Abstract
The present study was undertaken to investigate an effect of various concentrations of Spirulina maxima extract (SME) in BIOXcell® extender on Simmental bulls spermatozoa quality. Semen was collected from 20 healthy bulls using an artificial vagina. Semen was evaluated in terms of concentration, motility, viability and morphology. Samples that showed more than 70% motility, 60% viability and 70% morphologically accepted spermatozoa were selected for the experiment. SME at the concentrations of 2, 4, 6 and 8 µg/mL was added to bovine commercial BIOXcell® extender. The semen aliquots were cooled and preserved at 4 °C. Their qualities were evaluated during pre-freezing, then the cooled semen samples were packaged into 0.25 ml straws. The straws were frozen in the vapour of liquid nitrogen, and stored at −196 °C in container. The straws were thawed one day later and the characteristics of spermatozoa were examined. Results showed significant effect of SME on characteristics such as viability, Reactive Oxygen Species synthesis, DNA fragmentation and motility of spermatozoa in post freezing conditions (p < 0.05) and revealed that supplementation of SME extender improved the post-thaw spermatozoa quality in bulls.
Spirulina maxima extract has antioxidant effect on bovine sperm cells
The addition of Spirulina maxima extract improves the quality of bovine semen
The extender supplemented with 4 µg/mL Spirulina maxima extract improves the motility, viability and DNA integrity of the bovine spermatozoa
Highlights
Introduction
Mammalian spermatozoa are extremely sensitive to oxidative stress (Leod Citation1943). Many factors contribute to male infertility, and these include, among others, spermatozoa damage caused by low temperatures. Cold shock may damage mitochondria (Pena et al. Citation2009), as well as the acrosomal membrane of spermatozoa (Meyers Citation2005), inducing changes in the composition and permeability of plasma and membranes (Januskauskas et al. Citation2003). The causes of male infertility also include infective (Purvis and Christiansen Citation1993), genetic (Ferlin et al. Citation2006), and nutritional factors (Sinclair Citation2000). Another factor contributing to infertility is an oxidative stress (OS), resulting from imbalance between the reactive oxygen species (ROS) and antioxidants in the animal's body.
Balance between ROS production and their detoxification may be an important factor in sperm survival and function before, during and after cryopreservation. Moreover, ROS are associated with lipid peroxidation, DNA and protein damages ( Farber et al. Citation1990, Calamera et al. Citation2001, Neild et al. Citation2003 ), which also determines the final effectiveness of the semen. Lipid peroxidation plays a leading role in spermatozoon ageing, its life-span shortening in vivo and affects the preservation of semen for artificial insemination (Alvarez et al. Citation1982). Antioxidant molecules could reduce the impact of OS, and thus improve semen quality after thawing. The aim of antioxidant treatments should not be the complete ROS since oxidative mechanisms play an important role in the physiological control of mammalian sperm functions (Aitken et al. Citation2004; Ford Citation2004). ROS produced in excess is highly toxic to the cell, but when are found at low concentrations they act as mediators for normal sperm functioning.
Spirulina maxima is a blue–green microalga belonging to the Oscillatoraceae family which composition is rich in β-complex, vitamins, minerals, proteins, γ-linoleic acid and nutraceutical pigments (Keservani et al. Citation2015). It is used as a dietary supplement that has health benefits in preventing or managing hypercholesterolaemia (Iwata et al. 1990), hyperglycerolemia (Deng and Chow Citation2010), obesity (Becker et al. 1986), inflammation (Coskun et al. Citation2011), cancer (Ismail et al. Citation2009) and cardiovascular diseases (Khan et al. Citation2005). It is also known due to its antioxidant potential (El-Tantawy Citation2016), anti-diabetic effects involving decreasing blood glucose level, regulating cholesterol and improving insulin resistance (Samuels et al. 2002; Gupta et al 2010). The effect of Spirulina on reproductive functions is not well known. So far, studies conducted in rats have reported that S. maxima extracts (SME) increased the body and testis weights, metabolic parameters, normal seminiferous tubules, Leydig cell number, testosterone levels and steroidogenic enzymes mRNA (Won et al. 2012). The results of these studies are very promising. However, direct effects of Spirulina on sperm parameters have not been investigated. The objective of this study was to investigate the effects of SME on the viability, motility, DNA defragmentation and ROS synthesis in bull sperm.
Material and methods
The experiment has been performed as part of routine activities during the current semen production in the reproductive station and did not require the approval of the ethics committee. These experiments were performed on the Breeding and Insemination Centre ‘MCB’ (Krasne, Poland). The study included twenty healthy Simmental bulls with an average age of 3.5 ± 0.5 years housed individually in pens. Three ejaculates were collected from each bull using an artificial vagina at 7 a.m. The semen was held in a water bath at 37 °C, where the sperm concentration and initial percentage of motile spermatozoa were estimated. Sperm concentration was assessed using a digital photometer (Dr Lange, LP 300 SDM; Minitube, Tiefenbach b. Landshut, Germany) at 560 nm.
Semen processing
Semen samples were immediately after collection transferred into graduated test tubes, placed in a water bath at 37 °C. The fresh undiluted semen was then evaluated microscopically (Nikon E 200, China) for mass motility. Subsequently, the semen was extended with animal protein–free commercial BIOXcell® extender (IMV Technologies, L’aigle, France) to a final concentration of 120 × 106 spermatozoa/mL, and rated in terms of motile sperm percentage, progressive motility, viability and abnormality of spermatozoa. Semen samples that showed more than 70% morphologically accepted (without morphological changes), 70% motility and 60% viability, were selected for the experiment.
After a positive evaluation, semen samples were pooled to eliminate individual differences. The fresh semen was then divided into five equal fractions. The first fraction was left for the control group (without SME), and SME (Eco Spa, Poland) was added to the others (2, 4, 6 and 8 µg/mL). Semen was automatically packed (Bloc Machine FIN, IS 4, France) into polyvinyl chloride (PVC) straws (0.25 mL) (Biovet, France) which were filled and equilibrated for 1.5 h at 4 °C. After equilibration, the straws were frozen in liquid nitrogen vapour using a computer controlled automatic freezer from 4 °C to −15 °C at the rate of −3 °C/min and from −15 °C to −80 °C at the rate of −10 °C/min (IMV Technologies, France).
After reaching −80˚C, semen straws were plunged into liquid nitrogen and packaged in plastic goblets for 24 hours of storage in the liquid nitrogen container.
After one day, the straws were thawed in a water bath at 38 °C for 20 sec and then were examined.
Volume
The volume of the ejaculate was measured by reading the graduated tube.
Assessment of sperm DNA integrity
To further analyse the sperm DNA integrity, chromatin susceptibility to acid-induced denaturation in situ was assessed. The chromatin instability was then quantified by flow cytometric method (CytoFlex Beckman Coulter, B3-R1-V0, China) using Sperm Chromatin Structure Assay (SCSA) test. The samples were thawed in a water bath (26 °C for 30 sec). Thirteen microliters of semen and 487 µL of NaCl (0.9%) were placed in a glass tube on ice. Fifty microliters of thus prepared mixture was moved to the second tube on ice and 100 μL of acid detergent solution (0.08 M HCl, 0.15 M NaCl, 0.1% v/v Triton X-100, pH 1.2) was added. After exactly 30 sec (without a light), 300 µL of acridine orange (AO)-staining solution [6 μg AO (chromatographically purified) (Polysciences, Inc. – USA) per ml citrate buffer (0.037M citric acid, 0.126M Na2HPO4, 1.1mM EDTA disodium, 0.15M NaCl, pH 6.0] was added. The prepared sample was incubated 3 min on ice (without a light) then examined using flow cytometry method; 5000 spermatozoa were evaluated in each sample.
Sperm motility
Mass motility was examined in 20 µL of semen which was placed on a prewarmed slide without any cover slip and analysed under microscope (Nikon E 200, China) equipped with phase-contrast optics (100×). The mass motility was scored into four scales: + no motion, ++ free spermatozoa moving without forming any waves, +++ vigorous movement with moderately rapid waves, ++++ very rapidly moving waves.
Motility
Fifteen microliters of semen placed on a prewarmed slide and covered with a cover slip. The motility was determined by eye-estimation of the proportion of spermatozoa moving progressively straightforward at higher magnification (400×) (Nikon E 200, China).
Computerized assessment
Sperm motility was examined using a Sperm Class Analyzer (SCA, version 5.1, Microptic, Barcelona, Spain), a light microscope (Nikon Eclipse E200). Just prior to analysis, semen was diluted 1:10 in a warm (25 °C) physiological solution (sodium chlorate 0.9%). Then, 2 μL of the prepared sample was placed in a Leja 4 analysis chamber (Leja Products B.V., Holland) of a thickness of 20.0 μm. The slide was placed on a stage warmer (38 °C). The following motility parameters were included in this study: percentage of motile sperm, curvilinear velocity (VCL), straight-line velocity (VSL), path velocity (VAP), linearity (LIN) and amplitude of lateral head displacement (ALH). Minimum 500 cells were evaluated, and depending on sperm concentration, five analyses were performed per sample.
Viability
The double stain SYBR-14 with propidium iodide (L-7011 LIVE/DEAD Sperm Viability Kit; Invitrogen, Molecular Probes, Barcelona, Spain) using flow cytometer was applied (CytoFlex Beckman Coulter, B3-R1-V0, China). For this purpose, 50 µL of thawed semen was measured (37 °C for 20 sec) and 940 µL NaCl (0.9%) and 5 µL SYBR14 were added. The whole was thoroughly mixed and then incubated (36 °C for 10 min) without light access. Subsequently, 5 µL of PI was remixed and incubated 3 min without light, followed by a test.
Reactive oxygen species synthesis
Dichlorfluorescein-diacetate (DCFH-DA) was used in order to measure ROS level. For this purpose, 5 µL of DCFH-DA and 3 µL PI was added to 492 µL of diluted sperm. After addition of fluorescence dyes, semen samples were incubated at 37 °C for 30 min and mixed just before measurement. DCFH-DA was analysed using flow cytometric method (CytoFlex Beckman Coulter, B3-R1-V0, China).
Statistical analysis
Data are presented as mean standard error of the mean (SEM). Analysis of variance (ANOVA) was used to assess differences among stages of SME supplementation on all the semen characteristics. When the F ratio was significant (p < .05), Duncan’s multiple range test was used to compare treatment means. Statistical analysis of the results was performed using Statistica 12.0 (StatSoft, Poland). The experiment was repeated eight times.
Results
The influence of SME on the parameters of frozen/thawed semen collected from Simmental bulls is shown in Table and .
Table 1. CASA-obtained mean values of motility parameters from frozen-thawed bovine semen with or without the addition of SME.
Table 2. Flow cytometry – obtained mean values of the DCFH, DNA integrity and viability from frozen-thawed bovine semen samples in the presence and the absence of SME.
Progressive motility was significantly different (p < .05) in the samples with the addition of 2, 4 and 6 µg/mL of SME. It was higher by 3.36% in the samples with the addition of 2 µg/mL of SME than in the control group. The sample containing 4 µg/mL demonstrated 11.9% higher result than the result for a group without SME addition. The results of the progressive motility in the sample with the addition of 6 µg/mL were 4.88% higher than in the sample without SME addition. The results obtained within the parameters: VCL, VSL, VAP, LIN, STR and WOB in the sample supplemented with the addition of 4 µg/mL of SME were the highest of all tested samples and differed significantly (p < .05) compared to the control group. The results obtained in the sample with the addition of 8 µg/mL showed a significant reduction in all the examined motility parameters, which differed significantly (p < .05) compared to the control group and other SME concentrations. No significant differences (p < .05) within the ALH parameter were observed between samples supplemented with 2, 4 and 6 µg/mL of SME.
The results of the tests of DCFH, DNA integrity and viability on frozen-thawed bovine sperm are shown in Table .
The percentages of DNA-intact spermatozoa were significantly improved (p < .05) as a result of 2 µg/mL; 4 µg/mL and 6 µg/mL SME addition. Higher concentration (8 µg/mL) did not significantly affect (p < 0.05) DNA protection. The highest result was observed in the sample with the addition of 2 µg/mL of SME, and this value was higher by 6.55% than the result obtained in the sample without addition.
In addition, the sperm viability was not affected by the addition of 6 and 8 µg/mL of SME, but it was significantly increased as a result of an addition of 2 and 4 µg/mL. The viability of spermatozoa in the sample with the addition of 4 µg/mL was higher by 5.15%, and in the sample with the addition of 2 µg/mL by 4.01%, in relation to the result obtained in the group without the addition.
Samples with the addition of 2, 4 and 6 mg of SME revealed the reduction of DCFH-emitted fluorescence intensity, successively by 4.47 (2 µg/mL), 6.91 (4 µg/mL) and 6.72 (6 µg/mL). The above results were significantly different (p < 0.05) compared to the control group. No significant differences (p < 0.05) were observed in the sample with the addition of 8 µg/mL of SME.
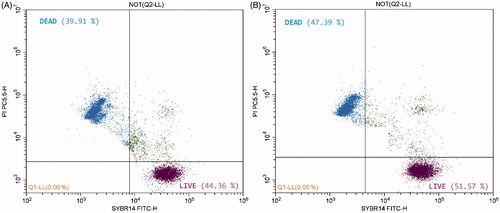
Figure shows the randomly selected charts, presenting sperm viability analysis: semen without the addition of SME and semen containing 4 µg/mL of SME.
Discussion
An effect of S. maxima extract on the characteristics of spermatozoa in Simmental bulls during freezing/thawing conditions was examined in this study. The results indicated that the addition of 4 µg/mL of SME to the BIOXcell® extender increased motility, viability, reduced ROS synthesis and protected DNA from defragmentation. Our observations indicate that SME protected the sperm during freezing conditions.
Spirulina is widely used in clinical practice. It has been proven to have anti-inflammatory activity by inhibiting the release of histamine from mast cells (Yang et al. Citation1997; Kim et al. Citation1998). Recent studies by Mao et al. (Citation2005) also showed that the high dose of Spirulina significantly lowered the level of interleukin-4 by 32%, which indicates the protective effect of this microalgae on allergic rhinitis. Cingi et al. (Citation2008) showed that Spirulina was clinically effective in the treatment of allergic rhinitis, and its consumption significantly reduced inflammatory symptoms. In vitro experiment carried out by Hayashi et al. (Citation1996) showed that the aqueous extract of Spirulina inhibited HIV-1 replication in human T lymphocytes, peripheral blood mononuclear cells and Langerhan cells. Other evidence of antiviral activity of Spirulina is described by Hernandez-Corona et al. (Citation2002). To initiate the isolation and identification of the compound that determines the antiviral activity of S. maxima, some extracts made using several solvents with different polarity were evaluated by microplate inhibition assay using HSV-2. The highest antiviral activity was detected in the methanol-water 3:1. Moreover, numerous studies (Shklar and Schwartz Citation1988a, Citation1988b; Mathew et al. Citation1995; Sung-Ho et al. Citation2011) suggest that the combined antioxidant and modulatory properties of the immune system of Spirulina may have a potential mechanism of cancer destruction and this play a role in the prevention of cancer in both animals and humans.
Rahman et al. (Citation2012) reported that Spirulina may increase the production of immunostimulatory and immuno-modulator chemicals. Moreover, the authors point to the significant effect of Spirulina on free radicals scavenging, thereby protecting the organs from damage caused by the exposure to lead.
Mammalian spermatozoa membranes are sensitive to oxygen-induced damage. Despite existing (limited) mechanisms aimed at these damages reversal, defective sperm function is difficult to evaluate and treat. The excessive formation of ROS by abnormal spermatozoa has been identified as one of the few defined aetiologies for male infertility. It was observed in our study (Table ), that the percentage of sperm with intact membrane in samples with the addition of 2, 4 and 6 mg of SME was higher than in the control group, which also was reflected in a reduction in fluorescence emitted by DCFH.
OS is a condition associated with an increased rate of cellular damage induced by oxygen and oxygen-derived oxidants commonly known as ROS (Sikka et al. 1996), that have been implicated in multiple of disease states such as infection, arthritis and connective tissue disorders to carcinogenesis, toxin exposure, aging, physical injury and acquired immunodeficiency syndrome (Joyce Citation1987).
Unfortunately, research on the role of OS in infertility and methods for counteracting its impact on reproductive tissues with antioxidants is still at the beginning stage. Our results (Table ) confirm the antioxidant effects of Spirulina. Similar antioxidant potential of Spirulina has been observed also by other authors (Gutiérrez-Rebolledo et al. 2015; Asghari et al. 2016; Bashandy et al. 2016). We have shown a significant effect of SME addition to semen extender (BIOXcell®) on ROS synthesis, DNA damage and spermatozoa viability and motility in the semen collected from Simmental bulls. Similar results were observed in the studies conducted by other authors, that report the positive effect of Spirulina on the quality of sperm in males (Granaci Citation2007a, Citation2007b; Kistanova et al. Citation2009). Granaci (Citation2007a) showed a 5% improvement in the viability of spermatozoa in boar in the group receiving Spirulina. In this study, 11% higher volume of semen was collected from males receiving a supplement of Spirulina extract compared to the group not receiving the supplement. As with boars, the quality of the bull sperm has been improved due to Spirulina. Sperm motility, their concentration and viability after storage were positively changed when bulls received a biological extract from Spirulina (Granaci Citation2007b). Spirulina supplementation also has a positive effect on the process of spermatogenesis in rats (Won et al. 2012; Esener et al. 2016). So far, the direct effects of the addition of Spirulina to semen have not been included in the studies.
Conclusions
Oxygen toxicity is an inherent challenge to aerobic life forms, including the spermatozoa. It is still unknown how toxicity influencing on interaction of sperm with the ovum is. Increased oxidative damage to sperm membranes, proteins and DNA and also lowering sperm motility directly affect the results of fertilisation. Taking into account the obtained results, we recommend an addition of 4 µg/mL of SME to the extender to improve the quality of bovine semen.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aitken RJ, Ryan AL, Baker MA, McLaughlin EA. 2004. Redox activity associated with the maturation and capacitation of mammalian spermatozoa. Free Radic Biol Med. 36:994–1010.
- Alvarez JG, Storey BT. 1982. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effect on sperm motility. Biol. Reprod. 27:1102–1108.
- Asghari A, Fazilati M, Latifi AM, Salavati H, Choopani A. 2016. Antioxidant properties of Spirulina. J Appl Biotechnol Rep. 3:345–351.
- Bashandy S, Mohamed M, Fatma A. 2016. Spirulina platensis, reduced liver and kidney injuries induced by sodium arsenite. Int J PharmTech Res. 11:35–48.
- Becker EW, Jakober R, Luft D, Schmülling RM. 1986. Clinical and biochemical evaluation of the alga Spirulina with regard to its application in the treatment of obesity. A double-blind cross-over study. Nutr Rep Int. 33:565–572.
- Calamera JC, Fernandez PJ, Buffone MG, Acosta AA, Doncel GF. 2001. Effects of long-term in vitro incubation of human spermatozoa: functional parameters and catalase effect. Andrologia. 33:79–86.
- Cingi C, Conk-Dalay M, Cakli H, Bal C. 2008. The effects of Spirulina on allergic rhinitis. Eur Arch Otorhinolaryngol. 265:1219–1223.
- Coskun ZK, Kerem M, Gurbuz N, Omeroglu S, Pasaoglu H, Demirtas C, Lortlar N, Salman B, Pasaoglu OT, Turgut HB. 2011. The study of biochemical and histopathological effects of Spirulina in rats with TNBS-induced colitis. Bratislavské Lekárske Listy. 112:235–243.
- Deng R, Chow TJ. 2010. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 28:33–45.
- El-Tantawy WH. 2016. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J Tradit Complement Med. 6:327–331.
- Esener OBB, Gurel-Gurevin E, Isbilen-Basok B, Yigit F, Bilal T, Altiner A, Yilmazer N, Armutak EI. 2016. Spirulina platensis affects factors involved in spermatogenesis and increases ghrelin receptors in testis tissue of rats fed a high-fat diet. Pol J Vet Sci. 20: 467–475.
- Farber JL, Kyle ME, Coleman JB. 1990. Mechanisms of cell injury by activated oxygen species. Lab Invest. 62:670–679.
- Ferlin A, Arredi B, Foresta C. 2006. Genetic causes of male infertility. Reprod. Toxicol. 22:133–2141.
- Ford WCL. 2004. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 10:387–399.
- Granaci V. 2007a. Achievements in the artificial insemi-nation of swine. Bulletin of University of Agricultural Sci-ences and Veterinary Medicine Cluj-Napoca. Anim Sci Biotechnol. 63/64:382–386.
- Granaci V. 2007b. Contributions on the study of thecryoresistance increase of the bull semen material. Bulletin of University of Agricultural Sciences and VeterinaryMedicine Cluj-Napoca. Anim Sci Biotechnol. 63/64:387–391.
- Gupta S, Hrishikeshvan HJ, Sehajpal PK. 2010. Spirulina protects against rosiglitazone induced osteoporosis in insulin resistance rats. Diabetes Res Clin Pract. 87:38–43.
- Gutiérrez-Rebolledo GA, Galar-Martínez M, García-Rodríguez RV, Chamorro-Cevallos GA, Hernández-Reyes AG, Martínez-Galero E. 2015. Antioxidant effect of Spirulina (Arthrospira) maxima on chronic inflammation induced by Freund's complete adjuvant in rats. J Med Food. 18:865–871.
- Hayashi T, Hayashi K, Maeda M, Kojima I. 1996. Calcium spirulan, an inhibitor of envelope virus replication, from a blue-green alga Spirulina platensis. J Nat Prod 59:83–87.
- Hernández-Corona A, Nieves I, Meckes M, Chamorro G, Barron BL. 2002. Antiviral activity of Spirulina maxima against herpes simplex virus type 2. Antiviral Res. 56:279–285.
- Ismail MF, Ali DA, Fernando A, Abdraboh ME, Gaur RL, Ibrahim WM, Raj MH, Ouhtit A. 2009. Chemoprevention of rat liver toxicity and carcinogenesis by Spirulina. Int J Biol Sci. 5:377–387.
- Iwata K, Inayama T, Kato T. 1990. Effects of spirulina platensis on plasma lipoprotein lipase activity in fructose-induced hyperlipidemic rats. J Nutr Sci Vitaminol. 36:165–171.
- Januskauskas A, Johannisson A, Rodriguez-Martinez H. 2003. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility. Theriogenology. 60:743–758.
- Joyce DA. 1987. Oxygen radicals in disease. Adverse Drug Reaction Bull. 127:476. 9
- Keservani RK, Kesharwani RK, Sharma AK, Jarouliya U. 2015. Dietary supplements, nutraceutical, and functional foods in immune response (immunomodulators). In: Bagchi D, Swaroop A, Preuss HG, editors. Nutraceutical and functional foods in human life and disease prevention. Boca Raton (FL): CRC Press, Taylor and Francis, Chapter 20; 343–360.
- Khan M, Shobha JC, Mohan IK, Naidu MU, Sundaram C, Singh S, Kuppusamy P, Kutala VK. 2005. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother Res. 19:1030–1037.
- Kim H, Lee E, Cho H, Moon Y. 1998. Inhibitory effect of mast cell-mediated immediate-type allergic reactions in rats by Spirulina. Biochem Pharmacol. 55:1071–1076. Vol. no.
- Kistanova E, Marchev Y, Nedeva R, Kacheva D, Shumkov K, Georgiev B, Shimkus A. 2009. Effect of the Spirulina platensis included in the main diet on the boar sperm quality. Biotechnol Anim Husbandry. 25:547–557.
- Leod M. 1943. The role of oxygen in the metabolism and motility of human spermatozoa. Am J Physiol. 138:512–518.
- Mao T, Van de Water J, Gershwin M. 2005. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food. 8:27–30.
- Mathew B, Sankaranarayanan R, Nair PP, Varghese C, Somanathan T, Amma BP, Amma NS, Nair MK. 1995. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer. 24:197–202.
- Meyers SA. 2005. Spermatozoal response to osmotic stress. Anim Reprod Sci. 89:57–64.
- Neild DM, Gadella BM, Chaves MG, Miragaya MH, Colenbrander B, Agüero A. 2003. Membrane changes during different stages of a freeze-thaw protocol for equine semen cryopreservation. Theriogenology. 59:1693–1705.
- Pena FJ, Rodriguez-Martinez H, Tapia JA, Ortega-Ferrusola C, Gonzalez FL, Macias GB. 2009. Mitochondria in mammalian sperm physiology and pathology: a review. Reprod Dom Anim. 44:345–349.
- Purvis K, Christiansen E. 1993. Review: infection in the male reproductive tract. Impact, diagnosis and treatment in relation to male infertility. Int J Androl. 16:1–13.
- Rahman MA, Moitry NF, Alam M, Yasmin Z, Debnath D, Mostofa M. 2012. Effects of Spirulina in lead induced toxicities in long evans rats. J Environ Sci Nat Res. 5:79–82.
- Samuels R, Mani UV, Iyer UM, Nayak US. 2002. Hypocholesterolemic effect of Spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food. 5:91–96.
- Schwartz J, Shklar G, Reid S, Trickler D. 1988. Prevention of experimental oral cancer by extracts of Spirulina-Dunaliella algae. Nutr Cancer. 11:127–134. no.
- Shklar G, Schwartz J. 1988. Tumor necrosis factor in experimental cancer regression with alphatocopherol, beta-carotene, canthaxanthin and algae extract. Eur J Cancer Clin Oncol. 24:839–850. Vol. no.
- Sikka SC, Rajasekaran M, Hellstrom WJ. 1995. Role of oxidative stress and antioxidants in male infertility. J Androl. 16:464–468.
- Sinclair S. 2000. Male infertility: nutritional and environmental considerations. Altern Med Rev. 5:28–38.
- Sung-Ho O, Juhee A, Do-Hyung K, Hyeon-Yong L. 2011. The effect of ultrasonificated extracts of Spirulina maxima on the anticancer activity. Mar Biotechnol. 13:205–214.
- Won HN, Il KK, Hae SA, Mi JK, Hee-Gyoo K, Jin HJ, Myung CG. 2012. Effect of Spirulina maxima on spermatogenesis and steroidogenesis in streptozotocin-induced type I diabetic male rats. Food Chem. 134:173–179.
- Yang H, Lee E, Kim H. 1997. Spirulina platensis inhibits anaphylactic reaction. Life Sci. 61:1237–1244.