Abstract
Studies over the past 30 years have confirmed the important role of metabolic hormones and metabolic substrates in reproductive function in female cattle. The emergence of metabolomics is providing a deeper understanding of the role of specific metabolites, and clusters of metabolites, in reproduction and also health and disease. Dairy cows undergo major fluctuations in metabolic health and metabolomics is helping to better understand the changes in metabolite profiles associated with negative energy balance and ketosis. New knowledge that emerges from this work should lead to improved nutritional management of dairy cows. In reproduction, it is now possible to gain a metabolomic signature of ovarian follicular fluid and of developing embryos. This should likewise lead to improvements in both natural and assisted reproduction in cattle. Systems biology integrates genomics, transcriptomics, proteomics and metabolomics, and contributes to gaining an understanding of complex biological networks.
Metabolic hormones and metabolic substrates have a major influence on reproduction in female cattle.
Negative energy balance and ketosis are associated with changes in the systemic and liver metabolome in dairy cows.
The metabolome of ovarian follicular fluid influences oocyte quality and embryo development.
Systems biology integrates genomics, transcriptomics, proteomics and metabolomics, and provides a deeper understanding of complex biological networks.
Highlights
Introduction
Studies over the past 30 years have demonstrated the fundamental importance of metabolic hormones (GH, IGF1, insulin, leptin, thyroxine) and metabolic factors (glucose, fatty acids) in female cattle reproduction (Lucy Citation2000, Citation2008; Velazquez et al. Citation2008; Silva et al. Citation2009; Roche et al. Citation2011; Castro et al. Citation2012; Samadi et al. Citation2013, Citation2014; Lucy et al. Citation2014; Sartori et al. Citation2016; Meikle et al. Citation2018; D'Occhio et al. Citation2019). Insulin-like growth factor-I (Ghanipoor-Samami et al. Citation2018) has received particular attention given its central roles in ovarian follicular growth (Wandji et al. Citation1992; Spicer and Echternkamp Citation1995; Yuan et al. Citation1998; Lucy Citation2000, Citation2008, Citation2011; Spicer and Aad Citation2007) and function of the corpus luteum (Lucy et al. Citation1999; Woad et al. Citation2000). Follicles can produce IGF1 (Adashi Citation1998; Yuan et al. Citation1998) but an important source is IGF1 secreted by the liver and sequestered from blood by follicles (Clemmons and Underwood Citation1991; Lucy Citation2011; Zhang, Wu et al. Citation2013). Insulin-like growth factor-I production by the liver is influenced by nutrition and metabolic status (Clemmons and Underwood Citation1991; Guggeri et al. Citation2014). Dysfunction of the liver, due to metabolic stress or liver disease, is associated with reduced IGF1 (Fenwick et al. Citation2008) and a disruption of ovarian folliculogenesis in cattle (O’Doherty et al. Citation2014; Mokhtari et al. Citation2016). Metabolic health and liver health are therefore critical for normal ovarian function and fertility in cattle.
The relationships between body condition, metabolic status, liver function and reproduction, have been studied extensively in periparturient and early lactation dairy cows (Roche et al. Citation2007, Citation2009, Citation2011, Citation2013, Citation2018; LeBlanc Citation2012; Sundstrum Citation2015; Overton et al. Citation2017). In the period before calving, there is an increase in glucose demand for foetal growth and after calving glucose is required for milk production. This is often accompanied by a decrease in appetite which can lead to negative energy balance (NEB) and lowered amounts of glucose in blood (Shaw Citation1956; Lucy et al. Citation2014; www.farmhealthonline.com/US/disease-management/cattle-diseases/ketosis/). The rumen converts carbohydrates in feed to volatile fatty acids that include propionate, acetate and butyrate. Propionate is used by the liver to produce glucose that is used as a source of energy for oxidative processes in the liver. If carbohydrate intake and propionate levels are low due to decreased appetite, the liver can switch to energy production by utilising non-esterified fatty acids (NEFAs) that diffuse into blood from fat because of the NEB (Adewuyi et al. Citation2005). NEFAs are oxidised by the liver to acetyl-CoA. Acetyl-CoA can undergo complete oxidation through the tricarboxylic acid cycle (TCA), converted to very-low density lipoprotein (VLDL) and exported, or converted to triglycerides (TAG) and stored by the liver (White Citation2015). The liver has a limited capacity to metabolise acetyl-CoA and if complete oxidation is not possible then acetyl-CoA is converted to ketone bodies that are exported as acetone, acetoacetate and beta-hydroxybutyrate (Aschenbach et al. Citation2010). The latter causes the metabolic disease of ketosis (acetonemia) in dairy cows. Common features of ketosis are low blood concentrations of glucose, insulin and IGFI, with elevated NEFAs and ketone bodies (Walsh et al. Citation2007; LeBlanc Citation2010; Bisinotto et al. Citation2012; Esposito et al. Citation2014). At the same time, excess TAG is stored by the liver giving rise to ‘fatty liver’ syndrome. This metabolic picture is typically associated with anoestrus (Bisinotto et al. Citation2012). Major disruption of glucose homeostasis before calving can cause a clinical condition in cows similar to pregnancy toxaemia in smaller ruminants (Marteniuk and Herdt Citation1988; Rook Citation2000). Ketosis and pregnancy toxaemia are uncommon in beef cows.
Metabolomics
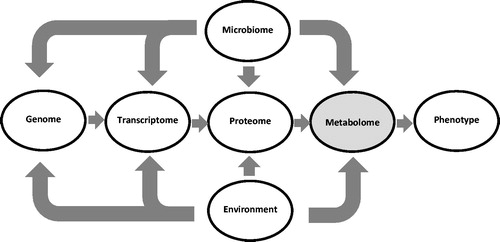
The study of relationships between metabolic condition and reproduction in cattle entered a new era with the advent of metabolomics (Ryan et al. Citation2013; Canovas et al. Citation2014; Fontanesi Citation2016; Goldansaz et al. Citation2017; Li et al. Citation2017). Metabolomics is the study of the metabolome which comprises the myriad of low molecular weight metabolites (lipids, amino acids, vitamins) that influence cellular, tissue and organ function (Patti et al. Citation2012; Dona et al. Citation2014; Goldansaz et al. Citation2017). The metabolome is downstream of the genome, transcriptome and proteome, and is considered the closet ‘omic’ to the phenotype (Figure ) (Fontanesi Citation2016; Pantophlet et al. Citation2017). The latter has led to the suggestion that the metabolome is a particularly important indicator of changes in biological function. Another significant feature of the blood metabolome is that it represents the integration of external (e.g. diet) and internal (e.g. genotype) factors that influence metabolism (Figure ). Hence, the metabolome acts as an integrator of endogenous and exogenous processes to shape the phenotype. This includes the reproductive phenotype. The following sections provide a synthesis of how metabolomics is leading to a deeper understanding of relationships between metabolic condition and reproduction in cattle.
Figure 1. Diagram illustrating the positioning of the metabolome downstream of the genome and positioned closely to the phenome. The myriad of low molecular weight metabolites that comprise the blood metabolome is derived from endogenous processes and exogenously from the diet and activity of the gut microbiome. The environment and microbiome can also impact the genome through mutations or epigenetic effects.
Ruminal metabolome
As illustrated in Figure , feed consumed by cattle impacts the ruminal microbial metabolome (Saleem et al. Citation2013; Khiaosa-ard and Zebeli Citation2014) which ultimately influences the systemic metabolome. Holstein dairy cows fed either corn stover or a mixture of alfalfa hay and corn silage showed differences in ruminal fluid levels of key metabolic factors such as acetate, glucose and propionate (Zhao et al. Citation2014). In a second study in Holstein dairy cows, low concentrate and high concentrate diets produced differences in the ruminal amino acid profile that included alanine, leucine and glycine (Zhang, Zhu et al. Citation2017). Dairy cows fed a high grain diet early in lactation tend to have a higher incidence of metabolic disorders. It was found that Holstein cows on a total mixed ration (TMR) with a high grain diet (30 or 45% barley on a dry matter basis) from day 60 of lactation had an altered ruminal microbiome compared with cows fed a low grain diet (15% barley), and the former cows also had differences in ruminal levels of short-chain fatty acids and a range of amino acids (Saleem et al. Citation2012). In several studies, high grain diets (TMR and 30 or 45% barley on a dry matter basis) increased the ruminal levels of potentially toxic and inflammatory compounds such as putrescine and methylated amines in lactating Holstein cows (Ametaj et al. Citation2010; Saleem et al. Citation2012). Lactating Simmental cows with subacute ruminal acidosis from a grain-rich diet had an altered systemic metabolome response to intramammary challenge with lipopolysaccharide (Aditya et al. Citation2018). However, this was not observed in a second study (Humer et al. Citation2018). Lactating Friesian cows that grazed ryegrass and white clover pastures had greater ruminal concentrations of isoacids compared with contemporary cows that were fed a TMR based on maize silage (O’Callaghan et al. Citation2018). Isoacids are branched-chain fatty acids (isobutyric, isovaleric) and straight-chain valeric acid, which serve as nutrients for ruminal cellulolytic bacteria (Andries et al. Citation1987). The ruminal metabolite profile of Holstein-Friesian cows differed to Hanwoo steers independent of diet, and this was interpreted to suggested that genotype can have a predominant influence on the ruminal metabolome in cattle (Lee et al. Citation2012).
Diet influences the milk metabolome in dairy cows in addition to the systemic metabolome (Sun et al. Citation2015a, Citation2015b). In a recent study, the systemic metabolome in Holstein cows was predictive of the milk protein profile (Wu et al. Citation2018). However, an earlier study reported that milk was a distinct metabolic compartment and had a metabolite composition different to blood (Maher et al. Citation2013). Further studies are required to ascertain relationships between the blood and milk metabolomes. This is important as accurate indices are required to determine the metabolite profile of milk in relation to human nutrition and health. In one study, the ruminal metabolome was related to feed efficiency in Angus crossbred steers (Artegoitia et al. Citation2017). Similar to ruminants, diet influenced the systemic metabolome in a monogastric species, the pig (Sun et al. Citation2015a, Citation2015b).
The above presents only a summary of changes in the ruminal and systemic microbiomes in response to diet, and in all studies the complete picture is far more complex. This underscores the necessity for advanced metabolomics analysis platforms in order to understand the biology of very large metabolite datasets (Dona et al Citation2014).
Metabolome and ketosis
The nutritional management of dairy cows to prevent or mitigate ketosis is important for production and animal welfare (Littledike et al. Citation1981; Miettinen and Setala Citation1993; Lu et al. Citation2013). The ability to use metabolomics to identify cows susceptible to ketosis is a preferred strategy, compared with the application of metabolomics as a diagnostic technology (Kenez et al. Citation2016; Ceciliani et al. Citation2018). In Holstein-Friesian cows, a milk glycerophosphocholine/phosphocholine ratio of ≥ 2.5 early in lactation was associated with a very low risk of developing ketosis (Klein et al. Citation2012). Plasma metabolomic profiling was able to distinguish between lactating clinically normal cows, cows with subclinical ketosis, and cows with clinical ketosis (Zhang, Davis et al. Citation2013; Li et al. Citation2014; Sun et al. Citation2014). Hepatic lipidosis could also be diagnosed using the plasma metabolome in transition Holstein-Friesian and Red-Holstein cows (Imhasly et al. Citation2014) and early lactation Holstein-Friesian and Simmental cows (Humer et al. Citation2016). The systemic metabolome was able to identify Holstein dairy cows at-risk for a retained placenta (Dervishi et al. Citation2018) and metritis (Zhang, Deng et al. Citation2017). A relatively large study with lactating Danish Holstein-Friesian and Jersey cows revealed an association between the milk metabolome and somatic cell count (Sundekilde et al. Citation2013). Other studies have shown changes in the systemic metabolome of Holstein-Friesian calves after experimental infection (De Buck et al. Citation2014) and vaccination (Gray et al. Citation2015).
Transcriptome and negative energy balance
Whilst the metabolome is the focus of this review, it is relevant to consider the transcriptome within the context of metabolic health in cattle. Using RNA-seq technology, major differences in liver RNA linked to fatty acid metabolism were found between mild NEB and severe NEB in lactating Holstein-Friesian cows (McCabe et al. Citation2012). Differences were also reported for liver micro RNA (miRNA) expression related to NEB severity in lactating Holstein-Friesian cows (Fatima et al. Citation2014a, Citation2014b). In lactating Lacaune ewes, the systemic transcriptome was altered by NEB (Bouvier-Muller et al. Citation2017). The incorporation of transcription factors and miRNAs in an integrated computational approach yielded information on gene networks involved in NEB in cattle (Mozduri et al. Citation2018).
The science of systems biology (Woelders et al. Citation2011) brings together transcriptomic, proteomic and metabolomic information to build integrated gene networks that underpin biological processes linked to phenotypes (Homuth et al. Citation2012; Krumsiek et al. Citation2012; Widmann et al. Citation2013, Citation2015; Cho et al. Citation2014; Wang et al. Citation2019). Application of this approach in dairy cows has been reviewed in relation to nutrition (Loor et al. Citation2013), lactation (Li et al. Citation2017), breeding (Berry et al. Citation2011) and fertility (Ceciliani et al. Citation2017). The integration of ‘omics’ technologies has the potential to provide new knowledge that informs changes in management that enhance both production and wellbeing in dairy cattle and other livestock (Crowe et al. Citation2018).
Metabolome and reproduction
Ovarian follicles
Ovarian function in mammals is acutely sensitive to metabolic homeostasis, and the important role of the GH-IGF1 axis was noted above. It is now emerging that the metabolome, both systemic and follicular, influences follicle growth, oocyte quality and embryo developmental competency (Singh and Sinclair Citation2007; Nel-Themaat and Nagy Citation2011; Collado-Fernandez et al. Citation2012; Wallace et al. Citation2012; Bertoldo et al. Citation2013; Gerard et al. Citation2015; Gu et al. Citation2015; Krisher et al. Citation2015). Follicular fluid provides a metabolomic micro-environment that supports oocyte growth and development (Gerard et al. Citation2002; Pinero-Sagredo et al. Citation2010; Sirard Citation2011; Hennet and Combelles Citation2012; Leroy et al. Citation2012; Dumesic et al. Citation2015; El-Hayek and Clarke Citation2016; Guerreiro et al. Citation2018). In a study that utilised abattoir cow ovaries, palmitic acid and total fatty acids were reduced, and linoleic acid increased, in follicular fluid of follicles that contained competent oocytes (Matoba et al. Citation2014). Differences in follicular fluid concentrations of saturated fatty acids between Holstein-Friesian heifers and lactating cows were associated with differences in fertility (Bender et al. Citation2010). Predominantly, Holstein cows with either a positive or negative estimated progeny difference (EPD) for fertility had differences in follicular fluid content of saturated fatty acids, mono-unsaturated fatty acids and poly-unsaturated fatty acids (Moore et al. Citation2017). Lactating Holstein-Friesian cows had different profiles of amino acids and fatty acids in follicular fluid compared with non-lactating cows and heifers (Forde et al. Citation2016). Follicular fluid influences oocyte development through the cumulus layer (Zhang et al. Citation1995) and the metabolome profile of cumulus undergoes changes during follicular growth in cattle (Uhde et al. Citation2018). These studies are providing new insight into the metabolite environment of follicles that is optimal for oocyte development and should lead to targeted nutritional strategies that enhance fertility in cattle.
Assisted reproduction
Metabolomics has also been combined with assisted reproduction in cattle. Metabolomic analysis of spent culture media of IVF produced cattle embryos was able to distinguish between male and female embryos (Uyar et al. Citation2013; Gomez et al. Citation2016). The accuracy in predicting sex using spent culture media of bovine IVF embryos increased from early blastocysts (74%) to expanded blastocysts (86%) (Munoz et al. Citation2014b). Metabolomics, proteomics and miRNA have also been applied to assess stage of embryo development and embryo quality (Al Naib et al. Citation2009; Rodgaard et al. Citation2015). In a recent study, IVF and ICSI derived cattle embryos were associated with spent culture media with a different metabolomic signature (Li et al. Citation2018). Dual assessment of the systemic metabolome of recipient cows, together with the metabolome of spent culture media, could predict the pregnancy outcome for transferred IVF embryos (Munoz et al. Citation2014a) and conventional superovulated embryos (Munoz et al. Citation2014c). Notwithstanding recent progress with application of metabolomics in IVF, limitations have been identified and the field remains at an early stage (Cuperlovic-Culf et al. Citation2010; McRae et al. Citation2013; Uyar and Seli Citation2014; Munoz et al. Citation2014c; Krisher et al. Citation2015; Rodgaard et al. Citation2015).
Conclusions and future direction
The important role of metabolic hormones and metabolic substrates in health and reproductive function in female cattle has been clearly established over the past 30 years. The advent of metabolomics has brought this field into a new phase that will deliver an unprecedented increase in knowledge of the role of individual metabolites, and networks of metabolites, in reproduction. Metabolomics will similarly allow major advances in understanding of the biology of metabolic health and metabolic disease. Maintaining livestock in good metabolic health is important for production and reproduction, and it is also vital for animal wellbeing.
For the future, metabolomics will be integrated together with genomics, transcriptomics and proteomics into a systems biology framework (Dumas Citation2012). The unique strength of metabolomics is that biochemical pathways and networks are largely known, which means that information on the metabolome can guide gene discovery and also provide information on gene function (Gauguier Citation2016). Parallel study of the metabolomes of the rumen microbiome and host will provide new knowledge on the impact of the environment on metabolic health and disease, and gene function (Aguiar-Pulido et al. Citation2016; Scharen et al. Citation2018).
Acknowledgements
The authors acknowledge with gratitude the many postgraduates who contributed to research and thinking in our laboratories that is included in this review.
Disclosure statement
All authors declare there is no conflict of interest.
References
- Adashi EY. 1998. The IGF family and folliculogenesis. J Reprod Immunol. 39:13–19.
- Adewuyi AA, Gruys E, van Eerdenburg F. 2005. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Quart. 27:117–126.
- Aditya S, Humer E, Pourazad P, Khiaosa-ard R, Zebeli Q. 2018. Metabolic and stress responses in dairy cows fed a concentrate-rich diet and submitted to intramammary lipopolysaccharide challenge. Animal. 12:741–749.
- Aguiar-Pulido V, Huang W, Suarez-Ulloa V, Cickovski T, Mathee K, Narasimhan G. 2016. Metagenomics, metatranscriptomics, and metabolomics approaches for microbiome analysis. Evol Bioinform. 12:5–16.
- Al Naib A, Wallace M, Brennan L, Fair T, Lonergan P. 2009. Metabolomic analysis of bovine pre-implantation embryos. Reprod Fertil Dev. 22:230.
- Ametaj BN, Zebeli Q, Saleem F, Psychogios N, Lewis MJ, Dunn SM, Xia J, Wishart DS. 2010. Metabolomics reveals unhealthy alterations in rumen metabolism with increased proportion of cereal grain in the diet of dairy cows. Metabolomics. 6:583–594.
- Andries JI, Buysse FX, De Brabander DL, Cottyn BG. 1987. Isoacids in ruminant nutrition: their role in ruminal and intermediary metabolism and possible influences on performance – a review. Anim Feed Sci Technol. 18:169–180.
- Artegoitia VM, Foote AP, Lewis RM, Freetly HC. 2017. Rumen fluid metabolomics analysis associated with feed efficiency on crossbred steers. Nat Sci Rep. 7:2864.
- Aschenbach JR, Kristensen NB, Donkin SS, Hammon HM, Penner GB. 2010. Gluconeogenesis in dairy cows: the secret of making sweet milk from sour dough. IUBMB Life. 62:869–877.
- Bender K, Walsh S, Evans ACO, Fair T, Brennan L. 2010. Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction. 139:1047–1055.
- Berry DP, Meade KG, Mullen MP, Butler S, Diskin MG, Morris D, Creevey CJ. 2011. The integration of 'omic' disciplines and systems biology in cattle breeding. Animal. 5:493–505.
- Bertoldo MJ, Nadal-Desbarats L, Gerard N, Dubois A, Holyoake PK, Grupen CG. 2013. Differences in the metabolomic signatures of porcine follicular fluid collected from environments associated with good and poor oocyte quality. Reproduction. 146:221–231.
- Bisinotto RS, Greco LF, Ribeiro ES, Martinez N, Lima FS, Staples CR, Thatcher WW, Santos FS. 2012. Influences of nutrition and metabolism on fertility of dairy cows. Anim Reprod. 9:260–272.
- Bouvier-Muller J, Allain C, Tabouret G, Enjalbert F, Portes D, Noirot C, Rupp R, Foucras G. 2017. Whole blood transcriptome analysis reveals potential competition in metabolic pathways between negative energy balance and response to inflammatory challenge. Nat Sci Rep. 7:2379.
- Canovas A, Reverter A, DeAtley KL, Ashley RL, Colgrave ML, Fortes MRS, Islas-Trejo A, Lehnert S, Porto-Neto L, Rincon G, et al. 2014. Multi-tissue omics analyses reveal molecular regulatory networks for puberty in composite beef cattle. PLoS ONE. 9:e102551.
- Castro N, Kawashima C, van Dorland HA, Morel I, Miyamoto A, Bruckmaier RM. 2012. Metabolic and energy status during the dry period is crucial for the resumption of ovarian activity postpartum in dairy cows. J Dairy Sci. 95:5804–5812.
- Ceciliani F, Lecchi C, Urh C, Sauerwein H. 2018. Proteomics and metabolomics characterizing the pathophysiology of adaptive reactions to the metabolic challenges during the transition from late pregnancy to early lactation in dairy cows. J Proteom. 178:92–106.
- Ceciliani F, Vecchio D, De Carlo E, Martucciello A, Lecchi C. 2017. A systems biology approach to dairy cattle subfertility and infertility. In: Ametaj B, editor. Periparturient diseases of dairy cows. Springer International Publishing, Switzerland: Springer; p. 93–119.
- Cho K, Mahieu NG, Johnson SL, Patti GJ. 2014. After the feature presentation: technologies bridging untargeted metabolomics in biology. Curr Opinion Biotechnol. 28:143–148.
- Clemmons DR, Underwood LE. 1991. Nutritional regulation of IGF-I and IGF binding proteins. Annu Rev Nutr. 11:393–412.
- Collado-Fernandez E, Picton HM, Dumollard R. 2012. Metabolism throughout follicle and oocyte development in mammals. Int J Dev Biol. 56:799–808.
- Crowe MA, Hostens M, Opsomer G. 2018. Reproductive management in dairy cows – the future. Ir Vet J. 71:1.
- Cuperlovic-Culf M, Barnett DA, Culf AS, Chute I. 2010. Cell culture metabolomics: applications and future directions. Drug Discov Today. 15:610–621.
- D'Occhio MJ, Baruselli PS, Campanile G. 2019. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: a review. Theriogenology. 125:277–284.
- De Buck J, Shaykhutdinov R, Barkema HW, Vogel HJ. 2014. Metabolomic profiling in cattle experimentally infected with Mycobacterium avium subsp. paratuberculosis. PLoS ONE. 9:e111872.
- Dervishi E, Zhang G, Mandal R, Wishart DS, Ametaj BN. 2018. Targeted metabolomics: new insights into pathobiology of retained placenta in dairy cows and potential risk biomarkers. Animal. 12:1050–1059.
- Dona AC, Jimenez B, Schafer H, Humpfer E, Spraul M, Lewis MR, Pearce JT, Holmes E, Lindon JC, Nicholson JK. 2014. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal Chem. 86:9887–9894.
- Dumas ME. 2012. Metabolome 2.0: quantitative genetics and network biology of metabolic phenotypes. Mol BioSyst. 8:2494–2502.
- Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. 2015. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 103:303–316.
- El-Hayek S, Clarke HJ. 2016. Control of oocyte growth and development by intercellular communication within the follicular niche. In: Piprek RP, editor. Molecular mechanisms of cell differentiation in gonadal development, results and problems in cell differentiation. Springer International Publishing, Switzerland, Vol. 58. p. 191–224.
- Esposito G, Irons PC, Webb EC, Chapwanya A. 2014. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim Reprod Sci. 144:60–71.
- Fatima A, Lynn DJ, O'Boyle P, Seoighe C, Morris D. 2014a. The miRNAome of the postpartum dairy cow liver in negative energy balance. BMC Genomics. 15:279.
- Fatima A, Waters S, O’Boyle P, Seoighe C, Morris DG. 2014b. Alterations in hepatic miRNA expression during negative energy balance in postpartum dairy cattle. BMC Genomics. 15:28.
- Fenwick MA, Fitzpatrick R, Kenny DA, Diskin MG, Patton J, Murphy JJ, Wathes DC. 2008. Interrelationships between negative energy balance (NEB) and IGF regulation in liver of lactating dairy cows. Dom Anim Endocrinol. 34:31–44.
- Fontanesi L. 2016. Metabolomics and livestock genomics: insights into a phenotyping frontier and its applications in animal breeding. Anim Frontiers. 6:73–79.
- Forde N, O'Gorman A, Whelan H, Duffy P, O'Hara L, Kelly AK, Havlicek V, Besenfelder U, Brennan L, Lonergan P. 2016. Lactation-induced changes in metabolic status and follicular-fluid metabolomic profile in postpartum dairy cows. Reprod Fertil Dev. 28:1882–1892.
- Gauguier D. 2016. Application of quantitative metabolomics in systems genetics in rodent models of complex phenotypes. Arch Biochem Biophys. 589:158–167.
- Gerard N, Fahiminiya S, Grupen CG, Nadal-Desbarats L. 2015. Reproductive physiology and ovarian folliculogenesis examined via 1H-NMR metabolomics signatures: a comparative study of large and small follicles in three mammalian species (Bos taurus, Sus scrofa domesticus and Equus ferus caballus). OMICS. 19:31–40.
- Gerard N, Loiseau S, Duchamp G, Seguin F. 2002. Analysis of the variation of follicular fluid composition during follicular growth and maturation in the mare using proton nuclear magnetic resonance (1H NMR). Reproduction. 124:241–248.
- Ghanipoor-Samami M, Javadmanesh A, Burns BM, Thomsen DA, Nattrass GS, Estrella CAS, Kind KL, Hiendleder S. 2018. Atlas of tissue- and developmental stage specific gene expression for the bovine insulin-like growth factor (IGF) system. PLoS ONE. 13:e0200466.
- Goldansaz SA, Guo AC, Sajed T, Steele MA, Plastow GS, Wishart DS. 2017. Livestock metabolomics and the livestock metabolome: a systematic review. PLoS ONE. 12:e0177675.
- Gomez E, Munoz M, Simo C, Ibanez C, Carrocera S, Martin-Gonzalez D, Cifuentes A. 2016. Non-invasive metabolomics for improved determination of embryonic sex markers in chemically defined culture medium. J Chromatogr A. 1474:138–144.
- Gray DW, Welsh MD, Doherty S, Mansoor F, Chevallier OP, Elliott CT, Mooney MH. 2015. Identification of systemic immune response markers through metabolomics profiling of plasma from calves given an intra-nasally delivered respiratory vaccine. Vet Res. 46(7):1–16.
- Gu L, Liu H, Gu X, Boots C, Moley KH, Wang Q. 2015. Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci. 72:251–271.
- Guerreiro TM, Goncalves RF, Melo C, de Oliveira DN, Lima EO, Visintin JA, de Achilles MA, Catharino RR. 2018. A metabolomic overview of follicular fluid in cows. Front Vet Sci. 5:10.
- Guggeri D, Meikle A, Carriquiry M, Montossi F, De Barbieri I, Viñoles C. 2014. Effect of different management systems on growth, endocrine parameters and puberty in Hereford female calves grazing Campos grassland. Liv Sci. 167:455–462.
- Hennet ML, Combelles C. 2012. The antral follicle: a microenvironment for oocyte differentiation. Int J Dev Biol. 56:819–831.
- Homuth G, Teumer A, Volker U, Nauck M. 2012. A description of large-scale metabolomics studies: increasing value by combining metabolomics with genome-wide SNP genotyping and transcriptional profiling. J Endocrinol. 215:17–28.
- Humer E, Aditya S, Zebeli Q. 2018. Innate immunity and metabolomic responses in dairy cows challenged intramammarily with lipopolysaccharide after subacute ruminal acidosis. Animal. 12:2551.
- Humer E, Khol-Parisini A, Metzler-Zebeli BU, Gruber L, Zebeli Q. 2016. Alterations of the lipid metabolome in dairy cows experiencing excessive lipolysis early postpartum. PLoS ONE. 11:e0158633.
- Imhasly S, Naegeli H, Baumann S, von Bergen M, Luch A, Jungnickel H, Potratz S, Gerspach C. 2014. Metabolomic biomarkers correlating with hepatic lipidosis in dairy cows. BMC Vet Res. 10:122.
- Kenez A, Danicke S, Rolle-Kampczyk U, von Bergen M, Huber K. 2016. A metabolomics approach to characterize phenotypes of metabolic transition from late pregnancy to early lactation in dairy cows. Metabolomics. 12:1–11.
- Khiaosa-ard R, Zebeli Q. 2014. Cattle’s variation in rumen ecology and metabolism and its contribution to feed efficiency. Liv Sci. 162:66–75.
- Klein MS, Buttchereit N, Miemczyk SP, Immervoll AK, Louis C, Wiedemann S, Junge W, Thaller G, Oefner PJ, Gronwald W. 2012. NMR metabolomic analysis of dairy cows reveals milk glycerophosphocholine to phosphocholine ratio as prognostic biomarker for risk of ketosis. J Proteome Res. 11:1373–1381.
- Krisher RL, Schoolcraft WB, Katz-Jaffe MG. 2015. Omics as a window to view embryo viability. Fertil Steril. 103:333–341.
- Krumsiek J, Suhre K, Evans AM, Mitchell MW, Mohney RP, Milburn MV, Wagele B, Romisch-Margl W, Illig T, Adamski J, et al. 2012. Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet. 8:e1003005.
- LeBlanc S. 2010. Monitoring metabolic health of dairy cattle in the transition period. J Reprod Fertil. 56:S29–S35.
- LeBlanc SJ. 2012. Interactions of metabolism, inflammation, and reproductive tract health in the postpartum period in dairy cattle. Reprod Dom Anim. 47:18–30.
- Lee HJ, Jung JY, Oh YK, Lee S-S, Madsen EL, Jeon CO. 2012. Comparative survey of rumen microbial communities and metabolites across one caprine and three bovine groups, using bar-coded pyrosequencing and 1H nuclear magnetic resonance spectroscopy. Appl Environ Microbiol. 78:5983–5993.
- Leroy JLM, Rizos D, Sturmey R, Bossaert P, Gutierrez-Adan A, van Hoeck V, Valckx S, Bols P. 2012. Intrafollicular conditions as a major link between maternal metabolism and oocyte quality: a focus on dairy cow fertility. Reprod Fertil Dev. 24:1–12.
- Li X-X, Cao P-H, Han W-X, Xu Y-K, Wu H, Yu X-L, Chen J-Y, Zhang F, Li Y-H. 2018. Non-invasive metabolomics profiling of culture media of ICSI- and IVF-derived early developmental cattle embryos via Raman spectroscopy. Anim Reprod Sci. 196:99–110.
- Li S, Wang Q, Lin X, Jin X, Liu L, Wang C, Chen Q, Liu J, Liu H. 2017. The use of “omics” in lactation research in dairy cows. Int J Mol Sci. 18:983.
- Li Y, Xu C, Xia C, Zhang H, Sun L, Gao T. 2014. Plasma metabolic profiling of dairy cows affected with clinical ketosis using LC/MS technology. Vet Quart. 34:152–158.
- Littledike ET, Young JW, Beitz DC. 1981. Common metabolic diseases of cattle: ketosis, milk fever, grass tetany, and downer cow complex. J Dairy Sci. 64:1465–1482.
- Loor JJ, Bionaz M, Drackley JK. 2013. Systems physiology in dairy cattle: nutritional genomics and beyond. Annu Rev Anim Biosci. 1:365–392.
- Lu J, Antunes Fernandes E, Páez Cano AE, Vinitwatanakhun J, Boeren S, van Hooijdonk T, van Knegsel A, Vervoort J, Hettinga KA. 2013. Changes in milk proteome and metabolome associated with dry period length, energy balance, and lactation stage in postparturient dairy cows. J Proteome Res. 12:3288–3296.
- Lucy MC. 2000. Regulation of ovarian follicular growth by somatotropin and insulin-like growth factors in cattle. J Dairy Sci. 83:1635–1647.
- Lucy MC. 2008. Functional differences in the growth hormone and insulin-like growth factor axis in cattle and pigs: implications for post-partum nutrition and reproduction. Reprod Dom Anim. 43:31–39.
- Lucy MC. 2011. Growth hormone regulation of follicular growth. Reprod Fertil Dev. 24:19–28.
- Lucy MC, Bilby CR, Kirby CJ, Yuan W, Boyd CK. 1999. Role of growth hormone in development and maintenance of follicles and corpora lutea. J Reprod Fertil Suppl. 54:49–59.
- Lucy MC, Butler ST, Garverick HA. 2014. Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. Animal. 8:82–90.
- Maher AD, Hayes B, Cocks B, Marett L, Wales WJ, Rochfort SJ. 2013. Latent biochemical relationships in the blood-milk metabolic axis of dairy cows revealed by statistical integration of 1H NMR spectroscopic data. J Proteome Res. 12:1428–1435.
- Marteniuk JV, Herdt TH. 1988. Pregnancy toxemia and ketosis of ewes and does. Vet Clin N Am Food Anim Pract. 4:307–315.
- Matoba S, Bender K, Fahey AG, Mamo S, Brennan L, Lonergan P, Fair T. 2014. Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod Fertil Dev. 26:337–345.
- McCabe M, Waters S, Morris D, Kenny D, Lynn D, Creevey C. 2012. RNA-seq analysis of differential gene expression in liver from lactating dairy cows divergent in negative energy balance. BMC Genomics. 13:193.
- McRae C, Sharma V, Fisher J. 2013. Metabolite profiling in the pursuit of biomarkers for IVF outcome: the case for metabolomics studies. Int J Reprod Med. 2013:1.
- Meikle A, de Brun V, Carriquiry M, Soca P, Sosa C, Adrien ML, Chilibroste P, Abecia JA. 2018. Influences of nutrition and metabolism on reproduction of the female ruminant. Anim Reprod. 15:899–911.
- Miettinen PVA, Setala JJ. 1993. Relationship between subclinical ketosis, milk production and fertility in Finnish dairy cattle. Prev Vet Med. 17:1–8.
- Mokhtari A, Kafi M, Zamiri MJ, Akbari R. 2016. Factors affecting the size of ovulatory follicles and conception rate in high-yielding dairy cows. Theriogenology. 85:747–753.
- Moore SG, O'Gorman A, Brennan L, Fair T, Butler ST. 2017. Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility. Reprod Fertil Dev. 29:658–669.
- Mozduri Z, Bakhtiarizadeh MR, Salehi A. 2018. Integrated regulatory network reveals novel candidate regulators in the development of negative energy balance in cattle. Animal. 12:1196–1207.
- Munoz M, Uyar A, Correia E, Diez C, Fernandez-Gonzalez A, Caamano JN, Martinez-Bello D, Trigal B, Humblot P, Ponsart C, et al. 2014a. Prediction of pregnancy viability in bovine in vitro-produced embryos and recipient plasma with Fourier transform infrared spectroscopy. J Dairy Sci. 97:5497–5507.
- Munoz M, Uyar A, Correia E, Diez C, Fernandez-Gonzalez A, Caamano JN, Trigal B, Carrocera S, Seli E. 2014b. Non-invasive assessment of embryonic sex in cattle by metabolic fingerprinting of in vitro culture medium. Metabolomics. 10:443–451.
- Munoz M, Uyar A, Correia E, Ponsart C, Guyader-Joly C, Martinez-Bello D, Guienne BML, Fernandez-Gonzalez A, Carrocera S, Martin D, et al. 2014c. Metabolomic prediction of pregnancy viability in superovulated cattle embryos and recipients with Fourier transform infrared spectroscopy. BioMed Res Int. 1–8. doi:10.1155/2014/608579
- Nel-Themaat L, Nagy ZP. 2011. A review of the promises and pitfalls of oocyte and embryo metabolomics. Placenta. 32:S257–S263.
- O’Callaghan TF, Vazquez-Fresno R, Serra-Cayuela A, Dong E, Mandal R, Hennessy D, McAuliffe S, Dillon P, Wishart DS, Stanton C, et al. 2018. Pasture feeding changes the bovine rumen and milk metabolome. Metabolites. 8:27.
- O’Doherty AM, O’Gorman A, al Naib A, Brennan L, Daly E, Duffy P, Fair T. 2014. Negative energy balance affects imprint stability in oocytes recovered from postpartum dairy cows. Genomics. 104:177–185.
- Overton TR, McArt JAA, Nydam DV. 2017. A 100-year review: metabolic health indicators and management of dairy cattle. J Dairy Sci. 100:10398–10417.
- Pantophlet AJ, Roelofsen H, de Vries MP, Gerrits WJJ, van den Borne JJGC, Vonk RJ. 2017. The use of metabolic profiling to identify insulin resistance in veal calves. PLoS ONE. 12:e0179612.
- Patti GJ, Yanes O, Siuzdak G. 2012. Innovation: metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 13:263–269.
- Pinero-Sagredo E, Nunes S, de los Santos MJ, Celda B, Esteve V. 2010. NMR metabolic profile of human follicular fluid. NMR Biomed. 23:485–495.
- Roche JR, Bell AW, Overton TR, Loor JJ. 2013. Nutritional management of the transition cow in the 21st century – a paradigm shift in thinking. Anim Prod Sci. 53:1000–1023.
- Roche JR, Burke CR, Crookenden MA, Heiser A, Loor JJ, Meier S, Mitchell MD, Phyn CVC, Turner SA. 2018. Fertility and the transition dairy cow. Reprod Fertil Dev. 30:85–100.
- Roche JR, Burke CR, Meier S, Walker GG. 2011. Nutrition x reproduction interaction in pasture-based systems: is nutrition a factor in reproductive failure? Anim Prod Sci. 51:1045–1066.
- Roche JR, Friggens NC, Kay JK, Fisher MW, Stafford KJ, Berry DP. 2009. Invited review: body condition score and its association with dairy cow productivity, health, and welfare. J Dairy Sci. 92:5769–5801.
- Roche JR, Macdonald KA, Burke CR, Lee JM, Berry DP. 2007. Associations among body condition score, body weight, and reproductive performance in seasonally-calving dairy cattle. J Dairy Sci. 90:376–391.
- Rodgaard T, Heegaard PMH, Callesen H. 2015. Non-invasive assessment of in-vitro embryo quality to improve transfer success. Reprod Biomed Online. 31:585–592.
- Rook JS. 2000. Pregnancy toxemia of ewes, does, and beef cows. Vet Clin N Am Food Anim Pract. 16:293–317.
- Ryan EP, Heuberger AL, Broeckling CD, Borresen EC, Tillotson C, Prenni JE. 2013. Advances in nutritional metabolomics. Curr Metabolomics. 1:109–120.
- Saleem F, Ametaj BN, Bouatra S, Mandal R, Zebeli Q, Dunn SM, Wishart DS. 2012. A metabolomics approach to uncover the effects of grain diets on rumen health in dairy cows. J Dairy Sci. 95:6606–6623.
- Saleem F, Bouatra S, Guo AC, Psychogios N, Mandal R, Dunn SM, Ametaj BN, Wishart DS. 2013. The bovine ruminal fluid metabolome. Metabolomics. 9:360–378.
- Samadi F, Blache D, Martin GB, D'Occhio MJ. 2014. Nutrition, metabolic profiles and puberty in Brahman (Bos indicus) beef heifers. Anim Reprod Sci. 146:134–142.
- Samadi F, Phillips NJ, Blache D, Martin GB, D'Occhio MJ. 2013. Interrelationships of nutrition, metabolic hormones and resumption of ovulation in multiparous suckled beef cows on subtropical pastures. Anim Reprod Sci. 137:137–144.
- Sartori R, Gimenes LU, Monteiro PLJ Jr, Melo LF, Baruselli PS, Bastos MR. 2016. Metabolic and endocrine differences between Bos taurus and Bos indicus females that impact the interaction of nutrition with reproduction. Theriogenology. 86:32–40.
- Scharen M, Frahm J, Kersten S, Meyer U, Hummel J, Breves G, Danicke S. 2018. Interrelations between the rumen microbiota and production, behavioral, rumen fermentation, metabolic, and immunological attributes of dairy cows. J Dairy Sci. 101:4615–4637.
- Shaw JC. 1956. Ketosis in dairy cattle. A review. J Dairy Sci. 39:402–434.
- Silva JRV, Figueiredo JR, van den Hurk R. 2009. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology. 71:1193–1208.
- Singh R, Sinclair KD. 2007. Metabolomics: approaches to assessing oocyte and embryo quality. Theriogenology. 68:S56–S62.
- Sirard M-A. 2011. Follicle environment and quality of in vitro matured oocytes. J Assist Reprod Genet. 28:483–488.
- Spicer LJ, Aad PY. 2007. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 77:18–27.
- Spicer LJ, Echternkamp SE. 1995. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest Anim Endocrinol. 12:223–245.
- Sun J, Monagas M, Jang S, Molokin A, Harnly JM, Urban JF Jr, Solano-Aguilar G, Chen P. 2015a. A high fat, high cholesterol diet leads to changes in metabolite patters in pigs – a metabolomic study. Food Chem. 173:171–178.
- Sun H-Z, Wang D-M, Wang B, Wang J-K, Liu H-Y, Guan LL, Liu J-X. 2015b. Metabolomics of four biofluids from dairy cows: potential biomarkers for milk production and quality. J Proteome Res. 14:1287–1298.
- Sun LW, Zhang HY, Wu L, Shu S, Xia C, Xu C, Zheng JS. 2014. 1H-Nuclear magnetic resonance-based plasma metabolic profiling of dairy cows with clinical and subclinical ketosis. J Dairy Sci. 97:1552–1562.
- Sundekilde UK, Poulsen NA, Larsen LB, Bertram HC. 2013. Nuclear magnetic resonance metabonomics reveals strong association between milk metabolites and somatic cell count in bovine milk. J Dairy Sci. 96:290–299.
- Sundstrum A. 2015. Metabolic disorders in the transition period indicate that the dairy cow’s ability to adapt is overstressed. Animals. 5:978–1020.
- Uhde K, van Tol HTA, Stout TAE, Roelen BAJ. 2018. Metabolomic profiles of bovine cumulus cells and cumulus–oocyte-complex-conditioned medium during maturation in vitro. Nat Sci Rep. 8:9477.
- Uyar A, Munoz M, Diez C, Caamano JN, Seli E, Gomez E. 2013. Non-invasive prediction of embryo sex in cattle by metabolomics analysis of in vitro culture medium with Fourier transform infrared spectroscopy. Fertil Steril. 100:S483.
- Uyar A, Seli E. 2014. Metabolomic assessment of embryo viability. Semin Reprod Med. 32:141–152.
- Velazquez MA, Spicer LJ, Wathes DC. 2008. The role of endocrine insulin-like growth factor-I (IGF-I) in female bovine reproduction. Domest Anim Endocrinol. 35:325–342.
- Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. 2012. An investigation into the relationship between the metabolomics profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil Steril. 97:1078–1084.
- Walsh RB, Walton JS, Kelton DF, LeBlanc SJ, Leslie KE, Duffield TF. 2007. The effect of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J Dairy Sci. 90:2788–2796.
- Wandji SA, Pelletier G, Sirard MA. 1992. Ontogeny and cellular localization of 125I-labeled insulin-like growth factor-I, 125I-labeled follicle-stimulating hormone, and 125I-labeled human chorionic gonadotropin binding sites in ovaries from bovine fetuses and neonatal calves. Biol Reprod. 47:814–822.
- Wang Q, Wang K, Wu W, Giannoulatou E, Ho JWK, Li L. 2019. Host and microbiome multi-omics integration: applications and methodologies. Biophys Rev. 11:55–65.
- White HM. 2015. The role of TCA cycle anaplerosis in ketosis and fatty liver in periparturient dairy cows. Animals (Basel). 5:793–802.
- Widmann P, Reverter A, Fortes MRS, Weikard R, Suhre K, Hammon H, Albrecht E, Kuehn C. 2013. A systems biology approach using metabolomics data reveals genes and pathways interacting to modulate divergent growth in cattle. BMC Genomics. 14:798.
- Widmann P, Reverter A, Weikard R, Suhre K, Hammon HM, Albrecht E, Kuehn C. 2015. Systems biology analysis merging phenotype, metabolic and genomic data identifies non-SMC condensing I complex, subunit G (NCAPG) and cellular maintenance processes as major contributors to genetic variability in bovine feed efficiency. PLoS ONE. 10:e0124574.
- Woad KJ, Baxter G, Hogg CO, Bramley TA, Webb R, Armstrong DG. 2000. Expression of mRNA encoding insulin-like growth factors I and II and the type 1 IGF receptor in the bovine corpus luteum at defined stages of the oestrous cycle. J Reprod Fertil. 120:293–302.
- Woelders H, Te Pas MFW, Bannink A, Veerkamp RF, Smits MA. 2011. Systems biology in animal sciences. Animal. 5:1036–1047.
- Wu X, Sun H, Xu M, Wang D, Guan LL, Liu J. 2018. Serum metabolome profiling revealed potential biomarkers for milk protein yield in dairy cows. J Proteom. 184:54–61.
- Yuan W, Bao B, Garverick HA, Youngquist RS, Lucy MC. 1998. Follicular dominance in cattle is associated with divergent patterns of ovarian gene expression for insulin-like growth factor (IGF)-I, IGF-II, and IGF binding protein-2 in dominant and subordinate follicles. Dom Anim Endocrinol. 15:55–63.
- Zhang X, Davis ME, Moeller SJ, Ottobre JS. 2013. Effects of selection for blood serum IGF-I concentration on reproductive performance of female Angus beef cattle. J Anim Sci. 91:4104–4115.
- Zhang G, Deng Q, Mandal R, Wishart DS, Ametaj BN. 2017. DI/LC-MS/MS-based metabolic profiling for identification of early predictive serum biomarkers of metritis in transition dairy cows. J Agric Food Chem. 65:8510–8521.
- Zhang L, Jiang S, Wozniak PJ, Yang X, Godke RA. 1995. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol Reprod Dev. 40:338–344.
- Zhang H, Wu L, Xu C, Xia C, Sun L, Shu S. 2013. Plasma metabolomics profiling of dairy cows affected with ketosis using gas chromatography/mass spectrometry. BMC Vet Res. 9:186.
- Zhang R, Zhu W, Jiang L, Mao S. 2017. Comparative metabolome analysis of ruminal changes in Holstein dairy cows fed low- or high-concentrate diets. Metabolomics. 13:74.
- Zhao S, Zhao J, Bu D, Sun P, Wang J, Dong Z. 2014. Metabolomics analysis reveals large effect of roughage types on rumen microbial metabolic profile in dairy cows. Lett Appl Microbiol. 59:79–85.