Abstract
The aim of study was to evaluate whether butafosfan, associated or not with cyanocobalamin, alters the metabolic profile, hemogasometrics and acute phase proteins in recent postpartum dairy cows. Twenty-five cows (n = 25) were selected for study and divided into three groups: Butafosfan (BUT, n = 9); Butafosfan + Cyanocobalamin (BUTC, n = 9); and Control (CTL, n = 7). Treatments were applied daily from calving until 5 days postpartum. Blood samples for hemogasometry were performed on days 0, 1, 2 and 3, and for other metabolites on days 0, 1, 2, 3, 4, 5, 10, 15, 20 and 28 postpartum. Blood pH was lower in the BUT and BUTC groups than in the CTL group (p = .03) and carbon dioxide pressure was higher in the BUT and BUTC groups compared to CTL (p = .01). In addition, ionised calcium levels were higher in the BUT and BUTC groups (p = .0003) in comparison to CTL, whereas the BUT group had higher levels. The BUT group had the lowest levels of paraoxonase (p = .03). On the other hand, glucose levels tended to be highest in the BUT group (p = .08). The butafosfan with or without cyanocobalamin increased ionised calcium levels and tended to stabilise blood pH in the early post-partum cows, and butafosfan without cyanocobalamin tended to increase blood glucose levels.
Treated animals increase serum ionised calcium;
Treated animals have a reduction in blood pH;
Butafosfan tends to increase the glucose concentration.
Highlights
Introduction
The peripartum period, which comprises the 3 weeks before and after calving, is a challenging time for the dairy cow, and maintaining health and productivity during this period is one of the most difficult tasks in dairy production. It is known that most of the disorders that affect dairy cows occur in the recent postpartum period, the main diseases being displacement of the abomasum, hypocalcaemia, retention of the placenta, metritis, mastitis and ketosis (Burton et al. Citation2000; Martinez et al. Citation2012). During peripartum, the animal experiences intense metabolic and nutritional modifications, in addition to an increase in energetic requirements due to the growth of the foetus and the start of lactation, all accompanied by a decrease in dry matter intake (Chapinal et al. Citation2012). This imbalance leads to a negative energy balance (NEB), which is aggravated by foetal metabolic demands and also by the prioritisation of nutrients for the mammary gland (Leroy et al. Citation2008).
One of the main disorders of this period is ketosis, which is characterised by a high rate of fat mobilisation and leads to an increase in ketone bodies to toxic levels (Garro et al. Citation2014). Several factors can lead to the occurrence of this disorder, such as number of lactations, management and body condition score at calving (Nielen et al. Citation1994; Duffield et al. Citation2009). Several strategies have been developed to combat this condition, one of which is the use of butafosfan, a source of organic phosphorus, associated with cyanocobalamin, a variant of cobalamin (Vitamin B12) that is able to reduce fat mobilisation, as demonstrated by a reduction in levels of β-hydroxybutyrate (BHBA) and non-esterified fatty acids (NEFA), and increases the ingestion of dry matter (Pereira et al. Citation2013).
Beside this effect on energy metabolism, phosphorus may have the ability to stabilise blood pH through the phosphate-phosphoric acid system, which acts in the transport of sodium and hydrogen ions, being a potent acid-base balance stabiliser (Almosny Citation2003). It is of paramount importance to maintain this postpartum balance, because the alkalosis caused by an increase in bicarbonate retention (Ortolani Citation2003) can lead to a disruption of the parathyroid hormone receptor. On the other hand, in cases of mild acidosis, this receptor is stimulated (Campion et al. Citation2015), leaving the animal more prepared for the challenges of the peripartum period.
Cyanocobalamin is important for the formation of adenosine triphosphate (ATP) in ruminants, transforming methylmalonyl-CoA, a product of the transformation of propionate, into succinyl-CoA, which then enters the Krebs cycle (Girard and Matte Citation2005). In addition, it acts together with methionine synthase, which is important for methionine regeneration (Bässler Citation1997). However, its effect during the transition period of the dairy cow is not yet well understood.
During the peripartum period, an increase in serum NEFA causes a reduction in the immunological functionality of the animal, because these compounds can bind to the receptors that trigger inflammatory responses (Sordillo et al. Citation2009). Acute-phase proteins have been widely used as biomarkers that can assist in the determination of inflammatory and immunological conditions in the transition period (Eckersall and Bell Citation2010). Changes in these proteins are associated with several diseases such as uterine infections (Schneider et al. Citation2013), reduction in conception rate (Nightingale et al. Citation2015) and fatty liver and chronic liver damage (Bionaz et al. Citation2007) and can therefore be used to obtain an early diagnosis of the immune status of the animal (Reis et al. Citation2016). The association of butafofan and cyanocobalamin, favouring energy metabolism, may improve immunity in the animal by reducing NEFA levels (Pereira et al. Citation2013).
The aim of the study was to verify whether butafosfan associated or not with cyanocobalamin acts on metabolic parameters, hemogasometric and acute phase proteins in Holstein cows in the recent postpartum period.
Materials and methods
This study was approved by the Ethics Committee on Animal Experimentation of the Federal University of Pelotas (CEEA-UFPel) and is registered under number 1152.
The study was conducted at a commercial dairy farm, located in the municipality of Rio Grande, Rio Grande do Sul, Brazil, at the geographic coordinates 32° 16 'S, 52° 32' L. Primparous Holstein cows (n = 25), with an mean age of 3 years and body condition score (BCS) soon after calving in the range of 2.25 to 3.25, according to the scale described by Edmonson et al. (Citation1989), were used for the study. All animals were subjected to the same conditions of semiextensive management and received a standardised diet with 40% concentrate (composed of 35% soybean hull, 30% sorghum, 17% rice bran, 13% soybean meal, 4% mineral salt and 1% urea) and 60% of bulky (ryegrass hay), offered twice a day after milking. The animals had an average milk yield of 19.5 L milk/day.
After calving, the animals were divided into three groups: Butafosfan Group (BUT, n = 9), which received five applications of 20 mL of aqueous butafosfan 10% (Jinan Haohua Industry Co., Ltd., China); Butafosfan Associated with Cyanocobalamin Group (BUTC, n = 9), receiving five applications of 20 mL of commercial product based on 10% butafosfan and 5 mg of cyanocobalamin (Catosal® B12, Bayer Health Care, São Paulo, Brazil); and Control Group (CTL, n = 7), receiving five applications of 20 mL of NaCl at 0.9%. The treatments were administered intramuscularly daily from birth to the fourth day postpartum.
Blood samples for biochemical analysis were collected using the Vacutainer system (BD Diagnóstics, São Paulo, Brazil), using tubes containing clot activator. Blood was collected by puncture in the coccygeal vein, after milking before feeding, on the day of calving (day 0), daily on the first five days postpartum (days 1, 2, 3, 4, 5) and on days 10, 15, 20 and 28 postpartum, for the analysis of total calcium (Cat), phosphorus (P), beta-hydroxybutyrate (BHBA) and non-esterified fatty acids (NEFA). Likewise, samples were collected on days 0, 3, 5, 10, 15, 20 and 28 for analysis of cholesterol, bilirubin, albumin, haptoglobin and paraoxonase (PON-1).
Concentrations of total calcium, phosphorus, cholesterol, bilirubin, albumin (Labtest Diagnóstica S.A., Brazil) and NEFA (HR Series NEFA-HR (2), Wako Pure Chemical Industries, Ltd., Osaka, Japão) were measured using commercial kits by colorimetry, and the analysis of BHBA was performed using the method described by Ballou et al. (Citation2009) and a commercial kit (Ranbut, Randox, Oceanside, CA). To evaluate the activity of PON-1, the protocol described by Browne et al. (Citation2007) was used. The haptoglobin concentration was measured using the colorimetric method described by Jones and Mould (Citation1984), and adapted by Schneider et al. (Citation2013).
Five animals from each group were randomly selected for each evaluation of blood gas indices. Samples were collected into tubes containing heparin (BD Diagnóstics, São Paulo, Brazil) on days 0, 1, 2 and 3 after calving. The collected blood was analysed using the i-STAT®t1 Analyser (Abbott Point of Care Inc., Illinois, United States), which uses a CG8+ cartridge (Abbott Point of Care Inc., Illinois, United States) to evaluate pH, O2 pressure (pO2), CO2 pressure (pCO2), total CO2 (tCO2), bicarbonate, base excess (BE), O2 saturation (sO2), sodium, potassium, ionised calcium, glucose, haematocrit (HCT) and haemoglobin (HGB).
Statistical analyses were performed in the SAS Studio (SAS® Institute Inc., Cary, NC, EUA, 2014). The measured parameters were evaluated by analysis of variance (ANOVA) using the MIXED MODELS procedure, evaluating the effects of group, time and their interaction. To verify the normality of the data, the Shapiro–Wilk test was used, being considered as normal values of p > .90, if any data did not present normality was used log. The means were compared by the Tukey–Kramer method and values with p < .05 were considered significant.
Results
Regarding hemogasometry, the study showed tended of lower pH and higher pCO2 values in the BUTC and BUT groups compared to the CTL group (p = .07 and p = .01, respectively, Table ), but there was no significant difference between them. Differences in bicarbonate were observed on day 0, and the means of the BUTC and CTL groups were higher than that of the BUT group (p = .03). The values of pO2, BE, sO2, HCT and HGB did not differ significantly (p > .05; Table ).
Table 1. Mean (±standard error of the mean) of hemogasometrics, metabolic and acute phase protein parameters of dairy cows supplemented with butafosfan associated or not with cyanocobalamin during recent postpartum period.
Table 2. Mean (±standard error of the mean) in the days of pH, Carbon Dioxide Pressure and ionised calcium of dairy cows supplemented with butafosfan associated or not with cyanocobalamin during recent postpartum period.
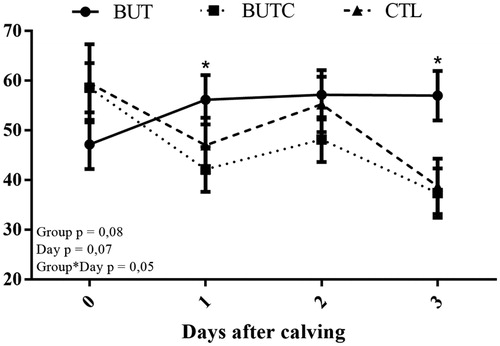
There was a tendency (p = .08) for glucose to be higher in the BUT group than in the BUTC and CTL groups (Table ). However, there was a significant interaction between group and day for glucose (p = .05), demonstrating that the BUT group had a higher concentration on day 1 in relation to BUTC, and on day 3 in relation to BUTC and CTL (Figure ). The mean of the BUT group was higher than those of the BUTC and CTL groups, and the BUTC group had higher levels of ionised calcium than the CTL group (p < .01, Table ). BHBA, NEFA, cholesterol, albumin, bilirubin and other minerals did not differ between groups (p > .05; Table ).
Figure 1. Serum glucose levels of dairy cows supplemented with butafosfan associated or not with cyanocobalamin in the recent postpartum period. Butafosfan (○ - BUT), Butafosfan + Cyanocobalamin (● - BUTC) and Control (△ - CTL). *p<.05.
Lower levels (p = .03) of PON-1 were found in the BUT group compared to BUTC and CTL, which did not differ from each other. There was no difference between groups for haptoglobin levels (p > .05; Table ).
Discussion
The beneficial effects of butafosfan in combination with cyanocobalamin during dairy cow peripartum have been studied for many years (Rollin et al. Citation2010; Pereira et al. Citation2013; Nuber et al. Citation2016). It is not yet known by which metabolic pathway these substances act, however, Fürll et al. (Citation2010) reported that this combination reduced the NEB, confirmed by a reduction in the levels of BHBA and NEFA. However, the effect of cyanocobalamin on this metabolic response is unclear, and there are no studies to determine whether butafosfan with or without the association of cyanocobalamin may alter the hemogasometry of dairy cows in the transition period.
All groups were able to maintain pH levels within the physiological range, however the control group had higher values than the BUT and BUTC groups, as shown in Table . This may be due to higher levels of pCO2 in the BUT and BUTC groups, in addition to butafosfan being a source of organic phosphorus. In addition, phosphate, one of the major plasma acidifiers, contained in the butaphosphan molecule, may have aided in acidifying the plasma, increasing bone demineralisation. Some studies report that increased phosphorous may cause increased calcium excretion in the urine (Remer and Manz Citation1994; Sebastian et al. Citation2002); however, a meta-analysis has shown that increased neutral/alkaline phosphate may reduce calcium excretion, causing an increase in calcium balance (Fenton et al. Citation2009). Moreover, a reduction in blood pH prevents hypocalcaemia, because mild acidosis increases parathyroid hormone-mediated bone resorption activity, increasing the activity of its calcium receptor (Campion et al. Citation2015).
An increase in pH changes the conformation of the bone and kidney receptor for parathyroid hormone, thus reducing calcium reabsorption and leaving the animals more susceptible to calcium-related disorders, since maintenance of calcium levels at the time of calving is a challenge (Goff Citation2008), also the low pH can reduce calcium binding with calcium binding proteins, increasing ionised calcium (Alexander et al. Citation2016). In this study, the highest levels of ionised calcium were found in the BUT and BUTC groups, probably due to the lower blood pH in these groups versus the control, which may have caused better activation of the PTH receptors (Goff Citation2014) and realese of calcium of calcium binding proteins.
During the recent postpartum period, the large amount of energy required by the animal for lactogenesis causes their body to devise strategies to compensate for the large loss of energy, exacerbated by reduced dry matter intake (Drackley et al. Citation2001). Reducing glucose availability causes lipid mobilisation for energy production, thereby increasing levels of BHBA and NEFA (Drackley et al. Citation2001; Adewuyi et al. Citation2005). The results of this work demonstrated that the BUT group was able to maintain constant glucose levels. This may be due to a greater amount of glucagon, the hormone responsible for the production and release of glucose by the liver, which is influenced by the administration of butafosfan, as documented by Nuber et al. (Citation2016). Phosphorus supplied by butafosfan may be important for the activation of glycogenolysis, being a source of substrate for the formation of glucose-1-phosphate that can be converted to ATP by glycolysis and the citric acid cycle, as well as for glycogen replacement in liver, in addition to supplying inorganic phosphorus for the conversion of adenosine diphosphate (ADP) to ATP (Nelson and Cox Citation2014). The other metabolites did not differ between groups.
For PON-1, a negative acute-phase protein (Feingold et al. Citation1998) that is related to various inflammatory processes such as uterine infections (Schneider et al. Citation2013) and the induction of an inflammatory condition in the peripartum period (Drackley et al. Citation2005), the findings of this study demonstrated that the group BUT had lower values than the control group. However, no animal presented any disease during the study period, with similar values to those in healthy animals, as described by Schneider et al. (Citation2013).
Conclusions
The use of butafosfan, with or without cyanocobalamin, is a good alternative for metaphylaxis of the damage caused by the negative energy balance in the postpartum period, providing stabilisation of the blood pH, increased the serum levels of ionised calcium and glucose, thus helping to minimise the metabolic disorders that could arise during this period. With the promising results obtained, more studies should be conducted with a larger number of animals and with different number of lactations.
Acknowledgements
Thanks to the Núcleo de Pesquisa, Ensino e Extensão em Pecuária for the opportunity, Farm 4 Irmãos for providing the animals.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adewuyi A, Gruys E, Van Eerdenburg F. 2005. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet Q. 27:117–126.
- Alexander RT, Cordat E, Chambrey R, Dimke H, Eladari D. 2016. Acidosis and urinary calcium excretion: insights from genetic disorders. J Am Soc Nephrol. 27:3511–3520.
- Almosny N. 2003. Equilíbrio ácido-básico em medicina veterinária. Anais Do I Simpósio de Patologia Clínica Veterinária da Região Sul Do Brasil. 01:5–16.
- Ballou M, Gomes R, Juchem S, DePeters E. 2009. Effects of dietary supplemental fish oil during the peripartum period on blood metabolites and hepatic fatty acid compositions and total triacylglycerol concentrations of multiparous Holstein cows. J Dairy Sci. 92:657–669.
- Bässler K. 1997. Enzymatic effects of folic acid and vitamin B12. Int J Vitam Nutr Res. 67:385–388.
- Bionaz M, Trevisi E, Calamari L, Librandi F, Ferrari A, Bertoni G. 2007. Plasma paraoxonase, health, inflammatory conditions, and liver function in transition dairy cows. J Dairy Sci. 90:1740–1750.
- Browne RW, Koury ST, Marion S, Wilding G, Muti P, Trevisan M. 2007. Accuracy and biological variation of human serum paraoxonase 1 activity and polymorphism (Q192R) by kinetic enzyme assay. Clin Chem. 53:310–317.
- Burton J, Madsen S, Yao J, Sipkovsky S, Coussens P. 2000. An immunogenomics approach to understanding periparturient immunosuppression and mastitis susceptibility in dairy cows. Acta Veterinaria Scandinavica. 42:407–424.
- Campion KL, McCormick WD, Warwicker J, Khayat ME, Atkinson-Dell R, Steward MC, Delbridge LW, Mun HC, Conigrave AD, Ward DT. 2015. Pathophysiologic changes in extracellular ph modulate parathyroid calcium-sensing receptor activity and secretion via a histidine-independent mechanism. J Am Soc Nephrol. 26:2163–2171.
- Chapinal N, Carson M, LeBlanc S, Leslie K, Godden S, Capel M, Santos J, Overton M, Duffield T. 2012. The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. J Dairy Sci. 95:1301–1309.
- Drackley JK, Dann HM, Douglas GN, Guretzky NAJ, Litherland NB, Underwood JP, Loor JJ. 2005. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Italian J Anim Sci. 4:323–344.
- Drackley JK, Overton TR, Douglas GN. 2001. Adaptations of glucose and long-chain fatty acid metabolism in liver of dairy cows during the periparturient period. J Dairy Sci. 84:E100–E112.
- Duffield T, Lissemore K, McBride B, Leslie K. 2009. Impact of hyperketonemia in early lactation dairy cows on health and production. J Dairy Sci. 92:571–580.
- Eckersall P, Bell R. 2010. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet J. 185:23–27.
- Edmonson A, Lean I, Weaver L, Farver T, Webster G. 1989. A body condition scoring chart for Holstein dairy cows. J Dairy Sci. 72:68–78.
- Feingold KR, Memon RA, Moser AH, Grunfeld C. 1998. Paraoxonase activity in the serum and hepatic mRNA levels decrease during the acute phase response. Atherosclerosis. 139:307–315.
- Fenton TR, Lyon AW, Eliasziw M, Tough SC, Hanley DA. 2009. Phosphate decreases urine calcium and increases calcium balance: a meta-analysis of the osteoporosis acid-ash diet hypothesis. Nutr J. 8:41.
- Fürll M, Deniz A, Westphal B, Illing C, Constable P. 2010. Effect of multiple intravenous injections of butaphosphan and cyanocobalamin on the metabolism of periparturient dairy cows. J Dairy Sci. 93:4155–4164.
- Garro C, Mian L, Cobos Roldan M. 2014. Subclinical ketosis in dairy cows: prevalence and risk factors in grazing production system. J Anim Physiol Anim Nutr. 98:838–844.
- Girard C, Matte J. 2005. Effects of intramuscular injections of vitamin B12 on lactation performance of dairy cows fed dietary supplements of folic acid and rumen-protected methionine. J Dairy Sci. 88:671–676.
- Goff JP. 2008. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. The Vet J. 176:50–57.
- Goff JP. 2014. Calcium and magnesium disorders. Vet Clin North Am Food Anim Pract. 30:359–381.
- Jones G, Mould D. 1984. Adaptation of the guaiacol (peroxidase) test for haptoglobins to a microtitration plate system. Res Vet Sci. 37:87.
- Leroy J, Vanholder T, Van Knegsel A, Garcia‐Ispierto I, Bols P. 2008. Nutrient prioritization in dairy cows early postpartum: mismatch between metabolism and fertility? Reprod Domest Anim. 43:96–103.
- Martinez N, Risco C, Lima F, Bisinotto R, Greco L, Ribeiro E, Maunsell F, Galvão K, Santos J. 2012. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J Dairy Sci. 95:7158–7172.
- Nelson DL, Cox MM. 2014. Veiga ABGd, Consiglio AR, Termignoni CDC et al., translators. Artmed, editor. Princípios de Bioquímica de Lehninger. 6° ed. Vol. 6a Edição. Porto Alegre.
- Nielen M, Aarts M, Jonkers A, Wensing T, Schukken YH. 1994. Evaluation of two cowside tests for the detection of subclinical ketosis in dairy cows. Can Vet J. 35:229.
- Nightingale CR, Sellers MD, Ballou MA. 2015. Elevated plasma haptoglobin concentrations following parturition are associated with elevated leukocyte responses and decreased subsequent reproductive efficiency in multiparous Holstein dairy cows. Vet Immunol Immunopathol. 164:16–23.
- Nuber U, Dorland H, Bruckmaier R. 2016. Effects of butafosfan with or without cyanocobalamin on the metabolism of early lactating cows with subclinical ketosis. J Anim Physiol Anim Nutr. 100:146–155.
- Ortolani EL. 2003. Diagnóstico e tratamento de alterações ácido-básicas em ruminantes. Simpósio de Patologia Clínica Veterinária da Região Sul Do Brasil. 1:17–29.
- Pereira RA, Fensterseifer S, Barcelos VB, Martins CF, Schneider A, Schmitt E, Pfeifer LFM, Del Pino FAB, Corrêa MN. 2013. Metabolic parameters and dry matter intake of ewes treated with butaphosphan and cyanocobalamin in the early postpartum period. Small Rumin Res. 114:140–145.
- Reis J, Madureira K, Silva C, Baldacim V, Fagliari J, Gomes V. 2016. Perfil sérico proteico de vacas Holandesas no período de transição. Arq Bras Med Vet Zootec. 68:587–595.
- Remer T, Manz F. 1994. Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr. 59:1356–1361.
- Rollin E, Berghaus R, Rapnicki P, Godden S, Overton M. 2010. The effect of injectable butaphosphan and cyanocobalamin on postpartum serum beta-hydroxybutyrate, calcium, and phosphorus concentrations in dairy cattle. J Dairy Sci. 93:978–987.
- Schneider A, Corrêa M, Butler W. 2013. Short communication: acute phase proteins in Holstein cows diagnosed with uterine infection. Res Veterinary Sci. 95:269–271.
- Sebastian A, Frassetto LA, Sellmeyer DE, Merriam RL, Morris RC. Jr 2002. Estimation of the net acid load of the diet of ancestral preagricultural Homo sapiens and their hominid ancestors. Am J Clin Nutr. 76:1308–1316.
- Sordillo LM, Contreras G, Aitken SL. 2009. Metabolic factors affecting the inflammatory response of periparturient dairy cows. Animal Health Res Rev. 10:53–63.
