Abstract
The aim of the present study was to compare the effects of two different nitrogen-rich ingredients such as hydrolysed fish protein and autolysed yeast, on gilthead sea bream (Sparus aurata) growth performances and histological gut morphology. Animals were allocated to three experimental groups: the first received a fishmeal-based diet (FM), the second and the third received hydrolysed fish protein (HFP) and autolysed yeast (AY), respectively, to replace an equal amount of fishmeal. The experiment lasted 92 days. No significant differences in body weight and mortality were observed among the different groups. Villus branching, intraepithelial lymphocytes and inflammatory infiltrate of the submucosa were more prominent in AY than HFP and FM. The gut absorbent surface ratio was 5.94, 6.44 and 7.28 for group FM, HFP and AY, respectively, with statistical significant difference between FM and AY and between HFP and AY. A significant increment in the goblet cell density was observed for HFP and AY in comparison to FM. A statistically significant increase in small-sized goblet cells was observed in AY compared to FM. All our findings suggest that it is possible to use either HFP or AY, as partial replacer of FM in the S. aurata diet.
Yeast-derived products as a suitable alternative nitrogen source.
Autolyzed yeast as a novel approach in the use of yeast products.
Use of autolysed yeast for replacing fishmeal in aqua feed.
Use of hydrolysed fish protein for replacing fishmeal in aqua feed.
Highlights
Introduction
Nutrition is by far the most relevant tool for enhancing fish growth performance and health. Composition, nutritional parameters, as well as methods of administration of diets, are key factors to reach positive results from an economic point of view (NRC Citation2011). When fish are reared under stressful conditions and/or when dietary nitrogen formulation is sub-optimal, amino acid (AA) metabolism may be negatively affected and fish welfare dramatically compromised (Conceicao et al. Citation2012); in these conditions an increase in the requirement of specific AA may occur (Aragao et al. Citation2008). Hence, a suitable diet nitrogen composition in term of protein, amino acids, digestible protein/digestible energy ratio, may be crucial to maintain an appropriate fish welfare. Although their precise mechanism of action is not yet fully understood (Verri et al. Citation2017), it has been demonstrated that certain peptides may modulate nutrient digestion and absorption, enhance mineral absorption and/or bioavailability (Kitts and Weiler Citation2003), trigger the immune system against pathogen infection, have antibacterial properties and highly stimulate head kidney leucocytes (Kitts and Weiler Citation2003; Hartmann and Meisel Citation2007; Madureira et al. Citation2010).
Since, teleost can efficiently use dietary di/tri-peptides for development, growth, and metabolism, a balanced inclusion of peptides in fish feed might positively improve diet efficiency (Verri et al. Citation2011). Similarly, it has been reported that nucleotides and RNA extracted from Kluyveromyces fragilis induce transcriptional changes, modulate the physiological control of growth and inflammatory response, determine a higher survival ratio and improve the intestinal architecture (Falcinelli et al. Citation2018).
To cope with acute stress and diseases, and improve fish health and immune status, in recent years additives such as prebiotics (e.g. mannanooligosaccharydes – MOS), probiotics (e.g. Lactobacillus spp.), immunostimulants (e.g. β-glucans) and nucleotides have also been included in aquafeed (Piaget et al. Citation2007; Staykov et al. Citation2007; Tahmasebi-Kohyani et al. Citation2012; Torrecillas et al. Citation2014; Udayangani et al. Citation2017) to enhance diet digestibility and absorption, fish wellness and gut conditions (Torrecillas, Makol, Benitez-Santana et al. Citation2011; Torrecillas, Makol, Cabllero et al. Citation2011).
Compared to the number of studies that evaluates growth performances after administration of peptides and additives in aquafeed, few studies have included the histological evaluation of gut traits.
Epithelial cells of fish intestine are interspersed by mucus-producing goblet cells (Feist Citation2009) and intestinal barrier efficiency depends on mucus production, epithelial integrity and the presence and balance among commensal bacteria (Faure et al. Citation2006). The mucus protects and lubricates the epithelium, and it is part of the innate defence system protecting fish against endogenous and exogenous aetiological agents (Johansson et al. Citation2013; Kim and Khan Citation2013). Several studies have demonstrated that gut mucus secretion may be affected by diet and its ingredients. In the European sea bass (Dicentrarchus labrax), dietary MOS supplementation increased gut goblet cell number and mucus production (Torrecillas, Makol, Benitez-Santana et al. Citation2011). Besides, MOS supplementation to on-growing diets enhanced innate cellular and humoral immune parameters (Staykov et al. Citation2007; Torrecillas et al. Citation2007; Torrecillas, Makol, Cabllero et al. Citation2011) and reduced infection percentages in gut inoculated fish (Torrecillas et al. Citation2007). Moreover, including MOS in the diet significantly increases mucosal fold height, width and, as a consequence, the total surface area, accompanied by a significant increase of goblet cells (Torrecillas, Makol, Cabllero et al. Citation2011).
For these reasons and according to their nutritional characteristics, it is important to evaluate the effects of the wide array of nitrogen sources (alternative to fishmeal) already available to the aquafeed industry. Chemical, enzymatic, or microbial hydrolysis of proteins in animal by-products or plant-source feedstuffs generate high-quality peptides (Hou et al. Citation2017). Among others, Hydrolyzed Fish Protein (HFP) and Autolyzed Yeast (AY) are relevant nitrogen sources candidates for fishmeal replacement in aquafeeds (Day et al. Citation1997; Li and Gatlin Citation2003; Cahu et al. Citation2004; Espe et al. Citation2012; Sönmez Citation2017); this is particularly true for carnivorous species such as European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata), finfish species that are by far the most reared in the Mediterranean area.
The aim of the present study was thus to compare the effects of two nitrogen-rich ingredients such as HFP and autolysed yeast AY, to a control fishmeal-based diet (FM) on gilthead sea bream (Sparus aurata) growth performances and gut morphology.
Materials and methods
Experimental design, measurements and sampling
All the procedures were conducted in accordance with the National legislation (DL 26/2014) and European Union Guidelines for the ethical care and handling of animals under experimental conditions (2010/63/EU). The trial was carried out at the Experimental Center of VRM srl Naturalleva®, located in Civitavecchia (Italy).
A total number of 720 gilthead sea bream with an initial mean body weight of 122.18 ± 6.226 g (Mean ± SD) were randomly distributed into 9 fibreglass 2000 L tanks (80 fish/tank, at a rearing density of 1.83 ± 0.042 kg/m3) connected to a flow-through fish rearing system with three complete daily water changing in each tank. Experimental tanks were supplied with water with an approximately constant temperature of 24.5 ± 2.26 °C (Mean ± SD) throughout the experimental period (June–September); the oxygen level was maintained around 10 ± 1 mg/L (Mean ± SD) by use of pure oxygen. Fish were acclimatised for seven days under 12/12 D/L photoperiod and fed to visual satiety with a standard commercial diet (VRM S.r.l, Naturalleva, Verona, Italy). After the acclimation period, fish were distributed into 3 groups and 3 replicates, fed ad libitum twice per day, for a 92 days experimental period.
During this period, the following parameters were individually measured: (a) initial body weight (BWi); (b) final body weight (BWf); (c) total length (TL); (d) standard length (SL); liver weight (LW); moreover, the following parameters were calculated as follows: (e) body weight gain (BWg): BWg = BWf – BWi; f) average individual feed consumption (FC): as consumed feed at the end of the trial, (e) feed conversion rate (FCR): FCR = FC – BWg; (g) Specific Growth Rate (SGR): (% day−1) = 100 × [(ln BWf) – (ln BWi)]/days fed; condition factor (k): k = BWf/TL; h) hepatosomatic index (HSI): HIS = 100×(liver weight/BWf); j) mortality events were recorded on a daily bases and dead fish BWf recorded for FCR calculation. FC, FCR and SGR were calculated on as-fed bases.
Diets
The fishes allocated to the first group were fed a fishmeal-based diet as control group (FM); as alternative sources of dietary nitrogen, either hydrolysed fish protein (HFP; Crude Protein content 82%) and autolysed yeast (HiCell®, Biorigin©, Brazil; supplied by Albitalia Srl, Milan, Italy; Crude Protein content 40%) were used to replace an equal amount of fishmeal in treatments HFP and AY, respectively; HiCell® is a yeast-derived source of nitrogen in the form of proteins, amino acids, peptides, nucleotides, and also contains functional carbohydrates (MOS). Diets were isoenergetic, isoproteic and isolipidic and their details are shown in Table . Chemical analyses of the diets were performed according to the Commission Regulation (EC) No 152/2009 of 27 January 2009, that lays down the methods of sampling and analysis for the official control of feed and data are shown in Table ; on the same Table , additional information provided by the supplier and related to the tested raw materials, are also given. The three different feeds were extruded (4.5 mm diameter pellets), produced by VRM srl Naturalleva® (Verona, Italy) by means of a Wenger X-180 extruder. The diets were all formulated to cover the nutritional requirements suggested for Gilthead Sea Bream (NRC Citation2011).
Table 1. detailed diet formulations.
Table 2. Amino acid content (%, as fed basis) of HFP, HiCell® and diets.
Sampling procedures for histopathology
On day 92nd, 10 fish per replicate were randomly caught and four of them (n = 12 per group) were then sacrificed, dissected according to the procedures described below and samples of the anterior part of the mid-gut taken to perform the histological analysis. To this purpose, fish were dissected longitudinally from the anal orifice, the abdominal wall was partially removed, the liver raised and the visceral package gently isolated; a 2-cm long segment of the intestine behind the pyloric ceca was dissected and promptly fixed in a 10% buffered formalin solution (pH 7.4). Two transversal sections of each intestine were then processed and embedded in paraffin perpendicularly to the surface of the embedding mould, to obtain gut transversal sections. Five µm thick sections were then cut and stained with hematoxylin and eosin (H&E) and Alcian blue (pH 2.5) to stain the acidic goblet cells according to the manufacturer instructions.
Gut morphology
General morphology of the gut was investigated on H&E stained sections under a light microscope to assess the following parameters: (i) villus thickening (ii) villus branching, (iii) villus tip oedema, (iv) intraepithelial lymphocytes and (v) inflammatory infiltrate in the lamina propria of the intestinal mucosa. For parameters (i), (ii), (iii) presence/absence was recorded (presence was recorded only if the feature appeared in more than 10% of the examined sample); a scoring system of 0 = none to scant, 1 = moderate, 2 = severe was used to assess the parameter (iv) and (v).
Gut absorbent surface and acidic goblet cells morphometry
Sections were examined using a light microscope (Nikon, Eclipse 80i, Nikon Instruments, Calenzano, Italy) connected to a personal computer via a Nikon digital camera (Digital Sight DS-U1) and representative images were acquired using the NIS-Elements Br accompanying software to perform morphometrical analysis on the following parameters: (1) gut absorbent surface; (2) number and size of acidic goblet cells.
To evaluate the gut absorbent surface H&E stained sections were used and two measures were taken per field at low magnification (in order to include the entire section): (i) the epithelial surface of the mucosa along the profile of the villi epithelium and (ii) the perimeter along the junction between the inner muscle layer and the mucosa. The gut absorbent surface was thus expressed in arbitrary units as the ratio between the two measures (ii/i): this allowed to minimise differences related to the size of the gut among different animals.
Acidic goblet cells were counted and their size (µm2) measured on Alcian blue stained sections, in 4 consecutive fields (200× magnification); hence, the goblet cell density (number/mm2) was also calculated. After the qualitative observation indicating the presence of small goblet cell in some specimens, a cut-off value was arbitrarily established to investigate differences among groups as follows: all goblet cell sizes recorded (FM, HFP, AY) were averaged and cells measuring less than one half of this obtained value were considered ‘small’.
Statistical analysis
Fisher’s exact test was used to analyses gut morphology findings (thickening, branching and oedema). One-Way ANOVA followed by Tukey-Kramer HSD (Honestly Significant Difference) test was used for statistical analysis of growth performance parameters and to investigate the effect of the treatment on goblet cell size and density. Mortality events were analysed by a non-parametric test (Chi2 test). All statistical significance was set at p < .05.
Results
Mortality and growth performances
The observed mortality rate was 4.2%, 5.8% and 8.75% for FM, HFP and AY, respectively and no statistically significant differences were observed.
During the experimental period, all the groups doubled their BWi (122.9 g to 248.2 g for HF, 122.4 g to 245.5 g for HFP and 120.8 g to 252.0 g for AY). Statistically significant differences among groups were not observed for BWg, FCR TL, FC, FCR and SGR (Table ). A statistically significant difference was observed for SL only since fish belonging to FM group appeared to be significantly longer than fish of the groups HFP and AY.
Table 3. Growth performances of gilthead sea bream (Sparus aurata) fed hydrolysed fish protein (HFP) and autolysed yeast (AY) as alternative nitrogen sources to fishmeal.
Gut morphology
In FM the elongated villi showed focal thickening and their profile displayed a few lateral villus branching occupying less than the 10% of the transverse intestinal section (Figure ). Small intraepithelial lymphocytes were observed mainly in the abluminal region, aligned along the basal membrane or rarely in migration between the enterocytes (Figure ). In two specimens, the tip of a few villi appeared dilated because of oedema. A scant to mild inflammatory infiltrate was observed in all cases but one, predominantly consisting in acidophilic granular cells and present in most of the subdomain at the base of the villi (Figure ). In the remaining cases, the infiltrate was moderate.
Figure 1. Photomicrographs reporting histological observations; images in the first row are from FM, images in the middle row are from HFP and images in the bottom row are from AY. (a, d, g) low magnification of a transversal gut section, where villus branching and oedema of the tip can be observed (arrow); (b, e, h) longitudinal view of villi to show the presence of intraepithelial lymphocytes; (c, f, i) high magnification images showing the lamina propria inflammatory infiltrate. Scale bar is equal to 1 mm (a, d, g) and 50 µm (b, c, e, f, h, i). FM: fishmeal based diet; HFP: hydrolyzed fish protein; AY: autolyzed yeast.
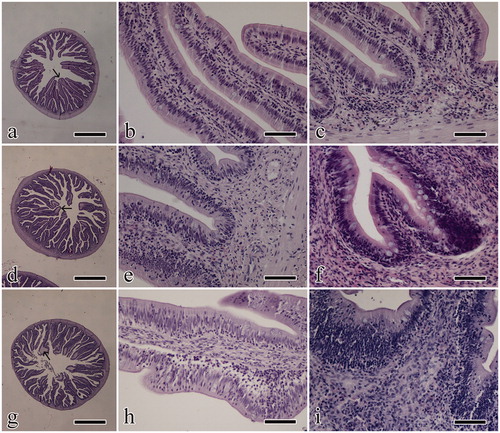
In HFP, elongated villi were slightly thickened in only one case; lateral branching from the main villi was prominent in half of the cases (Figure ). In the enterocyte covering, lymphocytes were aligned near the basal membrane or sparse in migration through the mucosal thickness (Figure ). An inflammatory infiltrate was observed, predominantly consisting of acidophilic granular cells accumulating in the lamina propria and giving a thickened aspect of the base of the villi (Figure ). Oedema of the tip of the villi was also seen in four cases.
In AY the elongated villi appear slightly and focally thickened and extensively branched in most of the cases (Figure ). Intraepithelial lymphocytes were distributed close to the basal membrane or in migration; in one case small foci of granulocyte migration were also observed (Figure ). In the lamina propria, an inflammatory infiltrate was observed, mainly consisting of acidophilic granular cells; the infiltrate accumulated in the submucosa being associated to thickening of the villus base (Figure ). Three of the specimens showed multifocal oedema of the tip of the villi.
Only for the parameter, ‘branching’ statistical differences were observed between either FM vs HFP and FM vs AY. For all the considered groups, presence of villus thickening, branching and tip oedema, as well as intraepithelial lymphocyte and inflammatory infiltrate scores, are reported in Table .
Table 4. Gut histological changes in gilthead sea bream (Sparus aurata) fed hydrolysed fish protein (HFP) and autolysed yeast (AY) as alternative nitrogen sources to fishmeal (FM).
Gut absorbent surface and acidic goblet cell morphometry
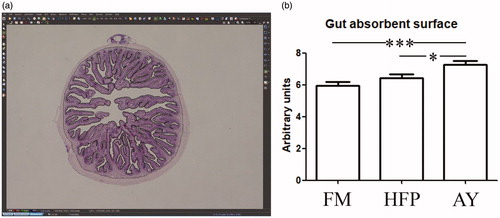
The gut absorbent surface ratio was 5.94, 6.44 and 7.28 for FM, HFP and AY, respectively. Differences achieved a statistical significance of p < .001 between FM and AY, of p < .05 between HFP and AY while FM did not differ from HFP (Figure ).
Figure 2. Gut absorbent surface measurement; (a) screenshot obtained from the image processing software where both (i) the epithelial surface of the mucosa along the profile of the villi and (ii) the perimeter along the junction between the inner muscle layer and the mucosa are selected; (b) histogram showing the gut absorbent surface (ii/i) in the experimental groups: asterisks indicate statistical differences (*p < .05; ***p < .001). FM: fishmeal based diet; HFP: hydrolyzed fish protein; AY: autolyzed yeast.
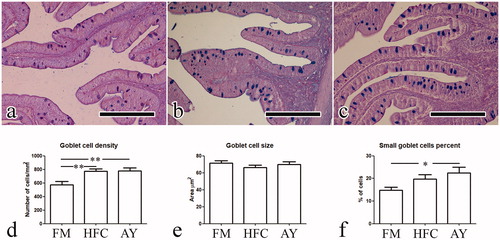
In FM, goblet cells were confined to the apical surface of the intestine epithelium while in HFP and AY they often appeared stratified within the epithelial thickness (Figure ). A significant (p < .01) increment in the goblet cell density was observed for HFP and AY in comparison to FM (mean values were 575.4, 774.4 and 778.7 for FM, HFP and AY respectively). The mean size of goblet cells in the three groups did not show significant differences. A statistically significant increase (p < .05) in small sized goblet cells was observed in AY compared to FM (Figure ).
Figure 3. Goblet cell morphometry; (a, b, c) photomicrographs obtained from group a, b, and c respectively: presence of goblet cells stratified within the epithelial thickness are visible in b and c; (d, e, f) histograms showing results of the parameters recorded for goblet cells: asterisks indicate statistical differences (*p < .05; **p < .01). Scale bar in a, b, and c is equal to 200 µm. FM: fishmeal based diet; HFP: hydrolyzed fish protein; AY: autolyzed yeast.
Discussion
Fish grew indifferently when fed HFP or AY. Hence, this finding suggests that it is possible to use either of them as partial replacer of FM in the S. aurata diet. The growth performances observed in the present experiment were consistent with those observed in other studies on the same species (Mihelakakis et al. Citation2002; Sitjà-Bobadilla et al. Citation2005). Also in trout (Oncorhynchus mykiss), Hauptman et al. (Citation2014) used dried yeast to replace FM without affecting fish body weight gain and FCR up to an inclusion level of 11.2% (37.5% FM replacement rate). Conversely, in a similar trial carried out on sea bream, plant protein sources were used to replace FM with detrimental effects on growth performances (Gómez-Requeni et al. Citation2004); similarly, a significant reduction of body weight, standard length, DGI (Daily growth index), condition factor and feed efficiency were observed on sea bass when FM and fish oil (FO) were totally replaced by plant sources, already after 45 days of administration (Torrecillas et al. Citation2017). Hence, these evidence hypothetically suggest that HFP and AY could be used as FM replacers in aquafeed more efficiently than plant protein sources.
So far autolysed yeast have rarely been used as a bulk ingredient for FM replacement but rather as potential immunostimulant. A reduction of growth performances in Senegalese sole (Solea senegalensis) was observed when autolysed yeast (S. cerevisiae) was included in a plant-based diet (Batista et al. Citation2016). These results are likely related to the replacement of fish meal with plant sources while our study suggests that when HPF or AY are used, no negative effect on fish growth performances are observed.
Unexpectedly, in our study a reduction of fish standard length was observed upon feeding fish either HFP or AY; similar results have been observed also in the above-mentioned study carried out on sea bass (Torrecillas et al. Citation2017); however, to our knowledge, the biological meaning of this observation remains unclear.
In a trial carried out on salmon (Salmo salar), fish were fed a control diet containing 20% FM and 5 additional experimental diets where FM was replaced up to 75% with fish protein concentrate (FPC), for a period of 79 days (Espe et al. Citation2012). In the remaining diet’s protein was a mixture of plant proteins; the results of the trial showed that none of the experimental diets affected, neither negatively nor positively, feed intake and the other growth performances. Also, these results are consistent with those observed in the present work in relation to the use of HFP for replacing FM in gilthead sea bream.
Regarding the gut histological findings, few studies have been carried out on the effects of dietary nitrogen compounds on intestine morphology; on the other hand, these effects have been widely described in relation to the diet inclusion of pre- and pro-biotics (Balcazar et al. Citation2006; Dimitroglou et al. Citation2010; Zhou et al. Citation2010). The higher absorbent surface ratio observed for the fish fed autolysed yeast in comparison to the other groups, can likely depend on the enhanced villi branching and thickening. Quite similar morphological aspects were reported upon feeding MOS to European sea bass (Dicentrarchus labrax) and these findings were considered as a positive enhancement of gut performances (Torrecillas, Makol, Benitez-Santana et al. Citation2011). Moreover, in relation to the thickening of the intestine wall, this latter study reported that MOS supplementation significantly enlarged intestine folds’ height and width, with that suggesting an enhanced intestinal barrier. Also, a study in red drum (Sciaenops ocellatus) gut morphology, revealed increased dimensions for intestinal fold height when supplemented with MOS (Zhou et al. Citation2010) and this enlargement seems to be related to the lamina propria engrossment because of the higher infiltrating eosinophilic granulocytes observed on the posterior gut. In agreement, in other vertebrates the increase of the lamina propria thickness is related to the gut immune system stimulation and gut maturation promotion (de los Santos et al. Citation2007), these providing a more efficient anatomical barrier against bacterial translocation (Ringo et al. Citation2007). All these findings seem to agree with the thickening of the intestinal wall observed in the present study, specifically in the experimental groups. However, it is worth to remind that an investigation on intestinal Defense mechanisms was not the aim of the present study. Lastly, the increased number of fish positive to villus branching seems to suggest an improved nutrient absorbing area and this parameter showed a significant increase in animals fed autolysed yeast rather than fishmeal or hydrolysed fish protein. As stated in the result section, the increased villus thickening and branching was associated with moderate increase in the intra-epithelial lymphocyte presence and the inflammatory infiltrate in the lamina propria. Our histological results are thus in accordance with the observation reported by the above-mentioned authors that supplemented the fish diet with MOS (Torrecillas, Makol, Benitez-Santana et al. Citation2011); however, a possible immunomodulatory effect should be demonstrated by mean of other techniques.
These effects on gut morphology seem to be confirmed by the higher goblet cell density observed for both, fish fed hydrolysed fish protein and fed autolysed yeast. It is worth to highlight that in the experimental groups, goblet cells were found not only on the epithelial surface but also stratified within the epithelial thickness. Apical localisation of Alcian positive goblets indicates a baseline secretion known as simple exocytosis; for a contrary, a stratified disposition of goblets within mucous cells may indicate exposure to a secretagogue with cells undergoing compound exocytosis (Deplancke and Gaskins Citation2001) even though in the present study the likely secretagogue stimulus remains unknown. As reported by other authors, several factors such as hormones, neuropeptides as well as inflammatory mediators can induce compound exocytosis (Laboisse et al. Citation1995, Citation1996); our results suggest that the inflammatory infiltrate is a possible trigger of acute mucin secretion in the experimental groups. Interleukin-1, a proinflammatory cytokine that can be released by immune cells activated by intestinal lamina propria during inflammation, has been shown to function as goblet-cell secretagogue in both cultures of mouse duodenum (Cohan et al. Citation1991) and a goblet cell line (C1.16E) from a human colonic adenocarcinoma (Laboisse et al. Citation1996). The role of mucin as a protective layer on the enterocyte surface has been emphasised in previous studies, but the role of the increased mucin production as a protective phenomenon in the groups considered in the present study deserves further investigations. Since authors suggest that the intestinal barrier efficiency markedly depends on mucins production lead by goblet cells (Torrecillas, Makol, Benitez-Santana et al. Citation2011), it is possible to hypothesise that this observed change positively affects the general intestinal function; no negative effects on growth performance were observed in our experimental groups, that would have been severely affected in case of lower nutrients absorption due to a severe gut inflammation.
Finally, considering the peculiar characteristics of the autolysed yeast, which contain yeast cell wall (typically rich in MOS) in addition to a wide range of amino acids, peptides and nucleotides, and the effect of MOS on the intestinal mucosa described by several studies (Piaget et al. Citation2007; Torrecillas et al. Citation2007; Dimitroglou et al. Citation2010; Torrecillas, Makol, Benitez-Santana et al. Citation2011; Torrecillas, Makol, Cabllero et al. Citation2011; Torrecillas et al. Citation2014), one can speculate that MOS might also play a role in the enhancement of the fish gut traits.
Conclusions
The present study showed that growth performances were not affected (neither positively nor negatively) using either hydrolysed fish protein or autolysed yeast as FM replacers in diet for gilthead sea bream. Likely depending on the enhanced villi branching and thickening, fish fed autolysed yeast showed a higher absorbent surface ratio in comparison to both fish fed FM and HFP. Based on these observations, it seems that autolysed yeast might be considered the most valuable solution for efficiently replace fishmeal in the diet for gilthead sea bream.
Thus, when autolysed yeast can be obtained at a favourable price, the aquaculture sector might significantly benefit on the economical perspective. Although encouraging, our results require independent confirmation by different techniques: further studies would indeed be helpful to better discriminate the biological role of its nitrogen fractions and other yeast cell wall compounds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aragao C, Corte-Real J, Costas B, Dinis MT, Conceicao LE. 2008. Stress response and changes in amino acid requirements in Senegalese sole (Solea senegalensis Kaup 1858). Amino Acids. 34:143–148.
- Balcazar JL, Vendrell D, de Blas I, Ruiz-Zarzuela I, Girones O, Muzquiz JL. 2006. Immune modulation by probiotic strains: quantification of phagocytosis of Aeromonas salmonicida by leukocytes isolated from gut of rainbow trout (Oncorhynchus mykiss) using a radiolabelling assay. Comp Immunol Microbiol Infect Dis. 29:335–343.
- Batista S, Medina A, Pires MA, Morinigo MA, Sansuwan K, Fernandes JM, Valente LM, Ozorio RO. 2016. Innate immune response, intestinal morphology and microbiota changes in Senegalese sole fed plant protein diets with probiotics or autolysed yeast. Appl Microbiol Biotechnol. 100:7223–7238.
- Cahu C, Rønnestad I, Grangier V, Zambonino Infante JL. 2004. Expression and activities of pancreatic enzymes in developing sea bass larvae (Dicentrarchus labrax) in relation to intact and hydrolyzed dietary protein; involvement of cholecystokinin. Aquaculture. 238:295–308.
- Cohan VL, Scott AL, Dinarello CA, Prendergast RA. 1991. Interleukin-1 is a mucus secretagogue. Cell Immunol. 136:425–434.
- Conceicao LE, Aragao C, Dias J, Costas B, Terova G, Martins C, Tort L. 2012. Dietary nitrogen and fish welfare. Fish Physiol Biochem. 38:119–141.
- Day OJ, Howell BR, Jones DA. 1997. The effect of dietary hydrolysed fish protein concentrate on the survival and growth of juvenile Dover sole, Solea solea (L.), during and after weaning. Aqua Res. 28:911–921.
- de los Santos FS, Donoghue AM, Farnell MB, Huff GR, Huff WE, Donoghue DJ. 2007. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poultry Sci. 86:921–930.
- Deplancke B, Gaskins HR. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 73:1131S–1141S.
- Dimitroglou A, Merrifield DL, Spring P, Sweetman J, Moate R, Davies SJ. 2010. Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture. 300:182–188.
- Espe M, Ruohonen K, El-Mowafi A. 2012. Hydrolysed fish protein concentrate (FPC) reduces viscera mass in Atlantic salmon (Salmo salar) fed plant-protein-based diets. Aqua Nutr. 18:599–609.
- Falcinelli S, Randazzo B, Vargas Abúndez JA, Cangiotti G, Olivotto I, Carnevali O. 2018. Kluyveromyces fragilis RNA extract supplementation promotes growth, modulates stress and inflammatory response in zebrafish. Aquac. Res 49:1521–1534.
- Faure M, Mettraux C, Moennoz D, Godin JP, Vuichoud J, Rochat F, Breuille D, Obled C, Corthesy-Theulaz I. 2006. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J Nutr. 136:1558–1564.
- Feist SW. 2009. Atlas of fish histology - Edited by F. Genten, E. Terwinghe and A. Danguy. J Fish Biol. 75:757–758.
- Gómez-Requeni P, Mingarro M, Calduch-Giner JA, Médale F, Martin SAM, Houlihan DF, Kaushik S, Pérez-Sánchez J. 2004. Protein growth performance, amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata). Aquaculture. 232:493–510.
- Hartmann R, Meisel H. 2007. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 18:163–169.
- Hauptman BS, Barrows FT, Block SS, Gaylord TG, Paterson JA, Rawles SD, Sealey WM. 2014. Evaluation of grain distillers dried yeast as a fish meal substitute in practical-type diets of juvenile rainbow trout, Oncorhynchus mykiss. Aquaculture. 432:7–14.
- Hou Y, Wu Z, Dai Z, Wang G, Wu G. 2017. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J Anim Sci Biotechnol. 8:24.
- Johansson ME, Sjovall H, Hansson GC. 2013. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 10:352–361.
- Kim JJ, Khan WI. 2013. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens. 2:55–70.
- Kitts DD, Weiler K. 2003. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr Pharm Des. 9:1309–1323.
- Laboisse C, Jarry A, Branka JE, Merlin D, Bou-Hanna C, Vallette G. 1995. Regulation of mucin exocytosis from intestinal goblet cells. Biochem Soc Trans. 23:810–813.
- Laboisse C, Jarry A, Branka JE, Merlin D, Bou-Hanna C, Vallette G. 1996. Recent aspects of the regulation of intestinal mucus secretion. Proc Nutr Soc. 55:259–264.
- Li P, Gatlin IIIDM. 2003. Evaluation of brewers yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysopsxM. saxatilis). Aquaculture. 219:681–692.
- Madureira AR, Tavares T, Gomes AM, Pintado ME, Malcata FX. 2010. Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci. 93:437–455.
- Mihelakakis A, Tsolkas C, Yoshimatsu T. 2002. Optimization of feeding rate for hatchery-produced juvenile Gilthead Sea Bream Sparus aurata. J. World Aquacult Soc. 33:169–175.
- NRC. 2011. Nutrient Requirements of Fish and Shrimp. Washington, DC: The National Academies Press. Available from https://link.springer.com/article/10.1007/s10499-009-9312-0
- PDF) Growth and feed utilization of gilthead sea bream (Sparus aurata, L.) fed to satiation and restrictively at increasing dietary energy levels. Available from: https://www.researchgate.net/publication/227215649_Growth_and_feed_utilization_of_gilthead_sea_bream_Sparus_aurata_L_fed_to_satiation_and_restrictively_at_increasing_dietary_energy_levels [accessed 2019 Jan 13].
- Piaget N, Vega A, Silva A, Toledo P. 2007. Effect of the application of β-glucans and mannan-oligosaccharides (βG MOS) in an intensive larval rearing system of Paralichthys adspersus (Paralichthydae). Investigaciones Marinas. 35:35–43.
- Ringo E, Myklebust R, Mayhew TM, Olsen RE. 2007. Bacterial translocation and pathogenesis in the digestive tract of larvae and fry. Aquaculture. 268:251–264.
- Sitjà-Bobadilla A, Peña-Llopis S, Gómez-Requeni P, Médale F, Kaushik S, Pérez-Sánchez J. 2005. Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture. 240:387–400.
- Sönmez AY. 2017. Evaluating two different additive levels of fully autolyzed yeast, Saccharomyces cerevisiae, on rainbow trout (Oncorhynchus mykiss) growth performance, liver histology and fatty acid composition. Turkish J Fisheries Aqua Sci. 17:379–385.
- Staykov Y, Spring P, Denev S, Sweetman J. 2007. Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aqua Int. 15:153–161.
- Tahmasebi-Kohyani A, Keyvanshokooh S, Nematollahi A, Mahmoudi N, Pasha-Zanoosi H. 2012. Effects of dietary nucleotides supplementation on rainbow trout (Oncorhynchus mykiss) performance and acute stress nce and acute stress response. Fish Physiol Biochem. 38:431–440.
- Torrecillas S, Makol A, Benitez-Santana T, Caballero MJ, Montero D, Sweetman J, Izquierdo M. 2011. Reduced gut bacterial translocation in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Fish Shellfish Immunol. 30:674–681.
- Torrecillas S, Makol A, Caballero MJ, Montero D, GinÉS R, Sweetman J, Izquierdo M. 2011. Improved feed utilization, intestinal mucus production and immune parameters in sea bass (Dicentrarchus labrax) fed mannan oligosaccharides (MOS). Aqua Nutr. 17:223–233.
- Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L, Real F, Sweetman J, Tort L, Izquierdo MS. 2007. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol. 23:969–981.
- Torrecillas S, Montero D, Izquierdo M. 2014. Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish Shellfish Immunol. 36:525–544.
- Torrecillas S, Robaina L, Caballero MJ, Montero D, Calandra G, Mompel D, Karalazos V, Kaushik S, Izquierdo MS. 2017. Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax): production performance, tissue composition and liver morphology. Aquaculture. 474:101–112. English.
- Udayangani RM, Dananjaya SH, Fronte B, Kim CH, Lee J, De Zoysa M. 2017. Feeding of nano scale oats β-glucan enhances the host resistance against Edwardsiella tarda and protective immune modulation in zebrafish larvae. Fish Shellfish Immunol. 60:72–77.
- Verri T, Barca A, Pisani P, Piccinni B, Storelli C, Romano A. 2017. Di- and tripeptide transport in vertebrates: the contribution of teleost fish models. J Comp Physiol B Biochem Syst Environ Physiol. 187:395–462.
- Verri T, Terova G, Dabrowski K, Saroglia M. 2011. Peptide transport and animal growth: the fish paradigm. Biol Lett. 7:597–600.
- Zhou QC, Buentello JA, Gatlin DM. 2010. Effects of dietary prebiotics on growth performance, immune response and intestinal morphology of red drum (Sciaenops ocellatus). Aquaculture. 309:253–257.
