Abstract
In recent years, there has been an increase in the consumption of omega-6 fatty acids (FAs) over omega-3. Moreover, the antioxidant nature of oil supplemented diet in human and animal studies is not clear. In this work, the n-3 effects on hen ovarian cells, ovarian dynamics, serum FA profiles and expression level of apoptosis genes in the presence or absence of vitamin E were studied. Sixty-eight laying hens were randomly divided into four groups and the groups were fed the following diets: basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil +1.1 U alpha-tocopherol/hen/day (E); basal diet +1.5% fish oil +1.1 U alpha-tocopherol/hen/day (n-3 + E); and basal diet +1.5% fish oil (n-3). In E, n-3 + E and n-3, small yellow follicle numbers were higher than control (p < .05). Large yellow follicle numbers as well as total numbers of follicles of n-3 + E and n-3 were higher (p < .05) than control and E. The rate of ovulation was significantly (p < .05) higher in n-3 (96%) compared to the control (89%). The proportion of intact primordial follicles in n-3 and n-3 + E was significantly more than control and E (p < .01). The expression of Bak (Bcl2 antagonist/killer) was significantly (p < .05) higher in control than the other groups. There was significant reduction in expression of Cas9 in n-3 and E compared to the control and n-3 + E (p < .05). While omega-3 and vitamin E can protect ovarian cells from apoptotic death, omega-3 source could have promoting roles on follicular development and ovulation rate, being independent of dietary vitamin E.
Despite the perceived importance of supplementing antioxidant with fatty acids, our results indicated that alone low level of n-3 has a great influence on ovary performance.
Highlights
Introduction
Recent studies show convincingly that vitamin E is critical for the prevention of oxidation and it has been suggested that vitamin E requirements depend on dietary poly unsaturated fatty acid (PUFA) (Raederstorff et al. Citation2015). To some extent, this has been attributed to the alterations in the diet over the past century, noticeably with regard to the type and the amount of fat in the diet (Fontana and Torre Citation2016). The major adjusters of biological processes in diverse living tissues, particularly in reproductive systems, have been determined and known to be fatty acids (FAs) (Abayasekara and Wathes Citation1999). Among FA, omega-3 and omega-6 FA and their ratio to each other have been recognised as being a pivotal factor for reproductive systems. It has been reported that the consumption of omega-6 has recently become more prevalent than consumption of omega-3 FA (Blasbalg et al. Citation2011) and this has coincided with health problems as well as ovarian dysfunction in animal (Zachut et al. Citation2010) and human (Fontana and Torre Citation2016; Simopoulos Citation2016). Although it has been suggested that unsaturated FAs have various effects on ovarian function and numbers of follicles (Wathes et al. Citation2007), other dietary factors such as dietary antioxidants may affect these responses.
Laying hen is a useful model for ovarian studies (Damjanov Citation1989; Barua et al. Citation2009). Previously, Ebeid et al. (Citation2008) studied the effects of different amounts of supplemented dietary fish oil on ovarian follicular development in hen without dietary antioxidant. A few studies have shown that the number of follicles in bovine ovaries within the early postpartum period may be increased by dietary PUFA. However, they have not studied the role of dietary antioxidants, especially vitamin E (Leroy et al. Citation2014). Similarly, the number of preovulatory follicles and ovulation rates were notably higher in goats that had fed with fish oil supplement (Mahla et al. Citation2017), but antioxidant roles were ignored in this study. Researchers have posited that unique FAs such as Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA) provided by fish oil have effective roles in reproduction (Fouladi-Nashta et al. Citation2009). In addition, any variation in dietary FA intake causes changes in the FA profile of the serum and tissue (Zachut et al. Citation2010). Therefore, ovarian dynamics and biophysical behaviour of oocytes could be affected by PUFA modifications, but dietary antioxidant status and serum FA alterations have not been considered in most studies.
Since antioxidants inhibit the detrimental effects of reactive oxygen species on the reproductive organs, they are considered to be a major factor in avian reproduction. Raederstorff et al. (Citation2015) and Valk and Hornstra (Citation2000) suggested that vitamin E might be closely related to the dietary intake of PUFA and that the consumption increase of PUFA may affect the response to dietary PUFA by vitamin E supplementation. However, there have not been many studies on the antioxidant roles in diet with omega-3 or omega-6 PUFA. The objective of the current investigation was to study the effects of fish oil (omega-3 source) with or without vitamin E supplementation on ovarian follicular development, ovulation rate, and serum FA profiles of laying hen.
Materials and methods
Experimental design and dietary treatments
The type of study was interventional experiment and was carried out according to the guidelines of Royan Institute Ethical Committee (IR.ACECR.ROYAN.REC.1394.30). Sixty-eight Bovans White laying hens aged 29 weeks were domiciled individually in cages under conditions of 17h light and 7h dark periods, with lights on at 05:00 AM and lights off at 22:00 PM. Hens were fed with commercial basal diet [corn and soybean meal based diet without oils containing 17.4% crude protein (CP) and metabolisable energy (ME) 2550 kcal/kg] and fresh water adlibitum. The birds were randomly segregated into four groups (n = 17) in a completely randomised design and were fed by isoenergetic and isonitrogenous rations: basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil +1.1 U alpha-tocopherol/hen/day (E); basal diet+ 1.5% fish oil +1.1 U alpha-tocopherol/hen/day (n-3 + E) and basal diet +1.5% fish oil (n-3) from 29 weeks to 35 weeks old. Vitamin E (vitamin E premix; 5500 U alpha-tocopherol acetate) was provided by Amine Gostar Co. (Tehran, Iran). The FA profiles of the basal diet as well as sunflower and fish oils were determined by gas chromatography ().
Table 1. The major fatty acid profiles of oil and basal diet (% of total fatty acids).
Performance, ovary and oviduct morphology
Ten 35-week-old hens from each treatment were randomly selected, weighed and euthanized by cervical dislocation. Morphological parameters such as body weight, length and weight of oviduct, ovary weight, the number of normal large yellow follicles (LYF; ≥10 mm diameter), the number of small yellow follicles (SYF; 5–10 mm diameter), and large white follicles (LWF; 3–5 mm diameter) were recorded. The classification of follicle sizes was made as described by Renema et al. (Citation1995). Percent hen-day egg production was computed daily in order to specify the rate of ovulation (Moreng et al. Citation1980).
Histology and follicles structure
After dissection, ovarian tissues were removed from the bodies of the birds and drowned in phosphate buffered saline. Then all samples were immersed in fixative (Bouin’s fluid and formalin). Finally, slices were stained by Hematoxylin and Eosin. Based on a research conducted by Johnson and Woods (Citation2007), various types of follicles were determined and follicles with a maximum size of 80 μm and those between 0.08 and 1 mm were counted as primordial follicles and primary follicles, respectively. Furthermore, follicles larger than 1 mm in diameter were classified as prehierarchical follicles. It is worth mentioning that only the follicles with visible nucleus were counted in order to avoid double counting. In our previous study more information about appearance features of normal and atretic follicles has been presented (Nateghi et al. Citation2017).
Serum FAs profiles
Blood samples (8–10 mL from each hen) were obtained from the hens (10 hens from each group) by jugular vein bleeding into test tubes to quantify and determine their FA profiles. Serum was separated by using centrifuge device at 3800 g for 20 min and followed by freezing and keeping at −70 °C until the main assay is conducted.
After samples were thawed at room temperature for 3 min, FAs in serum were immediately methylated with 0.5 mL of 0.2N methanolic sodium hydroxide for 30 min. This process was followed by treatment with 1 mL of 14% boron trifluoride in methanol (BF3-methanol) at room temperature for 30 min (60 min overall). FA methyl esters were reconstituted in 1 mL of hexane (Loor et al. Citation2005). The concentration of FA was specified using gas chromatography (Model YL 6100, Make: Young Lin, Anyang, Korea) with a 60 m (0.25 mm I.D.) capillary column (Dikmacap-2330). The carrier gas was hydrogen, and the initial and final temperatures were set at 170 and 230 °C with detector and injector temperatures set at 300 and 260 °C, respectively.
Quantitative real time PCR for apoptotic genes
We used six hens from each group for gene expression. Immediately after the warming process, in order to extract RNA, fragments from each group were stored in RNA later reagent (Ambion, Foster City, USA) and preserved at −80 °C. More information and details about this method have been presented in our previous study (Nateghi et al. Citation2017).
The evaluation of the expression levels of Bak, Bcl-2, Cas3, Cas8, and Cas9 was performed. The housekeeping gene GAPDH was used for normalisation. The pattern of designed primers is presented in .
Table 2. The characteristic of primers used in real-time RT-PCR assays.
Statistical analysis
Statistical analysis was carried out by using SPSS (Version 22.0) (IBM Crop., Armonk, NY). The method used to demonstrate continuous variables was mean ± standard error of the mean (SEM). In addition, Duncan post-hoc test was used to compare groups with one another after a one-way ANOVA. All the statistical tests were two-sided and p < .05 was assumed as being statistically remarkable.
Results
Performance, ovary and oviduct morphology
The parameters of ovary and oviduct morphology of the laying hens fed experimental diets for 6 weeks were recorded (). Effects of dietary treatments on ovary weight, SYF, LYF and total follicles were significant (p < .05). In n-3 + E and n-3, number of SYF was higher than that of other groups (p < .05). Fish oil consumption stimulated the number of LYF more than sunflower oil regardless of vitamin E supplementation (p < .05). The total number of follicles in n-3 and n-3 + E groups were higher than those of control whereas E group was in the middle. Total egg production of control, E, n-3 + E and n-3 groups were 597,607,614 and 639, respectively, and the rate of ovulation was 89.5, 91, 92 and 96% in control, E, n-3 + E and n-3, respectively (). Although ovulation rate was significantly (p < .05) increased in n-3 than control group, the differences between E, n-3 + E and n-3 were not significant. Body weight, oviduct weight, oviduct weight/body weight, oviduct length, ovary weight/body weight, LWF and largest yellow follicle weight (F1 weight) were not significantly affected by dietary treatments.
Figure 1 Effects of dietary treatments on the rate of ovulation (hen-day egg production %). The rate of ovulation was 89.57, 91.08, 92.14 and 95.89% in control, E, n-3 + E and n-3 treatments, respectively. Experimental groups (n = 17 in each group): basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil +1.1 U alpha-tocopherol/hen/day (E); basal diet+ 1.5% fish oil +1.1 U alpha-tocopherol/hen/day (n-3 + E) and basal diet +1.5% fish oil (n-3). Data are presented as means ± SEM (a,bGroups followed by the same letter are not significantly different at the p < .05).
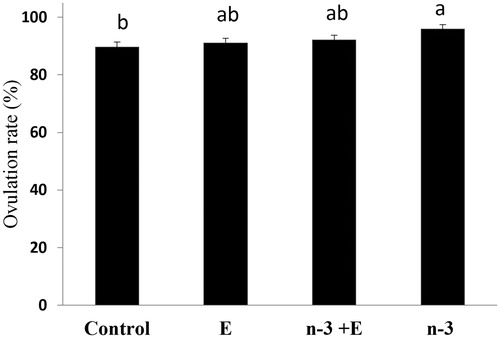
Table 3. Effects of diets on different reproductive morphology parameters in laying hens.
Histological evaluations
Histological assessment of ovarian tissues showed that the proportions of intact primordial follicles in n-3 and n-3 + E were significantly more than control and E groups (p < .01; ). The difference between the number and dispersion of primordial follicles in the groups receiving omega-3 (n-3, n-3 + E) in comparison with other groups is presented in .
Figure 2 The percentage of intact primordial, primary and prehierarchical follicles in different experimental groups (n = 10 in each group): basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil +1.1 U alpha-tocopherol/hen/day (E); basal diet+ 1.5% fish oil +1.1 U alpha-tocopherol/hen/day (n-3 + E) and basal diet +1.5% fish oil (n-3). Values are given as mean ± SEM. Groups followed by the same letter are not significantly different at p < .05.
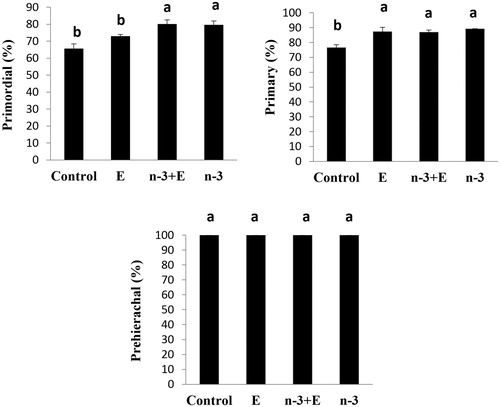
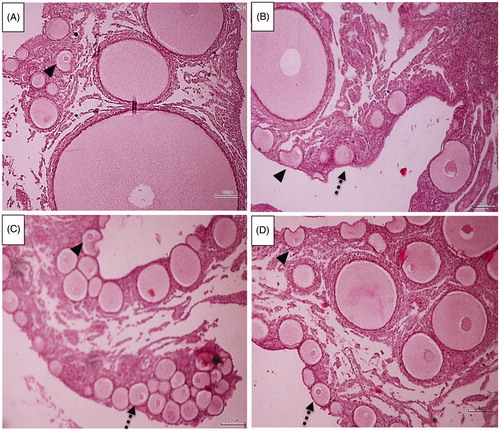
Figure 3 Laying hen ovarian tissues (n = 10 in each group). Intact primordial follicles (dash arrows) and atretic primordial follicles (arrow head). A: Control (basal diet), B: Vitamin E, C: n-3 + E, D: n-3. Basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil + 1.1 U alpha-tocopherol/hen/day (E); basal diet + 1.5% fish oil + 1.1 U alpha-tocopherol/hen/day (n-3 + E) and basal diet + 1.5% fish oil (n-3). Hematoxylin & Eosin staining (scale bar = 100 μm). Follicles with a maximum size of 80 μm were counted as primordial follicles. (Only the follicles with a visible nucleus were counted).
The lowest proportion of intact primary follicle was observed in the control (76.5%) in comparison to the other groups (p < .01; ). The differences in the proportion of prehierarchical follicles were not significant among the control and other experimental groups.
FA profiles in serum
shows the FA profiles of blood serum of the laying hen. Linoleic acid (C18:2 n-6c) levels in serum were significantly reduced in n-3 + E and n-3. The proportion of EPA in the serum of control, E and n-3 + E was analogous (0.16, 0.06 and 0.31, respectively), whereas EPA concentration in n-3 was 1.20% (p < .01). DHA concentration in the serum of n-3 group was about seven times more than that of the control. Total omega-3 FAs were the highest in fish oil groups (n-3 + E and n-3) (p < .01). Interestingly, arachidonic acid (AA) (C20:4 n-6c) concentration in serum of E, n-3 + E and n-3 groups was lower than control (p < .05). Monounsaturated fatty acid (MUFA), PUFA, odd chain FAs, total n-6 FAs and saturated FAs were unaltered by treatments.
Table 4. Effect of dietary treatments on fatty acid concentration of blood serum (% of total fatty acids).
Apoptotic gene expression
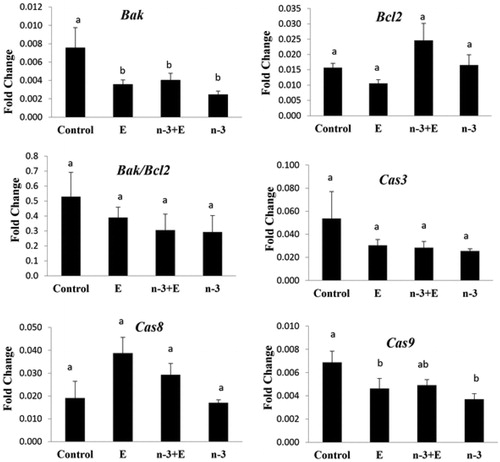
The expression of Bak was significantly higher in control than the other groups (p < .05; ). There was a significant reduction in expression of Cas9 in n-3 and E compared to the control and n-3 + E (p < .05; ). The expression of Bcl-2, Bak/Bcl-2, Cas3, and Cas8 were not significantly affected by dietary treatments.
Figure 4 Fold change of Bak, Bcl-2, Caspase 3, Caspase 8, and Caspase 9 in experimental groups (n = 6 in each group): basal diet +1.5% sunflower oil (control; C); basal diet +1.5% sunflower oil +1.1 U alpha-tocopherol/hen/day (E); basal diet+ 1.5% fish oil +1.1 U alpha-tocopherol/hen/day (n-3 + E) and basal diet +1.5% fish oil (n-3). Values are given as mean ± SEM. Groups followed by the same letter are not significantly different at p < .05.
Discussion
The results of this research proved that regardless of antioxidant supplementation, dietary fish oil stimulated follicular growth and the number, ovulation rate and ovarian weight. The survey on the effects of vitamin E with omega-3 and omega-6 sources could be considered as the novelty of this research.
Zeron et al. (Citation2002) noted that the number of primordial follicles was greater in ewes fed fish oil than those of control diet. Some other researchers who have focussed on omega-3 sources have mentioned that the number of follicles increased in cows fed with linseed (C18:3 n-3 source) (Petit et al. Citation2002; Robinson et al. Citation2002; Petit et al. Citation2004) and fish oil (DHA and EPA source) (Heravi Moussavi et al. Citation2007). Furthermore, in cows with diets rich in omega-3 and omega-6 FA increased the number of small follicles (Gandra et al. Citation2017). In rats, Broughton et al. Citation2010 have reported that consumption of fish oil ended in a higher ovulation rate. It has also been shown that omega-3 sources have a significant effect on ovarian weight in ruminants (Mattos et al. Citation2000). Eilati et al. (Citation2013) reported that laying hens fed fish oil for 3 weeks would increase egg production and down-regulated the expression of Cyclooxygenase (COX)-1 and COX-2 mRNA in comparison with that of control group. COX is the rate limiting enzyme in catalysing the conversion of AA to prostaglandins (PGs). PGs are biologically active lipids that are associated with inflammation, fever, pain and tissue injury (Eilati et al. Citation2013).
Our findings have supported the theory that omega-3 FAs, especially DHA, have a pivotal role in follicogenesis, and we have shown that this effect is independent of vitamin E supplementation.
Several mechanisms for the effects of omega-3 on ovary have been suggested in previous studies. Omega-3 is a stimulator of FSH leading to follicular growth, development and ability of follicular rupture and ovulation (Bender et al. Citation2010). Two unique biological signalling factors are EPA and DHA that cause ovulation and increase in blood flow to the ovary to elevate follicles growth and enhance ovarian weight (Senger Citation2005). Hypothalamic receptors of the reproductive system primarily mediate the influence of omega-3 on this system and the release of GnRH, which subsequently induces the synthesis of FSH and LH (Watanabe Citation2002). A high intake of dietary fish oil contain EPA and DHA leads to a significant rise in ovulation and follicles development, and these events depend on hypothalamic–pituitary function (Zidkova et al. Citation2004). Staples et al. (Citation1998) showed that PUFA affects ovarian dynamics leading to a few alterations in the numbers of follicles. It was shown that unsaturated FAs not only weakened follicular apoptosis, but also expedited the onset of puberty (Almog et al. Citation2001). Accordingly, Evans et al. (Citation2012) and Mossa et al. (Citation2012) indicated that dietary n-3 FAs accelerate follicogenesis in ovaries, which could favourably affect fertility efficiency despite dietary antioxidant function.
It was expected that vitamin E promotes the effect of fish oil because of its related antioxidant support. However, supplementation of vitamin E in the diet of laying hen appears to be ineffective in supporting FA function during the egg production period. Similar to our results, Cortinas et al. (Citation2003) demonstrated that dietary α-tocopherol acetate supplementation did not manipulate the FA profile of eggs when laying hens were fed with omega-3 FAs. Therefore, our results showed that in laying hens, vitamin E is unnecessary for the improvement of ovary morphological characteristics and there is no need for fish oil in the diet.
The increase in EPA, DHA, total n-3 PUFA, n-3: n-6 ratio, as well as decrease in C18:2 and C20:4 of serum were the major observations pertaining to FAs. In the present study, C20:4 decreased in n-3, n-3 + E, and E more than in the control group. Wada et al. (Citation2007) demonstrated that feeding fish oil as EPA and DHA source, a precursor for a competitive pathway, inhibits the synthesis of AA. Schreiner et al. (Citation2004) reported an almost complete exchange of AA and DHA after treating hens with omega-3 FA. Dietary omega-3 FAs may modulate substrate pools available to COX and lipoxygenase, thereby controlling the downstream eicosanoid formation and subsequent receptor activation (Moonen et al. Citation2004). COXs, convert AA to PGs. In fact, COX enzymes convert omega-6 FAs to 2-series PG products such as PGE2 whereas the end products of COX enzymes activity on omega-3 FAs are 3-series PGs such as PGE3. The 3-series PG products are generally less pro-inflammatory than the 2-series products (Kobel et al. Citation2010).
The amounts of C18:2 (linoleic acid) were reduced by fish oil supplementation. Our findings were in agreement with previous reports about FA content in serum of cows (Mattos et al. Citation2004) and egg yolk lipids (Ebeid Citation2011) that showed a reduction in n-6 FAs such as linoleic and AA due to fish oil consumption. C18:2 was transformed into AA, which is a primary precursor of PGs. The synthesis of PGF2α from AA could be adjusted by the key enzyme, prostaglandin endoperoxide synthase (PGHS).
Surprisingly, vitamin E had similar effects on AA metabolism. As a reported mechanism for omega-3 FAs, DHA is assumed to be a strong inhibitor of PGHS activity, and our findings in the n-3 group confirmed that omega-3 FA, especially DHA, plays a pivotal role in AA metabolism. It was reported that EPA and DHA could severely suppress synthesis of PGF2α by AA metabolism depression (Elattar and Lin Citation1989), and it was demonstrated that vitamin E may influence AA metabolism. This requires further studies.
Previous studies have suggested that n-6 FAs as well as n-3 FAs may have disruptive effects on reproductive function without dietary antioxidants support. In contrast, our study did not show any effects of consumption of fish oil. This could be because of the low quantities of fish oil consumed, and the short-term nature of the study. It must be mentioned that the absence of negative effects of low levels of fish oil (>2% in diet) has been shown earlier for cattle (Alizadeh et al. Citation2012), ram (Esmaeili et al. Citation2014; Habibi et al. Citation2017) and in the current study on hen.
n-3 PUFAs not only play a significant role in cell membrane composition, but also serve as natural ligands for certain nuclear receptors that affect gene expression. Programmed cell death process is known as apoptosis (Thomas Citation2009). It is believed that n-3 PUFAs, EPA and DHA are able to cause apoptosis in vitro, in tumour cell lines extracted from various kinds of tumours such as colorectal carcinoma (Chen and Istfan Citation2000), oesophageal (Kubota et al. Citation2013) and gastric cancers (Lee et al. Citation2009), hepatocellular carcinoma (Zhang et al. Citation2015), pancreatic cancer (Merendino et al. Citation2005), breast (Chamras et al. Citation2002), ovarian (Sharma et al. Citation2005), prostate (Narayanan et al. Citation2005) and lung cancer (Serini et al. Citation2008). Although many studies have investigated the effects of n-3 PUFAs on the function of cancer cells, few have been focussed on normal cells, such as the current study.
Our results showed that omega-3 was just able to lower the expression levels of Bak and Cas9 mRNA in compared to the control group. Nevertheless, Erol et al. (Citation2011) claimed that omega-3 FAs presented an extreme protective effect against apoptosis process in liver cells. Li et al. (Citation2017) showed that there is a major decrease in cardiomyocyte apoptosis after pre-treatment with n-3 PUFA. Another research showed that n-3 FAs play a role as an anti-apoptotic in developing cerebellum of hypothyroid rat (Sinha et al. Citation2009). These divergent observations may be explained as follows: n-3 could cause proapoptotic influences on pathological conditions, such as cancer. Although a few apoptotic pathways might be activated, this supposition is implausible and incorrect in normal tissues. However, there is a need for more research to better comprehend the precise mechanism of the effect of n-3 PUFA on gene expression modulation.
Conclusions
Inclusion fish oil in diet significantly increases the amounts of unique FAs (DHA, EPA) in blood, probably being a part of the ovarian stimulation response due to an increase in FA. Therefore, it could be supposed that antioxidant consumption would be unnecessary to achieve improvements in ovarian function; this was shown by the absence of effects when small quantities of fish oil were added to the diet of laying hens. However, n-3 PUFA and vitamin E may be able to protect ovarian cells from apoptotic death at the cellular level. FA and antioxidant crosstalk in ovarian function warrant further studies.
Ethical Statement
The project was approved by the Ethical Committee for the care and use of experimental animals of Royan Institute.
Acknowledgments
The authors thank the employees of the Halajerd Research Station (Dr. M. Haji Nasrollah and Mr. A. R. Nemati), Karaj, Iran for animal care and Tekno Azma laboratory (Dr. P. Kavousi) for collaboration for fatty acid analysis. The authors also thank Ms. Lakshmi Gopal (Valerdoc Company) for editing of manuscript.
Disclosure statement
The authors declare no conflicts of interest.
References
- Abayasekara DR, Wathes DC. 1999. Effects of altering dietary fatty acid composition on prostaglandin synthesis and fertility. Prostaglandins Leukot Essent Fatty Acids. 61:275–287.
- Alizadeh AR, Alikhani M, Ghorbani GR, Rahmani HR, Rashidi L, Loor JJ. 2012. Effects of feeding roasted safflower seeds (variety IL-111) and fish oil on dry matter intake, performance and milk fatty acid profiles in dairy cattle. J Anim Physiol Anim Nutr. 96:466–473.
- Almog B, Gold R, Tajima K, Dantes A, Salim K, Rubinstein M, Barkan D, Homburg R, Lessing JB, Nevo N, et al. 2001. Leptin attenuates follicular apoptosis and accelerates the onset of puberty in immature rats. Mol Cell Endocrinol. 183:179–191.
- Barua A, Bitterman P, Abramowicz JS, Dirks AL, Bahr JM, Hales DB, Bradaric MJ, Edassery SL, Rotmensch J, Luborsky JL. 2009. Histopathology of ovarian tumors in laying hens: a preclinical model of human ovarian cancer. Int J Gynecol Cancer. 19:531–539.
- Bender K, Walsh S, Evans ACO, Fair T, Brennan L. 2010. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction. 139:1047–1055.
- Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. 2011. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 93:950–962.
- Broughton KS, Bayes J, Culver B. 2010. High α-linolenic acid and fish oil ingestion promotes ovulation to the same extent in rats . Nutr Res. 30:731–738.
- Chamras H, Ardashian A, Heber D, Glaspy JA. 2002. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem. 13:711–716.
- Chen ZY, Istfan NW. 2000. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot Essent Fatty Acids. 63:301–308.
- Cortinas L, Galobart J, Barroeta AC, Baucells MD, Grashorn MA. 2003. Change in α-tocopherol contents, lipid oxidation and fatty acid profile in eggs enriched with linolenic acid or very long-chain ω3 polyunsaturated fatty acids after different processing methods. J Sci Food Agric. 83:820–829.
- Damjanov I. 1989. Ovarian tumours in laboratory and domestic animals. Curr Top Pathol. 78:1–10.
- Ebeid T. 2011. The impact of incorporation of n-3 fatty acids into eggs on ovarian follicular development, immune response, antioxidative status and tibial bone characteristics in aged laying hens. Animal. 5:1554–1562.
- Ebeid T, Eid Y, Saleh A, Abd El-Hamid H. 2008. Ovarian follicular development, lipid peroxidation, antioxidative status and immune response in laying hens fed fish oil-supplemented diets to produce n-3-enriched eggs. Animal. 2:84–91.
- Eilati E, Small CC, McGee SR, Kurrey NK, Hales DB. 2013. Anti-inflammatory effects of fish oil in ovaries of laying hens target prostaglandin pathways. Lipids Health Dis. 12:152
- Elattar TM, Lin HS. 1989. Comparison of the inhibitory effect of polyunsaturated fatty acids on prostaglandin synthesis I oral squamous carcinoma cells. Prostaglandins Leukot Essent Fatty Acids. 38:119–125.
- Erol AYG, Bulbul A, Avci G, Ozdemir M, Akkaya O. 2011. The protective effects of omega-3 fatty acids and sesame oil on cyclosporine-A induced liver apoptosis. J Acad Res Med. 1:8–11.
- Esmaeili V, Shahverdi AH, Alizadeh AR, Alipour H, Chehrazi M. 2014. Saturated, omega-6 and omega-3 dietary fatty acid effects on the characteristics of fresh, frozen-thawed semen and blood parameters in rams. Andrologia. 46:42–49.
- Evans AC, Mossa F, Walsh SW, Scheetz D, Jimenez-Krassel F, Ireland JL, Smith GW, Ireland JJ. 2012. Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod Domest Anim. 47:31–37.
- Fontana R, Torre SD. 2016. The deep correlation between energy metabolism and reproduction: a view on the effects of nutrition for women fertility. Nutrients. 8:87.
- Fouladi-Nashta AA, Wonnacott KE, Gutierrez CG, Gong JG, Sinclair KD, Garnsworthy PC, Webb R. 2009. Oocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acids. Reproduction. 138:771–781.
- Gandra JR, Verdurico LC, Mingoti RD, Takiya CS, Gardinal R, Vendramini THA, Barletta RV, Visintin JA, Renno FP. 2017. Whole flaxseed, raw soybeans, and calcium salts of fatty acids supplementation for transition cows: follicle development and embryo quality. Ital J Anim Sci. 16:538–545.
- Habibi M, Zamiri MJ, Akhlaghi A, Shahverdi AH, Alizadeh AR, Jaafarzadeh MR. 2017. Effect of dietary fish oil with or without vitamin E supplementation on fresh and cryopreserved ovine sperm. Anim Prod Sci. 57:441–447.
- Heravi Moussavi AR, Gilbert RO, Overton TR, Bauman DE, Butler WR. 2007. Effects of feeding fish meal and n-3 fatty acids on ovarian and uterine responses in early lactating dairy cows. J Dairy Sci. 90:145–154.
- Johnson AL, Woods DC. 2007. Chapter 6. Ovarian dynamics and follicle development. In: Jamieson BGM, editor. Reproductive biology and phylogeny of birds. Enfield (NH): Science Publishers, an imprint of Edenbridge Ltd; p. 243–277.
- Kobel M, Reuss A, Bois A, Kommoss S, Kommoss F, Gao D, Kalloger SE, Huntsman DG, Gilks CB. 2010. The biological and clinical value of p53 expression in pelvic high-grade serous carcinomas. J Pathol. 222:191–198.
- Kubota H, Matsumoto H, Higashida M, Murakami H, Nakashima H, Oka Y, Okumura H, Yamamura M, Nakamura M, Hirai T. 2013. Eicosapentaenoic acid modifies cytokine activity and inhibits cell proliferation in an oesophageal cancer cell line. Anticancer Res. 33:4319–4324.
- Lee SE, Lim JW, Kim H. 2009. Activator protein-1 mediates docosahexaenoic acid-induced apoptosis of human gastric cancer cells. Ann N Y Acad Sci. 1171:163–169.
- Leroy JL, Sturmey RG, Van Hoeck V, De Bie J, McKeegan PJ, Bols PE. 2014. Dietary fat supplementation and the consequences for oocyte and embryo quality: hype or significant benefit for dairy cow reproduction? Reprod Domest Anim. 49:353–361.
- Li Q, Yu Q, Na R, Liu B. 2017. Omega‑3 polyunsaturated fatty acids prevent murine dilated cardiomyopathy by reducing oxidative stress and cardiomyocyte apoptosis. Exp Ther Med. 14:6152–6158.
- Loor JJ, Ferlay A, Ollier A, Doreau M, Chilliard Y. 2005. Relationship among trans and conjugated fatty acids and bovine milk fat yield due to dietary concentrate and Linseed oil. J Dairy Sci. 88:726–740.
- Mahla AS, Chaudhari RK, Verma AK, Singh AK, Singh SK, Singh G, Sarkar M, Dutta N, Kumar H, Krishnaswamy N. 2017. Effect of dietary supplementation of omega-3 polyunsaturated fatty acid (PUFA) rich fish oil on reproductive performance of the goat (Capra hircus)). Theriogenology. 99:79–89.
- Mattos R, Staples CR, Arteche A, Wiltbank MC, Diaz FJ, Jenkins TC, Thatcher WW. 2004. The effects of feeding fish oil on uterine secretion of PGF2alpha, milk composition, and metabolic status of periparturient Holstein cows. J Dairy Sci. 87:921–932.
- Mattos R, Staples CR, Thatcher WW. 2000. Effects of dietary fatty acids on reproduction in ruminants. Rev Reprod. 5:38–45.
- Merendino N, Loppi B, D'Aquino M, Molinari R, Pessina G, Romano C, Velotti F. 2005. Docosahexaenoic acid induces apoptosis in the human PaCa-44 pancreatic cancer cell line by active reduced glutathione extrusion and lipid peroxidation. Nutr Cancer. 52:225–233.
- Moonen HJ, Dommels YE, van Zwam M, van Herwijnen MH, Kleinjans JC, Alink GM, de Kok TM. 2004. Effects of polyunsaturated fatty acids on prostaglandin synthesis and cyclooxygenase-mediated DNA adduct formation by heterocyclic aromatic amines in human adenocarcinoma colon cells. Mol Carcinog. 40:180–188.
- Moreng GR, Cain JR, Warner RL. 1980. The effect of adrenal glands on ovulation in Japanese quail (Coturnix coturnix japonica). Poult Sci. 59:2760–2764.
- Mossa F, Walsh SW, Butler ST, Berry DP, Carter F, Lonergan P, Smith GW, Ireland JJ, Evans AC. 2012. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J Dairy Sci. 95:2355–2361.
- Narayanan NK, Narayanan BA, Reddy BS. 2005. A combination of docosahexaenoic acid and celecoxib prevents prostate cancer cell growth in vitro and is associated with modulation of nuclear factor-k B, and steroid hormone receptors. Int J Oncol. 26:785–792.
- Nateghi R, Alizadeh AR, Jafari Ahangari Y, Fathi R, Akhlaghi A. 2017. Ethylene glycol and dimethyl sulfoxide combination reduces cryoinjuries and apoptotic gene expression in vitrified laying hen ovary. Biopreserv Biobank. 15:519–528.
- Petit HV, Dewhurst RJ, Scollan ND, Proulx JG, Khalid M, Haresign W, Twagiramungu H, Mann GE. 2002. Milk production and composition, ovarian function, and prostaglandin secretion of dairy cows fed omega-3 fats. J Dairy Sci. 85:889–899.
- Petit HV, Germiquet C, Lebel D. 2004. Effect of feeding whole, unprocessed sunflower seeds and flaxseed on milk production, milk composition, and prostaglandin secretion in dairy cows. J Dairy Sci. 87:3889–3898.
- Raederstorff D, Wyss A, Calder PC, Weber P, Eggersdorfer M. 2015. Vitamin E function and requirements in relation to PUFA. Br J Nutr. 114:1113–1122.
- Renema RA, Robinson FE, Melnychuk VL, Hardin RT, Bagley LG, Emmerson DA, Blackman JR. 1995. The use of feed restriction for improving reproductive traits in male-line large white turkey hens. 2. Ovary morphology and laying trait characteristics. Poult Sci. 74:102–120.
- Robinson RS, Pushpakumara PG, Cheng Z, Peters AR, Abayasekara DR, Wathes DC. 2002. Effects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cows. Reproduction. 124:119–131.
- Schreiner M, Hulan HW, Razzazi-Fazeli E, Bohm J, Iben C. 2004. Feeding laying hens seal blubber oil: effects on egg yolk incorporation, stereospecific distribution of omega-3 fatty acids, and sensory aspects. Poult Sci. 83:462–473.
- Senger PL. 2005. Pathways to pregnancy and parturition. Second revised edition. USA: Current Conceptions, Inc. p. 187–341.
- Serini S, Trombino S, Oliva F, Piccioni E, Monego G, Resci F, Boninsegna A, Picci N, Ranelletti FO, Calviello G. 2008. Docosahexaenoic acid induces apoptosis in lung cancer cells by increasing MKP-1 and down-regulating p-ERK1/2 and p-p38 expression. Apoptosis. 13:1172–1183.
- Sharma A, Belna J, Logan J, Espat J, Hurteau JA. 2005. The effects of Omega-3 fatty acids on growth regulation of epithelial ovarian cancer cell lines. Gynecol Oncol. 99:58–64.
- Simopoulos AP. 2016. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 8:128
- Sinha RA, Khare P, Rai A, Maurya SK, Pathak A, Mohan V, Nagar GK, Mudiam MKR, Godbole MM, Bandyopadhyay S. 2009. Anti-apoptotic role of omega-3-fatty acids in developing brain: perinatal hypothyroid rat cerebellum as apoptotic model. Int J Devl Neuroscience. 27:377–383.
- Staples CR, Burke JM, Thatcher WW. 1998. Influence of supplemental fats on reproductive tissues and performance of lactating cows. J Dairy Sci. 81:856–871.
- Thomas GC. 2009. Apoptosis and cancer: the genesis of a research field. Nat Rev. Cancer. 9:501–507.
- Valk EE, Hornstra G. 2000. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. Int J Vitam Nutr Res. 70:31–42.
- Wada M, DeLong CJ, Hong YH, Rieke CJ, Song I, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, et al. 2007. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem. 282:22254–22266.
- Watanabe K. 2002. Prostaglandin F synthase. Prostaglandins Other Lipid Mediat. 68–69:401–407.
- Wathes DC, Abayasekara DR, Aitken RJ. 2007. Polyunsaturated fatty acids in male and female reproduction. Biol Reprod. 77:190–201.
- Zachut M, Dekel I, Lehrer H, Arieli A, Arav A, Livshitz L, Yakoby S, Moallem U. 2010. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J Dairy Sci. 93:529–545.
- Zeron Y, Sklan D, Arav A. 2002. Effect of polyunsaturated fatty acid supplementation on biophysical parameters and chilling sensitivity of ewe oocytes. Mol Reprod Dev. 61:271–278.
- Zhang Y, Han L, Qi W, Cheng D, Ma X, Hou L, Cao X, Wang C. 2015. Eicosapentaenoic acid (EPA) induced apoptosis in HepG2 cells through ROS-Ca2+-JNK mitochondrial pathways. Biochem Biophys Res Commun. 456:926–932.
- Zidkova J, Sajdok J, Kontrova K, Kotrbova-Kozak A, Hanis T, Zidek V, Fucikova A. 2004. Effects of oxidised dietary cod liver oil on the reproductive functions of Wistar rat. Czech J Food Sci. 22:108–120.
