 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A 2 × 6 factorial experiment was carried out to evaluate the single and combined effects of oral gavage of Satureja khuzistanica essential oils (SkEO; 0, 200, 300, 400, 500 and 600 mg/bird/day) and dietary acetic acid (AA; 0 and 20 mg/kg) on selected parameters using 252 Ross-308 14-day-old broiler chicks. Effects of 12 treatments were examined in seven replicates of three birds each. Administration of SkEO at 400, 500 and 600 mg/day reduced ADFI by 8.99, 8.09 and 10.12% compared with those gavaged with 200 mg, respectively. In the birds gavaged with 400 mg SkEO, serum concentration of non-esterified fatty acids (NEFA) increased when compared with those who received 600 mg SkEO in Days 42 of age. Serum concentration of albumin (ALB) was greater by 8.38% in birds fed with AA-added diets than the control birds in Day 34 of age. Serum concentration of antioxidants increased in the birds receiving 400 mg of SkEO and fed with the non-acidified diet than those receiving 300 mg of SkEO and fed acidified diets. Gross kidney health improved by gavaging SkEO indicated by greater frequency of score zero (apparently healthy) and reduced fat percentage in the SkEO-fed birds. The kidney fat proportion in the birds gavaged SkEO at 300 to 500 mg were decreased when compared with control birds in Day 38 of age. In conclusion, SkEO can be fed to broiler chicken to improve kidney health at 200 to 400 mg/day with no adverse effect of performance.
Direct administration of SkEO into crop through oral gavageing lowered feed intake and improved gross kidney health.
Almost all productive performance indications were adversely affected in birds receiving AA-added diets.
No interaction was found between the SkEO and acetic acid in main productive traits.
HIGHLIGHTS
Introduction
Satureja khuzistanica Jamzad, a plant belonging to Labiatae family, grows mainly in the Middle East, Mediterranean region to Europe, West Asia, North Africa, the Canary Islands, South America (Cantino et al. Citation1992; Momtaz and Abdollahi Citation2010), and Iran (Hadian et al. Citation2011). A considerable level of essential oils, up to 4.5%, has been identified as a prominent characteristic of this plant (Khosravinia Citation2015). It has been shown that essential oils from Satureja khuzistanica contain a broad spectrum of phenols, flavones, triterpenoids, steroids, and tannins (Moghaddam et al. Citation2007). The extraordinarily high levels of a terpenic monophenolic entity named as carvacrol offer SkEO a great value, as a promising biological compound for pharmaceutical and food industries. Previous works reported carvacrol as the main component in Satureja khuzistanica essential oils (Farsam et al. Citation2004; Hadian et al. Citation2011; Khosravinia et al. Citation2013), ranging from 92.2 to 93.9%, the peculiarity causing Khosravinia et al. (Citation2013) describes the plane as a ‘bioreactor of carvacrol’.
Extensive works by many researchers (Abdollahi et al. Citation2003; Amanlou et al. Citation2005; Haeri et al. Citation2006), in particular, Khosravinia (Citation2013, Citation2015, Citation2016), demonstrated anti-inflammatory, anti-nociceptive, antibacterial, antioxidant and anti-hyperlipidemic effects for SkEO, the beneficial effects which mainly attributed to carvacrol. In a series of works, Khosravinia and his co-workers examined the effects of SkEO in feed or water in a wide dose-range on various aspects of broiler chicken metabolism. From their results, it can be concluded that water supplementation with 400 mg/L SkEO improved breast weight of broilers under a tropical climate (Parvar et al. Citation2013). Moreover, SkEO has a potential to affect the serum lipid profile in broiler chickens evidenced by decreased serum concentration of oestradiol and the weight of abdominal fat in the birds provided with SkEO-enriched drinking water (500 or 1000 mg/L). In addition, evidence showed Satureja khuzistanica ethanolic extract (Souri et al. Citation2015) or selected SkEO components, carvacrol and thymol (Major et al. Citation2011; Machado Junior et al. Citation2014), have immunomodulatory effects in broiler chickens.
Organic acids, in particular, acetic acid, are among the main non-antibiotic feed additives attracting greater attention form poultry industry (Kral et al. Citation2011; Nourmohammadi et al. Citation2015, Citation2016, Citation2018). It is generally believed that use of acetic acids in broiler diet may inhibit pathogens like Salmonella in both raw materials and feed (Choct Citation2001). The lower pH created, can protect the animal from infection especially at their younger ages (Nourmohammadi et al. Citation2018). Acetic acid at a wide dose range from 0.5 to 5% has been implemented in diets for broiler which mainly reduced growth of many pathogenic or non-pathogenic intestinal bacteria, therefore, reduced the risk of intestinal colonisation and infectious processes, ultimately decreased inflammatory processes at intestinal mucosa, a phenomenon which in turn, increases villus height and function of secretion, digestion and absorption of nutrients by the mucosa (Loddi et al. Citation2004; Pellicano et al. Citation2005). Acidification of diet with AA has been reported to reduce the production of toxic components by the bacteria and colonisation of pathogens on the intestinal wall, thus preventing the damage to epithelial cells (Langhout Citation2000). Apart from the antimicrobial activity, AA reduces pH of digesta, increases the pancreatic secretion, and have trophic effects on the mucosa of gastrointestinal tract (Dibner and Buttin Citation2002). Moreover, acetate anion has shown to form complex with Ca, P, Mg, and Zn, which results in an improved digestibility of these minerals and serve as substrates in the intermediary metabolism (Kishi et al. Citation1999; Kral et al. Citation2011; Galik and Rolinec Citation2011; Petruska et al. Citation2012). Besides all dietary impacts, short chain organic acids like acetic acid, are well known for their role in energy metabolism. Particularly, AA in the form of acetyl-coenzyme A is a vital metabolite for the ATP production and biosynthesis of long chain fatty acids (Hamano and Kurimoto Citation2016).
Despite huge experimental results in the literature on various feed additives for poultry industry mainly based on criticisms raised against administration of subtherapeutic doses of antibiotics in animal diets during the last decade, a meaningful scanty of reports on interaction of different feed additives is prominent. Today, even small scale broiler farms are using two, three or more feed additives in a single diet, where their interaction has never been characterised in detail.
Therefore, this study was carried out to evaluate the effects of oral gavage of SkEO and diet supplementation with acetic acid and their interactions on productive performance, certain blood and kidney health-related parameters in broiler chickens.
Material and methods
Birds and diets
A total number of 800 Ross-308 one-day-old broiler chicks were obtained from a local hatchery and raised on litter in a windowless power ventilated house up to Day 10 of age, following the protocols of Animal Care Committee of the Lorestan University, Iran. The environmental temperature and relative humidity were kept at 32 ± 1 °C and 60 ± 5%, respectively, during the raising period. The birds were provided with 23 hours of light and one hour of darkness, except for the first three days when illumination provided clock round. Throughout the same period, birds had free access to water a crumble diet containing 2942 kcal/kg metabolisable energy and 20.43 protein (Table ). Vaccinations were performed against Newcastle and Bronchitis viruses on the 4th day of the experiment.
Table 1. Ingredients and nutrient composition of basal diets.
At the beginning of the Day 11, 252 healthy birds with an average weight of 300 ± 5 g were chosen and transferred to 84 wire cages with 45 × 50 × 40 cm dimensions for length, width and attitude, respectively, arranged in seven blocks perpendicular to the air flow direction, where they spent three days for acclimatisation followed by four weeks experimental period. At Day 14, experimental period initiated by feeding starter (11 to 20 days), grower (21 to 35) and finisher (36 to 42 days) pelleted diets (Table ) with or without 20 g/kg acetic acid inclusion. Then, birds in each group received 0, 200, 300, 400, 500, 600 mg SkEO daily through oral gavage. Lightening schedule was followed as the pre-experiment period and ambient temperature during the first week of the experimentation period was kept at 29 °C and then gradually reduced by 2 to 3 °C weekly to reach about 24 °C at the end of the fourth week then it was kept constant. The experiment was conducted in a 2 × 6 factorial fashion with 12 treatments in seven replicates and three birds in each replicate (cage).
Data collection
Data on live body weight (BW) and feed intake were collected weekly during the experimental period and data were used to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR). Mortality was recorded upon occurrence. European economic efficiency index (EEEI) was calculated based on the following equation; (Euribrid Citation1994)
where LW is live weight (kg), S is survival rate (%), FCR is feed conversion ratio and AS is age of slaughter (day).
At Days 34, 38 and 42 of the experimentation period, one bird from each replicate was killed and 10 mL blood was collected from each bird by the brachial vein puncture and kept on slush-ice pending serum extraction. Samples of coagulated whole blood were centrifuged at 1800 × g for 15 min. The serum was collected and stored at −20 °C for pending chemical analysis. Concentrations of serum biochemical constituents including glucose (GLU), total triglycerides (TG), total cholesterol (TC), none esterified fatty acid (NEFA), high density lipoproteins (HDL) low density lipoproteins (LDL), Total Protein (TP), Albumin (ALB), total Bilirubin (BIL), were determined using an autoanalyser (Selects E Autoanalyzer, Sr. No. 8-7140, Vital Pharma BV, Maarheeze, TheNetherlands). This analyser employs enzymatic procedures using SEPPIM Diagnostic Kits (SEPPIM S.A.S., Sees, France) in two replicates, at 25 °C, that has been described by Elliott (Citation1984) and adopted by Khosravinia (Citation2015). Lipid peroxidation was assayed by the content of thiobarbituric acid reactive substances (TBARS) in the serum with MDA assay kit.
Extraction of lipids from kidney tissue was conducted using the method of Folch et al. (Citation1975) with slight modification. Briefly, a 1 g tissue was homogenised in 2:1 (vol/vol) chloroform-methanol mixture for 30 min, mixture was filtered with Whatman filter paper and then KCl (8%) was added for 10 min at room temperature (for formation of 2 phases); fat phase was taken and separated with separator hopper, then placed inside the laminar hood and finally kidney fat was expressed as percentages.
Kidney apparent health was appraised by assigning a score for 0 to 2 for each bird based on colour and inflammation (status in natural location) score in all the birds killed at Days 34, 38 and 42 of age. Score 0, was a sign of kidney health, Score 1 was a sign for low inflammation in kidney and Score 2, was an indication of acute inflammation in the kidney.
Statistical analysis
A complete randomised block design with a 6 × 2 factorial arrangement of treatments was used to evaluate the response of broiler chickens to SkEO (0, 200, 300, 400, 500 or 600 mg/bird/day) combined with 2 levels of dietary acetic acid in seven replicates (cages) of three birds each. All data were analysed using PROC Mixed in Statistical Analysis System, version 9.1 (SAS Institute Citation2003). The Tukey test was used for multiple treatment comparisons (Kramer Citation1956). Kidney health scores were subjected to frequency analysis using PROC FREQ in the same statistical analysis software (SAS Institute Citation2003). For all tests, the maximum likelihood for type-II error was set at 5% (p < .05). Specific orthogonal contrasts (linear and quadratic) were applied to determine the effects of varying inclusion levels (0, 200, 400 and 600 mg/kg) of SkEO.
Results
Enteral administration of different levels of SkEO through gavage showed no effect on ADG, FCR, and EEEI of birds during Days 14 to 28 of age, as well as ADG, FCR, ADFI and EEEI in Days 28 to 42 of age. Mean ADFI was adversely affected by gavage of SkEO (p < .05) in a nonlinear fashion in 14 to 28 days of age. Birds received SkEO at 400, 500 and 600 mg/day showed 8.99, 8.09 and 10.12% lowered ADFI compared with those gavaged with 200 mg, respectively (p < .05). Feeding acidified diets significantly reduced ADG of birds by 5.92 g (11.41%), ADFI by 4.64 g (4.97%), and EEEI by 16.77%, while FCR was greater (1.95 vs. 1.77) compared with the control birds, and ADG by 6.64 g (9.49%) and ADFI by 10.99 g (8.12%) while FCR and EEEI were not affected, during14 to 28 and 28 to 42 days of the growing period, respectively (p < .05; Table ).
Table 2. Means of average daily gain (ADG; g), average daily feed intake (ADFI; g), feed conversion ratio (FCR) and European economic efficiency index (EEEI) in broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 14 to 28 d and 28 to 42 d.
Oral administration of SkEO to broiler chickens and feeding the birds with diets containing AA, did not modify mean serum concentration of TG, TC, HDL, LDL and NEFA in broiler chicken at Days 34 (Table ) and 38 (Table ) of age and mean serum concentration of TG, TC, HDL and LDL at age 42 d. In the birds gavaged with 400 mg SkEO, serum concentration of NEFA increased when compared with those who received 600 mg SkEO, however, no difference was observed with the control birds in Days 42 of age (p < .05; Table ).
Table 3. Means of total triglycerides (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) (mg/dl) and none esterified fatty acid (NEFA) concentrations (mmol/ml) in blood broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 34 d.
Table 4. Means of total triglycerides (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) (mg/dl) and none esterified fatty acid (NEFA) concentrations (mmol/ml) in blood broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 38 d.
Table 5. Means of total triglycerides (TG), total cholesterol (TC), low density lipoprotein (LDL), high density lipoprotein (HDL) (mg/dl) and none esterified fatty acid (NEFA) concentrations (mmol/ml) in blood broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 42 d.
The mean serum concentration of TP, ALB, GLU and BIL did not differ in birds receiving SkEO through oral gavaging compared with control birds at Days 34, 38 and 42 of age (Table ). Serum concentration of BIL was lower in birds receiving 300 mg of SkEO and AA-supplemented diets than those receiving 300 mg and diets without AA and those gavaging 400 and 600 mg and grown on AA-supplemented diets (p < .05; Figure ).
Figure 1. Interaction effects of Satureja khuzistanica essential oils (SkEO) and Acetic acid on Bilirubin concentrations (mg/dl) of broiler chickens in age of 34 d.
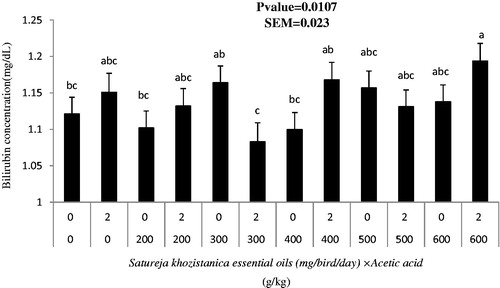
Table 6. Means of Protein (TP) and Albumin (ALB) concentrations (g/dl), Glucose (GLU) and Bilirubin (BIL) concentrations (mg/dl) in serum blood broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 34, 38 and 42 d.
Serum concentration of ALB was 8.38% greater in the birds grown on acidified diets than the control birds in Day 34 of age (p < .05; Table ). Feeding AA-included diets did not alter the mean serum concentration of TP, GLU and BIL in broiler chickens at age of 34 days, and concentration of TP, ALB, GLU and BIL at 38 and 42 days of the raising period (Table ).
Malondialdehyde and total antioxidant concentration in serum were not different among the birds receiving gavaged levels of SkEO and those grown on acidified diets in Days 34, 38 and 42 of the raising period (Table ). Blood serum concentration of antioxidants was greater in birds gavaging 400 mg of SkEO along with non-acidified diet than birds receiving 300 mg of SkEO and fed acidified diets (p < .05; Figure ).
Figure 2. Interaction effects of Satureja khuzistanica essential oils (SkEO) and acetic acid on total anti-oxidant concentrations (mmol/ml) in blood broiler chickens in age of 42 d.
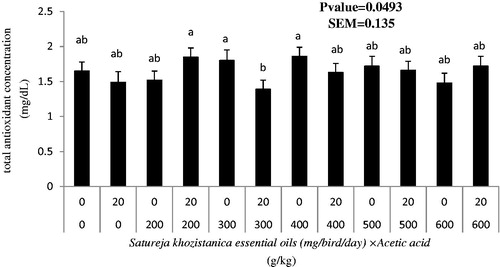
Table 7. Means of Malondialdehyde (MDA; nmol/ml) and Total anti-oxidant (mmol/ml) concentrations in blood broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 34, 38 and 42 d.
Gross kidney health appraised based on a 3-grade scale, was influenced by gavaging of various doses of SkEO as well as acidified diets (p < .05; Table ). The relative frequency of score zero, indicating a healthy kidney, was greater (21.62%) in birds receiving 600 mg SkEO. The birds receiving 500 and 600 mg SkEO showed lower frequency for Score 2 in Day 38 and those receiving 300 and 400 mg SkEO had a greater per cent of Score zero (18.03%), in Day 42 of age.
Table 8. Per cent of Kidney colour in broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 34, 38 and 42 d.
Kidney fat percentage in the birds receiving SkEO, as well as in the birds fed with acidified diets, showed no difference with the control birds in Days 34 and 42 of age (Table ). The kidney fat proportion in the birds receiving 300, 400 and 500 mg SkEO was significantly lower than the control birds and those receiving 200 mg SkEO, in Day 38 of age (p < .05; Table ).
Table 9. Means of Kidney fat per cent in broiler chickens received different levels of Satureja khuzistanica essential oils (SkEO) and Acetic acid in age of 34, 38 and 42 d.
Discussion
Many reports are available dealing with the study of SkEO or its major component carvacrol effects on broiler chicken performance when administrated through feed, water or gavage (Ocak et al. Citation2008; Mikaili et al. Citation2010; Abdel-Wareth et al. Citation2012). Supplementation of drinking water with a wide range of SkEO (from 500 to 2500 mg/L) adversely affected production performance of broiler chickens from Days 1 to 28 of age (Khosravinia et al. Citation2013). The similar results obtained in Basmacioglu et al. (Citation2004) study who reported dietary oregano extract (a natural product rich in carvacrol) at 0.15 g/kg decreased ADG in broiler chicken by 2% compared to the control birds, the findings which agree with results of the current study and altogether demonstrate that SkEO in high doses exert no or adverse effects on broiler performance. Adverse effects of SkEO on broiler performance are mainly attributed to the bitter and pungent taste of carvacrol on feed or water intake. In this study, SkEO was injected directly into the bird's crop using a 5-cm plastic tube adjusted with a syringe-needle assemble. We supposed that in this way, the bitter taste of SkEO is not sensible to the birds so that it could bring about improved ADFI in advanced ages, however, our expectation did not realise. It seems that the bitter and pungent taste of carvacrol and possibly other principle components of SkEO caused a significant drop in feed intake at the earlier ages through, modulating appetite of the birds as shown by Lee et al. (Citation2003) and not merely through affecting taste receptors. In the current study, the oral gavage of SkEO linearly improved FCR in Days 14 to 28, and 28 to 42 of age, however, the differences were not significant compared with the control birds. These results agree with the explanation of Windisch et al. (Citation2008), who in an overview on feeding phytogenic compounds in broiler chicken, revealed feed intake at largely unchanged body weight gain or final body weight, leading to an improved feed conversion ratio. A similar conclusion also reported by Brenes and Roura (Citation2010) indicating, an improvement in weight gain and FCR in broilers when feeding phytogenic comes as a result of a reduced feed intake at a largely unchanged body weight gain. Khosravinia (Citation2015) found a fact that SkEO may influence economic index in broiler production positively as it has been realised in the current study where EEEI was greater for the birds that received 400 mg/L of SkEO. Goodarzi et al. (Citation2014) observed no significant differences in serum concentrations of GLU, TG, total cholesterol, LDL-cholesterol and HDL-cholesterol in broiler chickens fed with diets supplemented with 500 mg/kg SKEO, which agree with the findings of Souri et al. (Citation2015) who reported no change in serum concentrations of the same constituents in broiler chickens grown on diets with 10 or 20 g/kg Satureja khuzistanica ethanolic extract. Our results disagree with Saadat Shad et al. (Citation2016), Ghalamkari et al. (Citation2011) and Nobakht et al. (Citation2011) and Beiranvand et al. (Citation2017) who indicated that serum cholesterol reduced in the broiler chickens fed diets containing carvacrol, thymol and menthol, while the blood serum concentration and ratio of TG, HDL, and LDL of chicks had no significant difference with control birds. The aforesaid workers attributed antihyperglycaemic effects of Satureja khuzistanica derivatizations to their ability to restore the function of pancreatic tissues by causing an increase in insulin output or a decrease in the intestinal absorption of glucose, protecting β-cells and smoothing out fluctuation in glucose levels (Malviya et al. Citation2010). Also, it was shown that SkEO is able to induce an alteration in the proportion of anabolic to catabolic steroids in mevalonate pathway in favour of the anabolic moieties (Khosravinia Citation2015).
Kidneys usually are the second target organ that may be damaged by most metabolic dysfunctions. In avian species, the evaluation of kidney function is mainly based on serum concentrations of uric acid, urea, and creatinine (Selvaraj et al. Citation1998). Khosravinia et al. (Citation2013) reported no elevation in uric acid and creatinine levels in birds received a wide range of SkEO through drinking water. Huff et al. (Citation1988) revealed that significant increases in serum uric acid and creatinine levels are indicative of nephrotoxicity in broiler chickens. Among them, uric acid is of prime importance. Like other birds, broiler chickens are uricotelic and eliminate 60 to 80% of excretory nitrogen in the form of uric acid. Among many other factors, serum levels of this acid alter with water consumption (Costa et al. Citation1993) and kidney health in terms of filtration rate.
On the other hand, acetic acid as a well-known and widely used organic acid in food and feed industry is mostly considered for promising effects on birds performance and health when included in diets by a wide range of 0.5 to 3%. The results of the current study did not confirm the same idea entirely where almost all productive performance results reduced in birds fed with AA-added diets. These findings agree Kopecky et al. (Citation2012) results who reported no change or slight decrease in performance of broilers grown on AA-added diets compared with the control birds in 21 and 28 days of age. The same results with AA-included diets have been reported by Afsharmanesh and Pourreza (Citation2005), Andrys et al. (Citation2003), Abdel-Fattah et al. (Citation2008), and Attia et al. (Citation2013). The adverse effects of AA are attributed to the reduced feed intake due to bitter taste of the acid (El-Hakim et al. Citation2009), and great change in hemostasis through altered pH and exchange of ions through biological membranes resulting in failure to establish internal balance causing deteriorated performance and gut mucosal health (Abdelrazek et al. Citation2016), a phenomenon speculated to happen in the birds in current study too. In the contrary to our results, Abdel-Fattah et al. (Citation2008) found that improved live body weight in broiler chicks received AA through diet compared with those fed un-supplemented diets, results which confirmed entirely or in part, by Owens et al. (Citation2008), Adil et al. (Citation2011) and Ghazalah et al. (Citation2011) who associated the promising effect of AA to its beneficial effect on gut microbiota. The organic acids may affect the integrity of microbial cell membrane or cell macromolecules or interfere with the nutrient transport and energy metabolism causing the bactericidal effect (Ricke Citation2003). In the current study, no blood TG and Cholesterol-lowering effect observed in AA-fed birds. However, AA and its common solution, vinegar, is believed to exert the same effects as Abdo and Zeinb (Citation2004) and Abdel-Fattah et al. (Citation2008), reported that blood total lipids and cholesterol decreased significantly by dietary organic acids. The beneficial role of organic acids in reducing the blood lipid profile may be interpreted through their influence in decreasing the microbial intracellular pH. Thus, inhibits the action of important microbial enzymes and forces the bacterial cell to use energy to release the acid protons, leading to an intracellular accumulation of acid anions (Young and Foegeding Citation1993).
The results of the current study with respect to kidney function are in harmony with findings of Abdel-Fattah et al. (Citation2008) who concluded that dietary supplementation of organic acids could be done up to the level of 3% in the diet of broiler chicken without causing adverse effects on the kidney and liver functions.
Despite huge works on implementation of herbal medicines and organic acids in broiler diets, there are absolutely scanty, if not at all, information on interaction of these two categories of the widely used feed additives in broiler diets, an idea which constitutes the main part of our hypothesis. We observed no interaction among dietary AA and administration of SkEO in broiler chicken except for blood concentration of BIL in Day 34 and total antioxidant capacity in Day 42 of age. Although carvacrol and almost all SkEO constituents are small, volatile entities same as acetic acid and they all effortlessly absorb into the mucosal cell wall all through digestive tract rapidly, however, their role in metabolism may differ greatly. While, AA participates directly in energy producing metabolic pathways by converting to acetyl-CoA in enterocytes, hepatocytes and likely many other cells, the same role never anticipates for carvacrol. It has been, however, suggested that carvacrol exhibit antioxidant activity and inhibit key enzymes in cholesterol and lipid synthesis (Qureshi et al. Citation1983; Elson and Qureshi Citation1995; Crowell Citation1999), the roles which have no interrelation with AA metabolic pathways. On the other hand, many clinical investigations have showed that carvacrol containing herbal extracts are able to alter reproductive functions in animals through affecting sex hormones secretion and their physiological balance (Dehghani et al. Citation2008; Grigorova et al. Citation2008), a concept which detailed by Khosravinia (Citation2015) resulting in the idea of “anabolic steroids-elevating effect for carvacrol”, again a physiological fact with no direct connection with AA metabolism and roles.
Conclusions
We hypothesised that there is an interaction between the SkEO and acetic acid as the representatives of the two major classes of the commonly used feed additives in broiler diets. The same hypothesis was not supported by our findings, with the exception of the results on few variables. However, the outcomes of the work could be noteworthy where we found; Direct administration of SkEO into crop through oral gavaging lowered feed intake, a phenomenon which cannot be attributed to the altered feed palatability due to bitter and pungent taste of carvacrol. Gross kidney health improved by gavaging SkEO as shown by greater frequency of apparently healthy score zero and reduced kidney fat percentage. Almost all productive performance indications were adversely affected in birds receiving AA-added diets. There was a SkEO × AA interaction for antioxidant capacity in serum, where greater antioxidant capacity in serum was found in birds receiving 400 mg of SkEO and fed on non-acidified diet.
Ethical approval
All procedures carried out in this experiment were reviewed and approved by the Animal Care and Use Committee of Lorestan University, Khorramabad, Iran.
Acknowledgements
The authors wish to thank Dr Ali Salehnia, Khorraman Medicinal Plants Company, Lorestan, Iran, for donating Satureja khuzistanica essential oils and Dr M. Alirezaei, Veterinary faculty, Lorestan University, for assistance in the conduction of antioxidant laboratory assessments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdo M, Zeinb A. 2004. Efficacy of acetic acid in improving the utilization of low protein-low energy broiler diets. Egypt Poult Sci. 24:123–141.
- Abdollahi M, Salehnia A, Mortazavi SH, Ebrahimi M, Shafiee A, Fouladian F, Keshavarz K, Sorouri S, Khorasani R, Kazemi A. 2003. Antioxidant, antidiabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja khuzistanica in rat in vivo: a toxicopharmacological study. Medic Sci Monit. 9:331–335.
- Abdel-Fattah SA, El-Sanhoury MH, El-Mednay NM, Abdel-Azeem F. 2008. Thyroid activity, some blood constituents, organs morphology and performance of broiler chicks fed supplemental organic acids. Int J Poultr Sci. 7:215–222.
- Abdelrazek HMA, Abuzead SMM, Ali SA, El-Genaidy HMA, Abdel-Hafez SA. 2016. Effect of citric and acetic acid water acidification on broiler’s performance with respect to thyroid hormones levels. Adv Anim Vet Sci. 4:271–278.
- Abdel-Wareth AAA, Kehraus S, Hippenstiel F, Sudekum KH. 2012. Effects of thyme and oregano on growth performance of broilers from 4 to 42 days of age and on microbial counts in crop, small intestine and caecum of 42-day-old broilers. Anim Feed Sci Technol. 178:198–202.
- Adil S, Banday T, Ahmad Bhat G, Salahuddin M, Raquib M, Shanaz S. 2011. Response of broiler chicken to dietary supplementation of organic acids. J Cent Europ Agric. 12:498–508.
- Afsharmanesh M, Pourreza J. 2005. Effects of calcium, citric acid, ascorbic acid, vitamin D3 on the efficacy of microbial phytase in broiler starters fed wheat-based diets I. Performance, bone mineralization and ileal digestibility. Int J Poult Sci. 4:418–424.
- Amanlou M, Dadkhah F, Salehnia A, Farsam H, Dehpour AR. 2005. An anti-inflammatory and anti-nociceptive effects of hydroalcoholic extract of Satureja khuzistanica Jamzad extract. J Pharm Pharm Sci. 8:102–106.
- Andrys R, Klecker D, Zeman L, Marecek E. 2003. The effect of changed pH values of feed in isophosphoric diets on chicken broiler performance. Czech J Anim Sci. 48:197–206.
- Attia YA, El-Hamid AEA, Ellakany HF, Bovera F, Al-Harthi MA, Ghazaly SA. 2013. Growing and laying performance of Japanese quail fed diet supplemented withdifferent concentrations of acetic acid. Ital J Anim Sci. 12:37.
- Basmacioglu H, Tokusoglu O, Ergul M. 2004. The effects of oregano and rosemary essential oils or alpha-tocopheryl acetate on performance and lipid oxidation of meat enriched with n-3 PUFAs in broilers. South Afric J Anim Sci. 34:197–210.
- Beiranvand MH, Khosravinia H, Azarfar A, Rafiei Alavai E. 2017. Lipids digestibility, blood serum concentrations of fat constituents and steroid hormones, carcass fat deposition and distribution pattern in broiler chickens fed carvacrol, menthol and thymol supplemented diets (In Persian). Iran J Anim Sci. 48:261–272.
- Brenes A, Roura E. 2010. Essential oils in poultry nutrition: main effects and modes of action. Anim Feed Sci Technol. 158:1–14.
- Cantino PD, Harley RM, Wagstaff SJ. 1992. Genera of Labiatae status and classification. In: Harley RM, Reynolds T, editors. Advances in Labiatae Science. Kew, UK: Royal Botanic Gardens Press; p. 511–522.
- Choct M. 2001. Enzymes supplementation of poultry diets based on viscous cereals in enzymes. Bedford MR Partridge GG: Farm Animal Nutrition, CABI Publishing, UK.
- Costa ND, McDonald DE, Swan RA. 1993. Age-related changes in plasma biochemical values of farmed emus (Dromaius novaehollandiae). Aust Vet J. 70:341–344.
- Crowell PL. 1999. Prevention and therapy of cancer by dietary monoterpenes. J Nut. 129:775–778.
- Dehghani F, Azizi M, Panjehshahin MR. 2008. Toxic effects of hydroalcoholic extract of Citrulluscolocynthis on pregnant mice. Iran J Vet Res. 9:42–45.
- Dibner JJ, Buttin P. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J App Poult Res. 11:453–463.
- Euribrid BV. 1994. Technical information for Hybro broilers. Boxmeer: Euribrid Poult Breeding Farm.
- El-Hakim AA, Cherian G, Ali M. 2009. Use of organic acid, herbs and their combination to improve the utilization of commercial low protein broiler diets. Int J Poult Sci. 8:14–20.
- Elliott RJ. 1984. Ektachem DT-60 Analyzer. Phys Leading Comput J. 2:6.
- Elson CE, Qureshi AA. 1995. Coupling the cholesterol- and tumor-suppressive actions of palmoil to the impact of its minor constituents on 3-hydroxy-3-methylglutaryl coenzyme A reductaseactivity. Prostaag Leukotr Ess. 52:205–208.
- Farsam H, Amanlou M, Radpour MR, Salehinia AN, Shafiee A. 2004. Composition of the essential oils of wild and cultivated Satureja khuzistanica Jamzad form Iran. Flavour Fragr J. 19:308–310.
- Folch J, Lees M, Stanley GHS. 1975. A simple method for the isolation and purification of total lipids from animal tissues. J Biolo Chem. 226:497–509.
- Galik B, Rolinec M. 2011. The effect of phytogenic feed additives on the performance of non-ruminants, Proc. International Ph.D. workshop on welfare, biotechnology and quality of animal production, Nitra, Slovak University of Agriculture. 19.
- Ghalamkari G, Toghyani M, Tavalaeian E, Landy N, Ghalamkari Z, Radnezhad H. 2011. Efficiency of different levels of Satureja hortensis L. (Savory) in comparison with an antibiotic growth promoter on performance, carcass traits, immune responses and serum biochemical parameters in broiler chickens. Afric J Biotech. 10:13318–13323.
- Ghazalah AA, Atta AM, KoutElkloub M, Moustafa EL, Shata RFH. 2011. Effect of dietary supplementation of organic acids on performance, nutrient digestibility and health of broiler chicks. Int J Poult Sci. 10:176–184.
- Goodarzi M, Pour N, Modiri D. 2014. The effect of savory (Satureja khuzistanica) essential oils on performance and some blood biochemical parameters of Ross and Cobb broilers. ARRB. 4:4336–4343.
- Grigorova G, Kashamov B, Sredkova V, Surdjiiska S, Zlatev H. 2008. Effect of tribulusterrestris extract on semen quality and serum total cholesterol content in white plymouth rock-mini cocks. Bio Anim Husb. 24:139–146.
- Haeri S, Minaie B, Amin G, Nikfar S, Khorasani R, Esmaily H, Salehnia A, Abdollahi M. 2006. Effect of Satureja khuzestanica essential oil on male rat fertility. Fitoterapia. 77:495–499.
- Hadian J, Mirjalili MH, Kanani MR, Salehnia A, Ganjipoor P. 2011. Phytochemical and morphological characterization of Satureja khuzistanica Jamzad populations from Iran. Chem Biodiv. 8:902–915.
- Hamano Y, Kurimoto Y. 2016. Effects of acetylated wood powder on growth performance, hepatic and mascular free amino acid profiles and inosine 5’-monophosphate concentration of breast meat in broiler chickens. Brit Poult Sci. 57:643–654.
- Huff WE, Kubena LF, Harvey RB. 1988. Progression of ochratoxicosis in broiler chickens. Poult Sci. 67:1139–1146.
- Khosravinia H, Ghasemi S, Rafiei Alavi E. 2013. The effect of savory (Satureja khuzistanica) essential oils on performance, liver and kidney functions in broiler chickens. J Anim Feed Sci. 22:50–55.
- Khosravinia H. 2015. Hypolipidemic effects of Satureja khuzistanica essential oil in broiler chicken are realized through alteration in steroid hormones. KafkasUniv Vet Faku. 21:203–209.
- Khosravinia H. 2016. Mortality, production performance, water intake and organ weight of the heat stressed broiler chicken given savory (Satureja khuzistanica) essential oils through drinking water. J Appl Anim Res. 44:273–280.
- Kishi M, Fukaya M, Tsukamoto Y, Nagasawa T, Takehana K, Nishizawa N. 1999. Enhancing effect of dietary vinegar on the intestinal absorption of calcium in overiectomized rats. BioSci Biotech Biochem. 63:905–910.
- Kopecky J, Hrncar C, Weis J. 2012. Effect of organic acids supplement on performance of broiler chickens. Anim Sci Biotech. 45:51–54.
- Kral M, Angeloviaova M, Mrazova L, Tkacova J, Kliment M. 2011. Probiotic and acetic acid of broiler chickens performance. Anim Sci Biotech. 44:149–152.
- Kramer CY. 1956. Extension of multiple range tests to group means with unequal number of replications. Biomet. 12:307–310.
- Langhout P. 2000. New additives for broiler chickens. Feed Mix. 16:24–27.
- Lee KW, Everts H, Kappert HJ, Frehner M, Losa R, Beynen AC. 2003. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Brit Poult Sci. 44:450–457.
- Loddi MM, Maraes VMB, Nakaghi ISO, Tucci F, Hannas MI, Ariki JA. 2004. Mannan oligosaccharide and organic acids on performance and intestinal morphometric characteristics of broiler chickens. Proceedings of the 20th annual symposium. Supplement.1, p. 45.
- Machado Junior PC, Beirão BCB, Filho TF, Lourenço MC, Joineau ML, Santin E, Caron LF. 2014. Use of blends of organic acids and oregano extracts in feed and water of broiler chickens to control Salmonella Enteritidis persistence in the crop and ceca of experimen-tally infected birds. J App Poult Res. 23:671–682.
- Major P, Revajova V, Levkut M, Sevcikova Z, Spisakova V, Faixova Z, Levkutova M, Kozarova I, Goldova M, Levkut M. 2011. Intestinal mucin dynamic and leukocytic responses of chickens infected with Eimeriaacervulina and fed oregano supplemented diet. Acta Vet Brno. 80:147–156.
- Malviya N, Jain S, Malviya S. 2010. Antidiabetic potential of medicinal plants. Acta Pol Pharm. 67:113–118.
- Mikaili P, Sarahroodi S, Hemmati A, Koochak M, Akbari Z. 2010. A histological study on the effects of aqueous extract of Althea officinalis on epithelial and submucosalmucocilliary system of rat trachea following inhalation of cigarette smoke. Iran J Pharm Res. 3:56–57.
- Moghaddam FM, Farimani MM, Salahvarzi S, Amin G. 2007. Chemical constituents of dichloromethane extract of cultivated Satureja khuzistanica. Evid Based Complement Alternat Med. 4:95–98.
- Momtaz S, Abdollahi M. 2010. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int J Pharm. 6:346–353.
- Nobakht A, Norany J, Safamehr AR. 2011. The effects of different amounts of Menthapulegium (pennyroyal) on performance, carcass traits, hematological and blood biochemical parameters of broilers. J Med Plant Res. 5:3763–3768.
- Nourmohammadi R, Khosravinia H, Afzali N. 2015. Effects of high dietary levels of citric acid on productive performance, serum enzyme activity, calcium and phosphorus retention and immune response in broiler chickens. Europ Poult Sci. 79:1612–9199.
- Nourmohammadi R, Khosravinia H, Afzali N, Manafi M. 2016. Effects on productive performance, tibia calcium and phosphorous retention, and liver enzymes activity of acidified and alkalinized diets in broiler chicken. Ann Anim Sci. 16:797–809.
- Nourmohammadi R, Khosravinia H, Afzali N. 2018. Effects of feed form and xylanase supplementation on metabolizable energy partitioning in broiler chicken fed wheat-based diets. J Anim Physiol Anim Nutr. 0:1–8.
- Ocak N, Erener G, Burak Ak F, Sungu M, Altop A, Ozmen A. 2008. Performance of broilers fed diets supplemented with dry peppermint (Mentha piperita) or thyme (Thymus vulgaris) leaves as growth promoter source. Czech J Anim Sci. 53:169–173.
- Owens B, Tucker L, Collins MA, McCracken KJ. 2008. Effects of different feed additives alone or in combination on broiler performance, gut microflora and ileal histology. Br. Poult. Sci. 49:202–212.
- Parvar R, Khosravini H, Azarfar A. 2013. Effect of Satureja khuzestanica essential oils on postmortem pH and antioxidative potential of breast muscle from heat stressed broiler. Asian J Poultr Sci. 7:83–89.
- Pellicano ERL, Souza PA, Souza HBA, Figueiredo DF, Boiago MM, Carvalho SR, Bordon VF. 2005. Intestinal mucosa development in broiler chicken fed natural growth promoters. Braz J Poultry Sci. 7:221–229.
- Petruska P, Tusimova E, Kalafova A, Hascik P, Kacaniova M, Kolesarova A, Capcarova M. 2012. Effect of propolis in chicken diet on selected parameters of mineral profile. J Micro Biotech Food Sci. 1:1090–1097.
- Qureshi AA, Din ZZ, Abuirmeileh N, Burger WC, Ahmad Y, Elson CE. 1983. Suppression of avian hepatic lipid metabolism by solvent extract of garlic impact on serum lipid. J Nutr. 113:1746–1755.
- Ricke SC. 2003. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult Sci. 82:632–639.
- Saadat Shad H, Mazhari M, Esmaeilipour O, Khosravinia H. 2016. Effects of thymol and carvacrol on productive performance, antioxidant enzyme activity and certain blood metabolites in heat stressed broilers. Iran J Appl Anim Sci. 6:195–202.
- SAS Institute. 2003. SAS Users Guide: Statistics. Ver. 6. Cary, NC.
- Selvaraj P, Thangavel A, Nanjappan K. 1998. Plasma biochemical profile of broiler chickens. India Vet J. 75:1026–1027.
- Souri H, Khatibjoo A, Taherpoor K, Hassan Abadi A, Fattahnia F, Askari M. 2015. Effect of Thymus vulgaris and Satureja khuzistanica ethanolic extracts on broiler chickens’ performance and immune response. Iran J Appl Anim Sci. 5:437–446.
- Windisch W, Schedle K, Plitzner C, Kroismayr A. 2008. Use of phytogenic products as feed additives for swine and Poultry. J Anim Sci. 86:140–148.
- Young KM, Foegeding PM. 1993. Acetic, lactic and citric acids and pH inhibition of Listeria monocytogenes Scott A. and the effect on intracellular pH. J Appl Bacteriol. 74:515–520.
