Abstract
Diffuse peritonitis is a potential fatal inflammation and considered the important common leading cause of peritonitis-related death in ruminants worldwide. This study was designed to find suitable biomarkers aid in diagnosis and differentiation between different causes of diffuse peritonitis in buffaloes. Clinical and ultrasonographic examinations were applied as well as blood samples were collected for biochemical analysis. Based on necropsy findings, diffuse peritonitis was found to be of digestive origin (traumatic reticuloperitonitis—TRP or perforated abomasum/intestine), or of non-digestive origin from ruptured uterus. Serum total protein (TP), globulins, Malondialdehyde and plasma fibrinogen were significantly increased in both digestive and non-digestive origins. Meanwhile, serum pepsinogen was significantly elevated in diffuse peritonitis associated with perforated abomasum and intestine. Serum haptoglobin (Hp) showed a highly significant increase in TRP rather than other groups. On the other hand, nitric oxide (NO) and catalase showed non-significant changes in all groups of diffuse peritonitis in buffaloes. In conclusion, our results suggested that Hp and pepsinogen could enable the veterinarians for better diagnosis of the different causes of diffuse peritonitis in buffaloes.
The current study suggested that Hp and pepsinogen could be a diagnostic markers for the differentiation between different causes of diffuse peritonitis in buffaloes, while, NO and CAT were of no value.
However estimation of Fb, globulins, Tp and MDA help in the diagnosis of diffuse peritonitis but it cannot help in the differentiation between different causes of diffuse peritonitis in buffaloes.
Highlights
Introduction
Peritonitis is defined as acute or chronic inflammation which affect the peritoneal cavity, it may be primary disease or secondary to many other pathological conditions also it may appear as localised or generalised inflammation (Andrews et al. Citation2008). In Egyptian buffaloes, diffuse peritonitis was recorded as a sequelae of traumatic reticuloperitonitis (TRP) (Abdelaal et al. Citation2009). This problem is considered a main pathological condition in the Egyptian small farms due to poor farm management. It is caused by ingestion of penetrating metallic foreign bodies in the reticulum and leakage of ingested material from a perforated abomasal ulcer or intestine (Mushonga et al. Citation2015). In dairy herds, this condition was documented as a secondary complication to abomasal disorders such as left and right abomasal displacement, abomasal ulcers and impaction or perforated intestine and ruptured uterus (Andrews et al. Citation2008). Bad management, stress and nutritional disorders are considered the major risk factors for such problem (Constable et al. Citation2016).
Peritonitis causes an extraordinary reduction in milk production and an increase in the number of cattle deaths, which results in severe economic losses (Constable et al. Citation2016). The clinical signs of peritonitis which result from toxaemia and gastrointestinal paresis are abdominal rigidity, tenderness, abdominal distension, fever, anorexia, sudden reduction in milk production, ruminal atony, scanty faeces and abdominal pain (Smith Citation2014). Ultrasonography is a common technique for diagnosis of peritonitis (Braun Citation2009). Meanwhile, the concentration of haptoglobin (Hp) and fibrinogen (Fb) as acute phase proteins (APPs) may be changed under several conditions such as stress, infection and inflammation (Murata et al. Citation2004). Nitric oxide (NO) has a number of important physiological functions such as regulation of blood flow, mucosal defence, leukocyte recruitment, and participation in acute and chronic inflammation (Sharma et al. Citation2007; Ni et al. Citation2010). Also NO is an oxidative stress marker that may be increased in cases of peritonitis and associated intra-abdominal sepsis (Koksal et al. Citation2004). Catalase is an antioxidant enzyme that converts hydrogen peroxide to water and oxygen (Chelikani et al. Citation2004). Malondialdehyde (MDA) is a commonly utilised marker for overall lipid peroxidation levels and an indicator of oxidative stress (Del Rio et al. Citation2005). The blood biochemistry such as Fb, protein concentrations, and enzymatic alterations have been reported to be a useful index for the early diagnosis of TRP in cattle (Alsaad Citation2011; Ramin et al. Citation2011). The high blood level of serum pepsinogen was previously stated in cattle with gastrointestinal nematodes (Charlier et al. Citation2011) and also in sheep with abomasal dysfunction (Kataria et al. Citation2008). Conversely, few studies have been conducted to evaluate NO, APPs, oxidative biomarkers and pepsinogen in buffaloes affected by peritonitis.
Although ultrasonography considered a gold tool for diagnosis of peritonitis and could evaluate the state of reticulum (Abdelaal et al. Citation2009; Abdelaal and Floeck Citation2015), it was a poor tool to diagnose other causes of peritonitis as abomasal ulcers and perforated intestine. Moreover, ultrasonography still unavailable tool for most farms and veterinary clinics in developing countries and require well trained veterinarian. Therefore, the objective of this research was to correlate between the use of different biomarkers to differentiate between TRP, perforated abomasum/intestine and ruptured uterus as different causes of diffuse peritonitis in buffaloes.
Materials and methods
Animal selection
Thirty female Egyptian buffaloes, at the age of 3–9 years and their weight varied from 450 to 600 kg, were examined during the period from January to December 2017. Twenty diseased buffaloes were examined at the Veterinary Teaching Hospital at the Faculty of Veterinary Medicine, Zagazig University. These buffaloes were sporadic cases from small holders from different localities along Sharkia Governorate, Egypt. The animals were examined with a history of illness from 7 to 10 days. Another 10 apparently healthy buffaloes were collected from Zagazig University farm and used as control buffaloes. Clinical comparisons were conducted between diseased and control buffaloes, then abdominal ultrasonography were performed on all animals. The disease was confirmed according to the results obtained from ultrasonographic examinations. This study follows and complies with the national guidelines of the Animal Use and care committee of Zagazig University, Egypt. All the national and institutional guidelines were followed. Plus, all the procedures and analysis utilised were approved by Zagazig University (ZU-IACUC/2/F/22/2018).
Clinical examination
The groups of diseased and control buffaloes were received a thorough clinical examination via methods that have been previously described in cattle (Rosenberger Citation1990). In brief, vital signs (rectal temperature, pulse rate, respiratory rate) beside, results of auscultation of the digestive tract, respiratory tract and the heart were recorded. Pain tests, including deep palpation of the abdominal wall behind the xiphoid with pinching of the withers, the side stick method and turning at an acute angle. Positive pain tests were indicated with a grunting sound that was audible by placing a stethoscope on the trachea while the tests were being conducted. A metal detector was also employed over the ventral and ventrolateral regions of the chest and abdomen to detect ferromagnetic foreign bodies.
Abdominal ultrasonography
Ultrasonographic scanning (Sonoscape, A5V, China) of the abdominal cavity was performed using a previously described method (Kofler and Braun Citation1997) with a 3.5-MHz convex transducer and 6-MHz linear transducers after hair shaving and applying transmission gel. The transducer was first applied to the ventral part of the abdomen and then to the left and right sides of the abdomen.
Blood samples
Blood samples were collected from all buffaloes through jugular vein puncture directly after the examination of the animals. Two millilitres of blood was added to tubes containing sodium citrate for Fb estimation. Five millilitres of blood was placed in tubes without anticoagulant for serum separation. Serum was extracted by centrifugation at 1000 xg (3000 rpm) for 20 min within 2 h of collection. Serum was stored at –20 °C until evaluation.
Biochemical parameters
The serum total protein (TP) and serum albumin level were measured by a colorimetric method using commercial test kits (Biodiagnostic, Dokki, Giza, Egypt) according to a previously described method (Henry Citation1964). Globulin was calculated according to an established method (Coles Citation1986). The Fb concentration was estimated in citrated plasma according to a previously described method (Benjamin Citation1978). Hp was measured by a nephelometric method using commercial test kits (Turbox, Orion Diagnostica Oy, Finland) according to a previously described method (Ojala et al. Citation1981). Serum NO, CAT and MDA level were measured with a colorimetric method using commercial test kits according to methods described in Aebi (Citation1984), Kei (Citation1978), Pertile et al. (Citation1995), respectively. The level of serum pepsinogen was calorimetrically determined (Edwards et al. Citation1960).
Post-mortem examination
The clinical investigation clarified a bad prognosis of the diseased buffaloes affected by diffuse peritonitis. Informed consents were obtained from the owners of these animals to submit their cases to the necropsy unit to be slaughtered by direct severance of the neck major blood vessel after relaxing of the animal according to the national law.
Statistical analysis
The statistical analysis was done using the Kruskal–Wallis test with post-hoc Dunn’s multiple comparison test because of the small size of the control group and the non-normal distribution of markers, which were tested for normality using the Shapiro–Wilk test. The results are presented as medians ± standard deviation. Differences between the control and diseased groups were considered statistically significant at p < .05. Data were analysed using stata version 13 (Stata Corp., College Station, Texas, USA).
Results
Clinical findings
The clinical presentations of affected buffaloes in comparison with control one are listed in Table . Overall, the buffaloes affected by diffuse peritonitis show an increase in the body temperature, respiratory rate and heart rate in 60%. Congested mucous membranes and lacrimation were observed in 90% of diseased buffaloes. Ruminal contractions was absent in 85% of the diseased buffaloes. Clinical observation was observed such as recurrent tympany, pain reactions and faecal abnormalities (scanty faeces and diarrhoea). Pain tests showed varying results in diseased buffaloes and the depression was observed in 90% of the buffaloes affected with peritonitis.
Table 1. Clinical findings in control and affected buffaloes by diffuse peritonitis.
Ultrasonographic examination
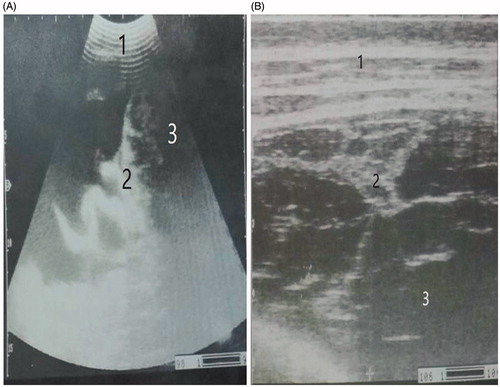
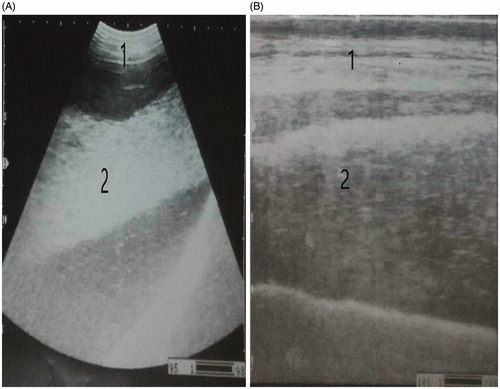
Diffuse peritonitis in this study appeared either with multiple echogenic strands of fibrin interspersed with anechoic fluid at whole abdominal cavity (n = 12) (Figure ) or as diffuse suppurative hypoechoic fluid in the whole abdominal cavity (n = 8) (Figure ).
Biochemical parameters
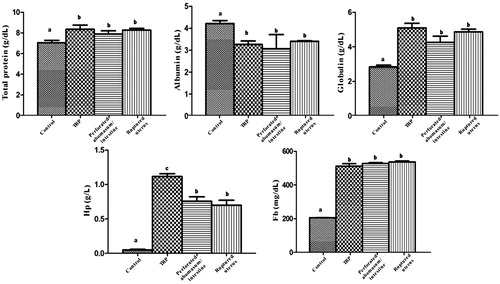
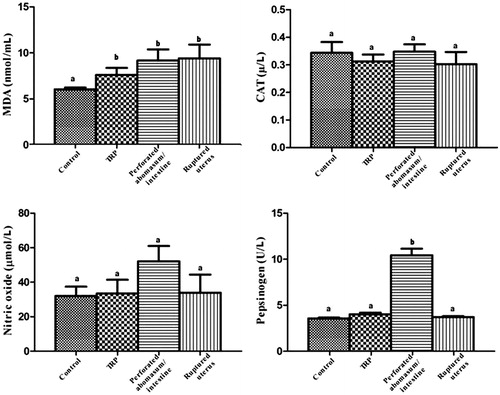
Serum biochemical assays were done utilising different biomarkers associated with different causes of diffuse peritonitis in buffaloes. Significant (p < .05) increase in the serum TP, globulin, Hp, MDA and plasma Fb levels with significant decrease in albumin were observed in all groups of diffuse peritonitis when compared with the control group. The serum NO and CAT levels showed a non-significant difference between the different groups of diffuse peritonitis and the control one. Serum Hp showed highly significant increases in buffaloes affected by peritonitis of TRP origin than other causes of diffuse peritonitis. While serum pepsinogen was significantly elevated in buffaloes with perforated abomasum/intestine than other groups (Figures ).
Necropsy findings
The origin of peritonitis and the associated pathological lesions during post-mortem examination are described in Table .
Table 2. Necropsy findings shows the different causes of 20 buffaloes affected by diffuse peritonitis.
Discussion
In appetence, depression and congested conjunctival mucous membranes with lacrimation were the most common signs in diseased buffaloes with diffuse peritonitis, and these results were similar to those previously reported (Abdelaal and Floeck Citation2015). In this study, variable degrees of pain and systemic reactions were observed in diseased buffaloes. This result is consistent with that observed previously in buffaloes with TRP (Saleh et al. Citation2008); therefore, it was difficult to diagnose this problem or differentiate between its different causes based only on the clinical signs.
Ultrasonography provides a gold standard tool for the diagnosis of diffuse peritonitis in this study, while it provides a poor value in the differentiation of TRP, perforated abomasum/intestine and or ruptured uterus. The diffuse peritonitis was either fibrinous or suppurative. The fibrinous peritonitis (n = 12), where the echogenic fibrins appeared, interspersed with anechoic peritoneal fluid. The suppurative peritonitis (n = 8) which characterised by appearance of large amount of hypoechoic peritoneal fluid due to excessive accumulation of pus. Similar results were previously reported (Abdelaal et al. Citation2009). On the other hand, the different causes of diffuse peritonitis were identified after necropsy; therefore, the necropsy examination was the gold standard tool in this study.
In this study, the reference ranges of TP, albumin and globulin of the control group were nearly similar to those previously reported in Egyptian buffaloes (El-Deeb and Iacob Citation2012) and cattle (Hussain and Uppal Citation2014). Buffaloes with diffuse peritonitis showed significant increases in TP and globulin with significant hypoalbuminaemia when compared with the control group. This result always occurs with inflammation (Dubensky and White Citation1983). The reference ranges of Fb and Hp in control buffaloes were in agreement with those previously reported in buffaloes (El-Deeb and Iacob Citation2012). When comparing our results to that reported in cattle (Alsaad Citation2011), we did found that Fb level was higher and Hp level was lower in buffaloes than that were reported in cows. In this study, buffaloes with TRP showed a highly significant increase in plasma Hp level than other groups. This result attributed to the severe inflammatory process following foreign body penetration in bovines affected by TRP resulting in hepatic synthesis of Hp (Roussel et al. Citation1997).
The results of oxidative biomarkers (NO, CAT, MDA) in healthy buffaloes were nearly similar to those previously reported in cattle (Atakisi et al. Citation2010). The most unexpected result in this study was the insignificant change in serum NO, which was described previously as a more sensitive biomarker for the diagnosis of inflammatory conditions in cattle with TRP (Atakisi et al. Citation2010). In general, NO has anti-inflammatory effect in normal physiological conditions. It induces several effects in the pathogenesis of the inflammatory pathways. It is also considered as a signalling molecule which induces a pro-inflammatory effect, vasodilatation in the cardiovascular system, and involves in the immune reaction by cytokine-activated macrophages (Sharma et al. Citation2007). On the other hand, MDA was significantly elevated in all groups of diffuse peritonitis, therefore it could not be specific for differentiation between different causes of diffuse peritonitis in buffaloes. MDA is a degradation product of lipid peroxidation after exposure to reactive oxygen species, and its concentration in the blood could be considered an indicator of the degree of lipid peroxidation (Kohen and Nyska Citation2002).
In this study, serum pepsinogen in the control buffaloes was slightly elevated when compared to that obtained in cattle (Charlier et al, Citation2011). Significant increase in the serum level of pepsinogen could be specific for diffuse peritonitis secondary to perforated abomasum and or intestine. The elevation of pepsinogen occurs mainly due to degeneration of abomasal mucosa which leads to an increase in the epithelial and vascular permeability allowing the leakage of pepsinogen into the blood (Jennings et al. Citation1966).
Conclusions
Our study detected the changes of APPs, oxidative stress markers and pepsinogen in buffaloes affected by diffuse peritonitis of different origin. The results of this study indicated that Hp and pepsinogen exhibited high degree of differentiation between different causes of diffuse peritonitis in buffaloes. Meanwhile, NO and CAT had no role in the diagnosis of such condition in buffaloes.
Consent for publication
All owners signed a consent form allowing us to use the animals and all the associated medical data for scientific analysis and publication.
Ethical approval
All institutional and national guidelines for the care and use of animals were followed according to the Egyptian Medical Research Ethics Committee.
Disclosure statement
The authors declare that they have no conflicts of interest.
References
- Abdelaal A, Floeck M. 2015. Clinical and sonographical findings in buffaloes (Bubalus bubalis) with traumatic reticuloperitonitis. Veterinarski Arhiv. 85:1–9.
- Abdelaal A, Floeck M, El Maghawry S, Baumgartner W. 2009. Clinical and ultrasonographic differences between cattle and buffaloes with various sequelae of traumatic reticuloperitonitis. Veterinarni Med. 54:399–406.
- Aebi H. 1984. Catalase in vitro. Methods in enzymology. Amsterdam: Elsevier; p. 121–126.
- Alsaad KM. 2011. Evaluation of hemogram, haptoglobine and clotting factors indices in cattle affected with acute and chronic peritonitis. J Anim Vet Adv. 10:11–17.
- Andrews AH, Blowey RW, Boyd H, Eddy RG. 2008. Bovine medicine: diseases and husbandry of cattle. New York: John Wiley & Sons.
- Atakisi E, Bozukluhan K, Atakisi O, Gokce HI. 2010. Total oxidant and antioxidant capacities and nitric oxide levels in cattle with traumatic reticuloperitonitis. Vet Rec. 167:908–909.
- Benjamin MM. 1978. Outline of veterinary clinical pathology. 3rd ed. Ames: Iowa State University Press; p. 351.
- Braun U. 2009. Ultrasonography of the gastrointestinal tract in cattle. Vet Clin North Am Food Anim Pract. 25:567–590.
- Charlier J, Dorny P, Levecke B, Demeler J, von Samson-Himmelstjerna G, Höglund J, Vercruysse J. 2011. Serum pepsinogen levels to monitor gastrointestinal nematode infections in cattle revisited. Res Vet Sci. 90:451–456.
- Chelikani P, Fita I, Loewen P. 2004. Diversity of structures and properties among catalases. Cell Mol Life Sci. 61:192–208.
- Coles E. 1986. Veterinary clinical pathology. 4th ed. London: WB Saunders company Philadelphia; p. 136–170.
- Constable PD, Hinchcliff KW, Done SH, Grünberg W. 2016. Veterinary medicine-e-book: a textbook of the diseases of cattle, horses, sheep, pigs and goats. Amsterdam: Elsevier Health Sciences.
- Del Rio D, Stewart AJ, Pellegrini N. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 15:316–328.
- Dubensky R, White M. 1983. The sensitivity, specificity and predictive value of total plasma protein in the diagnosis of traumatic reticuloperitonitis. Can J Comp Med. 47:241.
- Edwards K, Jepson RP, Wood KF. 1960. Value of plasma pepsinogen estimation. Br Med J. 1:30–32.
- El-Deeb WM, Iacob O. 2012. Serum acute phase proteins in control and Theileria annulata infected water buffaloes (Bubalus bubalis). Vet Parasitol. 190:12–18.
- Henry RJ. 1964. Clinical chemistry. 1st ed. New York: Harper & Row Publishers; p. 181.
- Hussain S, Uppal S. 2014. Haemato-biochemical changes and peritoneal fluid cytology in clinical cases of bovine peritonitis. Ind J Anim Res. 48:188–193.
- Jennings FW, Armour J, Lawson DD, Roberts R. 1966. Experimental Ostertagia ostertagia infections in calves: studies with abomasal cannulas. Am J Vet Res. 27:1249–1257.
- Kataria N, Kataria A, Gahlot A. 2008. Use of plasma gastrin and pepsinogen levels as diagnostic markers of abomasal dysfunction in Marwari sheep of arid tract. Slov Vet Res. 45:121–126.
- Kei S. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chimica Acta. 90:37–43.
- Kofler J, Braun U. 1997. Ultraschalluntersuchung am Bewegungsapparat. Berlin: Atlas und Lehrbuch der Ultraschalldiagnostik beim Rind. Parey Buchverlag; p. 253–268.
- Kohen R, Nyska A. 2002. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 30:620–650.
- Koksal G, Sayilgan C, Aydin S, Oz H, Uzun H. 2004. Correlation of plasma and tissue oxidative stresses in intra-abdominal sepsis. J Surg Res. 122:180–183.
- Murata H, Shimada N, Yoshioka M. 2004. Current research on acute phase proteins in veterinary diagnosis: an overview. Vet J. 168:28–40.
- Mushonga B, Habarugira G, Musabyemungu A, Udahemuka JC, Jaja FI, Pepe D. 2015. Investigations of foreign bodies in the fore-stomach of cattle at Ngoma Slaughterhouse, Rwanda. J S Afr Vet Assoc. 86:1–6.
- Ni J, McLoughlin RM, Brodovitch A, Moulin P, Brouckaert P, Casadei B, Feron O, Topley N, Balligand J-L, Devuyst O. 2010. Nitric oxide synthase isoforms play distinct roles during acute peritonitis. Nephrol Dial Transpl. 25:86–96.
- Ojala K, Weber T, Kauhala A. 1981. Immunoturbidimetric haptoglobin determination. J Clin Chem Clin Biochem. 19:788.
- Pertile TL, Sharma JM, Walser MM. 1995. Reovirus infection in chickens primes splenic adherent macrophages to produce nitric oxide in response to T cell-produced factors. Cell Immunol. 164:207–216.
- Ramin A, Hashemi A, Asri-Rezaie S, Batebi E, Tamadon A, Ramin S. 2011. Prediction of traumatic pericarditis in cows using some serum biochemical and enzyme parameters. Acta Veterinaria. 61:383–390.
- Rosenberger G. 1990. Die Klinische Untersuchung des Rindes. 3rd ed. Berlin: Paul Parey.
- Roussel AJ, Whitney M, Cole D. 1997. Interpreting a bovine serum chemistry profile. 2. Vet Med. 92: 559–566.
- Saleh MA, Rateb H, Misk N. 2008. Comparison of blood serum proteins in water buffaloes with traumatic reticuloperitonitis and sequellae. Res Vet Sci. 85:208–213.
- Sharma JN, Al-Omran A, Parvathy SS. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 15:252–259.
- Smith BP. 2014. Large animal internal medicine—e-book. St. Louis: Elsevier Health Sciences.