, Rice-Evans CA, Davies MJ, Gopinathan V, Milner A. 1993. A novel method for measuring antioxidant capacity and its application to monitoring antioxidant status in premature neonates. Clin Sci. 84:407–412.
- Mulinacci N, Romani A, Galardi C, Pinelli P, Giaccherini C, Vincieri FF. 2001. Polyphenolic content in olive oil waste waters and related olive samples. J Agric Food Chem. 49:3509–3514.
- Nazzaro F, Orlando P, Fratianni F, Coppola R. 2012. Microencapsulation in food science and biotechnology. Curr Opin Biotechnol. 23:182–186.
- Obied HK, Allen MS, Bedgood DR, Prenzler PD, Robards K, Stockmann R. 2005. Bioactivity and analysis of biophenols recovered from olive mill waste. J Agric Food Chem. 53:823–837.
- Obied HK, Bedgood DR, Prenzler PD, Robards K. 2007. Bioscreening of Australian olive mill waste extracts: biophenol content, antioxidant, antimicrobial and molluscicidal activities. Food Chem Toxicol. 45:1238–1248.
- Pagliarulo C, Sateriale D, Scioscia E, De Tommasi N, Colicchio R, Pagliuca C, Scaglione E, Jussila J, Makkonen J, Salvatore P, et al. 2018. Growth, survival and spore formation of the pathogenic aquatic oomycete Aphanomyces astaci and fungus Fusarium avenaceum are inhibited by Zanthoxylum rhoifolium bark extracts in vitro. Fishes. 3:12.
- Pastorelli G, Rossi R, Corino C. 2012. Influence of Lippia citriodora verbascoside on growth performance, antioxidant status, and serum immunoglobulins content in piglets. Czech J Anim Sci. 57:312–322.
- Pereira DM, Valentão P, Pereira JA, Andrade PB. 2009. Phenolics: from chemistry to biology. Molecules. 14:2202–2211.
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. 1999. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 18:6013–6020.
- Ramos P, Santos SAO, Guerra ÂR, Guerreiro O, Felício L, Jerónimo E, Silvestre AJD, Neto CP, Duarte M. 2013. Valorization of olive mill residues: antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind Crops Prod. 46:359–368.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26:1231–1237.
- Rice-Evans CA. 1995. Plant polyphenols: free radical scavengers or chain-breaking antioxidants? Biochem Soc Symp. 61:103–116.
- Rice-Evans CA, Miller NJ, Paganga G. 1996. Structure-antioxidant activity relationships of flavonoid and phenolic acids. Free Radic Biol Med. 21:417.
- Rossi R, Corino C, Pastorelli G, Durand P, Prost M. 2009. Assesment of antioxidant activity of natural extracts. Ital J Animal Sci. 8:655–657.
- Schrenk D. 2009. Dietary fiber, low-molecular-weight food constituents and colo-rectal inflammation in animal models – a review. Mol Nutr Food Res. 53:1281–1288.
- Sehm J, Lindermayer H, Dummer C, Treutter D, Pfaffl MW. 2007. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. J Anim Physiol Anim Nutr (Berl). 91:289–296.
- Sell DR, Reed WM, Chrisman CL, Rogler JC. 1985. Mucin excretion and morphology of the intestinal tract as influenced by sorghum tannins. Nutr Rep Int. 31:1369–1374.
- Servili M, Baldioli M, Selvaggini R, Miniati E, Macchioni A, Montedoro G. 1999. High-performance liquid chromatography evaluation of phenols in olive fruit, virgin olive oil, vegetation water and pomace and 1D- and 2D-nuclear magnetic resonance characterization. J Amer Oil Chem Soc. 76:873–882.
- Suárez M, Romero MP, Motilva MJ. 2010. Development of a phenol-enriched olive oil with phenolic compounds from olive cake. J Agric Food Chem. 58:10396–10403.
- Surai PF. 2014. Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol Anim Nutr (Berl). 98:19–31.
- Sureda A, Tejada S. 2015. Polyphenols and depression: from chemistry to medicine. Curr Pharm Biotechnol. 16:259–264.
- Varricchio E, Russo F, Coccia E, Turchini G, Francis D, de Girolamo P, Paolucci M. 2012. Immunohistochemical and immunological detection of ghrelin and leptin in rainbow trout Oncorhynchus mykiss and murray cod Maccullochella peelii peelii as affected by different dietary fatty acids. Microsc Res Tech. 75:771–780.
- Visioli F, Galli C. 1998. The effect of minor constituents of olive oil on cardiovascular disease: new findings. Nutr Rev. 56:142–147.
- Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A. 2011. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 90:566–578.
- Wang D, Dubois NR. 2010. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 29:781–788.
- Wang J, Tang L, Wang JS. 2015. Biomarkers of dietary polyphenols in cancer studies: current evidence and beyond. Oxid Med Cell Longev. 2015:732302.
- Willenberg I, Meschede AK, Gueler F, Jang MS, Shushakova N, Schebb NH. 2015. Food polyphenols fail to cause a biologically relevant reduction of COX-2 activity. PLoS One. 10:e0139147
- Zhang H, Tsao R. 2016. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 8:33–42.
