Abstract
The yellow-feather broiler is a popular poultry breed in Asia, particularly in China. In this study, we performed RNA-seq analysis to identify differentially expressed genes (DEGs) in the heart of yellow-feather broilers that had been subjected to heat stress treatment (38 ± 1 °C for 8 h, over 7 d) and determine the response of the heart to high temperature and its effects on yellow-feather broiler physiology. We found that body weight (BW) of the heat stress treatment group (BW28 = 354.8 ± 34.8 g) was significantly decreased (p = .033) compared with that of the control group (BW28 = 384.8 ± 58.9 g). However, there was no significant reduction in the heart relative weight (HRW) (p = .538). A total of 37 DEGs related to energy metabolism responded to heat stress in the heart of yellow-feather broiler. The results of KEGG pathways analysis indicated that these genes are involved in oxidative phosphorylation (KO: 00190), cardiac muscle contraction (KO: 04260) and carbon metabolism (KO: 01200). Analysis of the cardiac transcriptome of yellow-feather broilers subjected to heat stress indicated that the heart of these birds has specific physiological mechanisms for regulating body growth in response to high-temperature environments.
Yellow-feather broilers, a popular poultry breed in Asia, were used to determine how the heart responds to heat stress.
A total of 37 genes in the heart of yellow-feather broilers showed differential expression in response to heat stress.
The differentially expressed genes (DEGs) are associated with oxidative phosphorylation, cardiac muscle contraction and carbon metabolism pathways.
Highlights
Introduction
Global warming is affecting the broiler chicken industry worldwide (Windhorst Citation2006). In the past few decades, broiler production performance, particularly in terms of growth rates, has been significantly improved through genetic selection (Mckay et al. Citation2000; Deeb and Cahaner Citation2002). However, high environmental temperatures have a more detrimental effect on fast-growing broilers than on slow-growing broilers (Cahaner and Leenstra Citation1992; Lu et al. Citation2007; Cheng et al. Citation2018), particularly with regards to the body weight (BW) of these chickens (Sohail et al. Citation2012). Therefore, to enhance performance efficiency, it is important to gain an understanding of the genetic basis of the response to heat stress with respect to poultry physiology.
The yellow-feather broiler is a popular poultry breed in Asia, particularly in China, that is known for its slower growth than commercial broilers and unique meat flavour. Previous studies on chickens have shown that fast-growing broilers develop symptoms of injury to the left ventricle (Olkowski Citation2007), and failure of the left ventricle can cause loss of blood pumping function (Baghbanzadeh and Decuypere Citation2008). Birds have an efficient cardiovascular system that delivers the metabolic requirements of nutrients and heat. However, to date, there has been limited research on the response of yellow-feather broilers to heat stress. In this study, we investigated the expression of genes in the left ventricle of yellow-feather broilers with regards to the response to heat stress and analysed the effects of heat stress on the metabolism of these birds.
Materials and methods
Experimental design and animal management
We selected 17-day-old male yellow-feather broilers and raised them in two environmentally controlled rooms, each of which was divided into four cells per replicate. Each cell contained six male yellow-feather broilers, which were acclimated for 5 d. When these birds were 22 d old, those allotted to the heat stress treatment were subjected to a temperature of 38 ± 1 °C for 8 h/d over a period of 7 d and maintained at 25 ± 1 °C for the remainder of the time. Throughout this time, control birds were housed in a thermoneutral room maintained at 25 ± 1 °C. During the experiment, we provided the birds with ad libitum access to feed and water. All the experimental birds were cared for and treated according to the guidelines provided by the Guangdong Ocean University Animal Care and Use Committee (permit number: SYXK 2014-0053).
Phenotypic measurements and sample collection
A digital scale was used to determine the body weight (BW) of each bird at 21 and 28 d of age, and differences in the BW among groups were analysed with a two-way analysis of variance (ANOVA) and post hoc multiple comparisons using SPSS software version 19.0 (SPSS, Chicago, IL). We also determined the heart absolute weight (HAW) of each 28-day-old broiler and calculated the heart relative weight (HRW) as the ratio of HAW to BW (HRW = HAW/BW × 100%). In poultry, relative organ weights are typically assessed as indicators of physiological development (Ven et al. Citation2013). One 28-day-old yellow-feather broiler from each cell was euthanised at the midpoint of the final heat stress treatment and eight heart tissue samples (four thermoneutral and four heat stress treatment) were collected and stored at −80 °C.
Transcriptome sequencing analysis of gene expression in response to heat stress
We used the Illumina HiSeq 2500 platform (Illumina, San Diego, CA) to identify differentially expressed genes (DEGs) in the heart of yellow-feather broilers that had been subjected to heat stress. TRIzol Reagent (Invitrogen, Carlsbad, CA) was used to extract the total RNA from heart tissue samples from each yellow-feather broiler for sequencing, according to the manufacturer’s instructions. A NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, U.S.A) was used to prepare the sequencing libraries according to the manufacturer’s recommendations and index codes were added to attribute sequences to each sample. Briefly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads. Reads were mapped to the Gallus gallus genome assembly (ftp://ftp.ensembl.org/pub/release-92/fasta/gallus_gallus/dna/) using HISAT2 version 2.0.4 (Center for Computational Biology, Johns Hopkins University) (Kim et al. Citation2015). HTSeq version 0.9.1 (Python Software Foundation) was used to count the reads (Anders et al. Citation2015). The gene expression levels of the transcripts were quantified as fragments per kilobase of transcript sequence per million base pairs sequenced (fragments per kilobase million [FPKM]) (Trapnell et al. Citation2010). The DESeq R package version 1.18.0 (Bioconductor) (Anders and Huber Citation2010) was used to analyse DEGs in the heat-stressed heart samples (p < .05; false discovery rate (FDR) < 0.05 as a threshold). The GOseq R package (Young et al. Citation2010) and KOBAS software (Mao et al. Citation2005) were used to analyse gene ontology (GO) and the results of Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analysis, respectively.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and statistical analysis
We used qRT-PCR analysis to determine the expression levels of five genes (COX6A1, UQCRB, NDUFS6, MCEE and ALDOB). The primer pairs used to amplify the selected genes are shown in Table S1. The 2−ΔΔCT method (Livak and Schmittgen Citation2001) was used to analyse the relative levels of gene expression determined in qRT-PCR experiments. The mRNA expression levels were analysed with one-way ANOVA using SPSS software version 19.0 (SPSS, Chicago, IL).
Data deposition
The RNA-seq analysis data have been submitted to the Sequence Read Archive (SRA) at the National Centre for Biotechnology Information (NCBI), and assigned accession number SRP152925 (https://www.ncbi.nlm.nih.gov/sra/SRP152925).
Results and discussion
The results obtained for BW, HAW and HRW are presented in Figure S1A, B and C, respectively. After heat stress treatment (38 ± 1 °C, 8 h/d, for 7d), the BW of the heat stress treatment group (BW28 = 354.8 ± 34.8 g) was significantly decreased (p = .03) compared with that of the control group (BW28 = 384.8 ± 58.9 g). We found that heat stress decreased the BW of yellow-feather broilers by 7.8%. In comparison previous studies have shown a 12.8% reduction in the BW28 of commercial broilers subjected to heat stress (32 °C, period 2–4 weeks of age) (Geraert et al. Citation1996;), whereas in AIL chicken lines bred for thermo-tolerance (Deeb and Lamont Citation2002), exposure to heat stress (35 °C, 7 h/d, for 7d), resulted in a BW gain ratio (BW28–21) of 58.9% (Angelica et al. Citation2015). In this study, the BW gain ratio (BW28–21) in response to heat stress was 60.0% indicating that yellow-feather broilers tolerated a higher temperature (38 ± 1 °C, 8 h/d, 7d) environment. A decrease in growth performance in response to heat stress has previously been shown to be associated with various changes in certain organs, including intestinal damage, and relative weight reduction in immune-related organs and the heart (Quinteirofilho et al. Citation2010; Zhang et al. Citation2017). In this study, the HAW of the heat stress treatment group (HAW28 = 2.18 ± 0.16 g) showed a significant decrease (p = .001) compared with that of the control group (HAW28 = 2.75 ± 0.11 g).In contrast, however, the HRW of the heat stress treatment group (HRW28 = 0.61 ± 0.02%) did not differ significantly (p = .538) from that of the control group (HRW28 = 0.58 ± 0.08%). These results indicated normal physiological development of the heart in yellow-feather broilers subjected to a high-temperature environment (Ven et al. Citation2013).
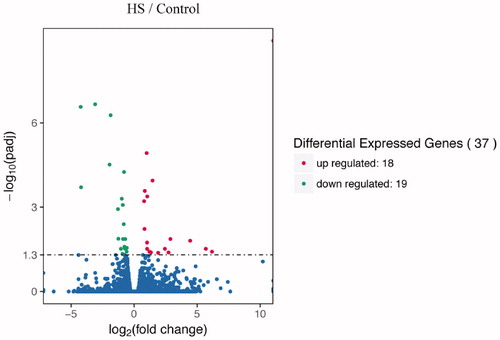
The number of total clean reads for the identified DEGs ranged from 53 to 62 million and approximately 90% of the clean reads were mapped on the chicken reference genome (Table S2), of which 78% of reads were mapped to exons (Figure S2). A total of 37 genes (FDR < 0.05) were differentially expressed in response to heat stress in the heart of yellow-feather broilers (Figure ). In contrast, 1089 DEGs (FDR < 0.1) were identified in the heart transcriptome of Ross broilers subjected to heat stress (35–37 °C, 8 h/d, for 14d) (Zhang et al. Citation2017). These findings indicate that the heat stress response of broilers is to a certain extent dependent on the duration of heat stress. Of the 37 DEGs identified in this study, 19 were downregulated and 18 were upregulated and the log2 (fold change) induced by heat ranged from −4.24 to 6.17.
Figure 1. Volcano plot statistics of differentially expressed genes (DEGs) in the heat stress group (38 ± 1 °C, 8 h/d, for 7 d) compared with the control group (25 ± 1 °C) group in yellow-feather broilers. Y-axis: the mean log10 (FDR) gene expression value. X-axis: the log2 fold change value of gene expression. The red and green dots represent up- and downregulated DEGs, respectively (FDR < 0.05).
We performed GO term enrichment analysis of the DEGs using the GOseq R package to investigate the biological processes related to heat stress. We accordingly found that the 30 most enriched biological process (BP) GO terms of the DEGs expressed in the heart are all related to energy metabolism. The heart requires large amounts of energy to maintain ionic homeostasis and contraction (Jafri et al. Citation2001), and in this regard, KEGG (Kanehisa and Goto Citation2000) pathway analysis of these DEGs revealed the enrichment of three pathways (p < .05) oxidative phosphorylation (KO: 00190), cardiac muscle contraction (KO: 04260) and carbon metabolism (KO: 01200) (Figure S3). The products of DEGs associated with the ‘oxidative phosphorylation’ pathway are: cytochrome c oxidase (COX) subunit 6A1 (COX6A1), ubiquinone oxidoreductase subunit S6 (NDUFS6) and ubiquinol-cytochrome c reductase-binding protein (UQCRB). Given that the heart has a high energy dependence, it is particularly susceptible to oxidative phosphorylation defects, which are considered oxidative phosphorylation diseases (Limongelli et al. Citation2010; Finsterer and Kothari Citation2014). Disruption of the function of the oxidative phosphorylation electron transport chain leads to mitochondrial dysfunction, and in this study, we found that the oxidative phosphorylation pathway was the most significantly enriched pathway, which supports this view. The interrelated oxidative phosphorylation DEGs (COX6A1, UQCRB and NDUFS6) may be involved in heat stress-related energy metabolism, and their differential expression indicates that yellow-feather broilers have specific physiologic mechanisms for regulating body growth in response to high-temperature environments.
COX is a key enzyme in cellular respiration (Brudvig and Wikström Citation2005). The COX6A1 gene encodes mitochondrial protein COX subunit 6A1, which drives ATP synthesis in the mitochondrial electron transport chain (Fornuskova et al. Citation2010). Another oxidative phosphorylation-related DEG, UQCRB, encodes subunit 7 of ubiquinol cytochrome c reductase. UQCRB plays a key role in hypoxia-induced angiogenesis and mitochondria-mediated metabolic disorders (Jung et al. Citation2011; Chang et al. Citation2014; Chang et al. Citation2015), and the loss of UQCRB function inhibits angiogenesis (Cho et al. Citation2013). NDUFS6 encodes NADH, ubiquinone oxidoreductase and dehydrogenase (ubiquinone) Fe-S protein 6 (Loeffen et al. Citation1998). Defects in NADH are associated with energy generation disorders (Gabaldon et al. Citation2005; Spiegel et al. Citation2009). NADH deficiency leads to neurodegeneration, muscle weakness, cardiac failure, liver failure and early death (Kirby et al. Citation1999; Loeffen et al. Citation2000). NDUFS6 mutations cause respiratory chain disorders (Kirby et al. Citation2004). Among these DEGs, COX6A1 and UQCRB are also involved in the cardiac muscle contraction pathway. In this study, three DEGs were downregulated in the heat stress treatment group and we conclude that these are involved in the energy metabolism of heat stress and the physiological response to high temperatures. The ‘carbon metabolism’ pathway includes methylmalonyl-CoA epimerase (MCEE) and fructose-bisphosphate aldolase B (ALDOB). The five selected genes associated with KEGG pathways were identified by qRT-PCR (Figure S4).
Conclusions
The findings of this study provide evidence for the transcriptomic regulation of heat stress responses in the heart of yellow-feather broiler chickens. Heat stress decreases the BW28 (p = .033) and HAW28 (p = .001) of these broilers, whereas the HRW28 was not significantly decreased (p = .538). A total of 37 DEGs related to energy metabolism were detected in heart tissues in response to heat stress. These results indicate that the heart of yellow-feather broilers has specific physiological mechanisms for regulating body growth in response to high-temperature environments.
Acknowledgements
The authors thank Prof. ZhuoCheng Hou, China Agricultural University, for his advice and review of this manuscript.
Disclosure statement
We certify that there is no conflict of interest with any financial organisation regarding the material discussed in the manuscript.
Additional information
Funding
References
- Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:1–12.
- Anders S, Pyl PT, Huber W. 2015. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics. 31:166–169.
- Angelica VG, Bolek KJ, Ashwell CM, Persia ME, Rothschild MF, Schmidt CJ, Lamont SJ. 2015. Identification of quantitative trait loci for body temperature, body weight, breast yield, and digestibility in an advanced intercross line of chickens under heat stress. Genet Sel Evol. 47:96.
- Baghbanzadeh A, Decuypere E. 2008. Ascites syndrome in broilers: physiological and nutritional perspectives. Avian Pathol. 37:117–126.
- Brudvig GW, Wikström M. 2005. Mechanistic comparisons between photosystem II and cytochrome c oxidase. Netherlands: Springer.
- Cahaner A, Leenstra F. 1992. Effects of high temperature on growth and efficiency of male and female broilers from lines selected for high weight gain, favorable feed conversion, and high or low fat content. Poult Sci. 71:1237.
- Chang J, Jung HJ, Jeong SH, Kim HK, Han J, Kwon HJ. 2014. A mutation in the mitochondrial protein UQCRB promotes angiogenesis through the generation of mitochondrial reactive oxygen species. Biochem Bioph Res Co. 455:290–297.
- Chang J, Jung HJ, Park HJ, Cho S, Lee SK, Kwon HJ. 2015. Cell-permeable mitochondrial ubiquinol-cytochrome c reductase binding protein induces angiogenesis in vitro and in vivo. Cancer Lett. 366:52–60.
- Cheng C, Tu W, Chen C, Chan H, Chen C, Chen H, Tang P, Lee YP, Chen S, Huang S. 2018. Functional genomics study of acute heat stress response in the small yellow follicles of layer-type chickens. Sci Rep. 8:1320.
- Cho YS, Jung HJ, Seok SH, Payumo AY, Chen JK, Kwon HJ. 2013. Functional inhibition of UQCRB suppresses angiogenesis in zebrafish. Biochem Biophys Res Co. 433:396–400.
- Deeb N, Cahaner A. 2002. Genotype-by-environment interaction with broiler genotypes differing in growth rate. 3. Growth rate and water consumption of broiler progeny from weight-selected versus nonselected parents under normal and high ambient temperatures. Poult Sci. 81:293.
- Deeb N, Lamont SJ. 2002. Genetic architecture of growth and body composition in unique chicken populations. J Hered. 93:107.
- Finsterer J, Kothari S. 2014. Cardiac manifestations of primary mitochondrial disorders. Int J Cardiol. 177:754–763.
- Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J. 2010. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem J. 428:363–374.
- Gabaldon T, Rainey D, Huynen MA. 2005. Tracing the evolution of a large protein complex in the eukaryotes, NADH: ubiquinone oxidoreductase (complex I). J Mol Biol. 348:857–870.
- Geraert PA, Padilha JC, Guillaumin S. 1996. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 75:195.
- Jafri MS, Dudycha SJ, O’Rourke B. 2001. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng. 3:57–81.
- Jung HJ, Kim KH, Kim ND, Han G, Kwon HJ. 2011. Identification of a novel small molecule targeting UQCRB of mitochondrial complex III and its anti-angiogenic activity. Bioorg Med Chem Lett. 21:1052–1056.
- Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
- Kim D, Langmead B, Salzberg SL. 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 12:357–360.
- Kirby DM, Crawford M, Cleary M, Dahl HHM, Dennett X, Thorburn DR. 1999. Respiratory chain complex I deficiency: an underdiagnosed energy generation disorder. Neurology. 52:1255–1255.
- Kirby DM, Salemi R, Sugiana C, Ohtake A, Parry L, Bell KM, Kirk EP, Boneh A, Taylor RW, Dahl HHM, et al. 2004. NDUFS6 mutations are a novel cause of lethal neonatal mitochondrial complex I deficiency. J Clin Invest. 114:837–845.
- Limongelli G, Tome-Esteban M, Dejthevaporn C, Rahman S, Hanna MG, Elliott PM. 2010. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur J Heart Fail. 12:114–121.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
- Loeffen J, Den Heuvel LV, Smeets R, Triepels R, Sengers RCA, Trijbels FJM, Smeitink J. 1998. cDNA sequence and chromosomal localization of the remaining three human nuclear encoded iron sulphur protein (IP) subunits of complex I: the human IP fraction is completed. Biochem Bioph Res Co. 247:751–758.
- Loeffen J, Smeitink JAM, Trijbels JMF, Janssen AJM, Triepels R, Sengers RCA, Den Heuvel L. 2000. Isolated complex I deficiency in children: clinical, biochemical and genetic aspects. Hum Mutat. 15:123–134.
- Lu Q, Wen J, Zhang H. 2007. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult Sci. 86:1059.
- Mao X, Cai T, Olyarchuk JG, Wei L. 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 21:3787–3793.
- Mckay JC, Barton NF, Koerhuis ANM, Mcadam J. 2000. The challenge of genetic change in the broiler chicken. 27:1–7.
- Olkowski AA. 2007. Pathophysiology of heart failure in broiler chickens: structural, biochemical, and molecular characteristics. Poult Sci. 86:999–1005.
- Quinteirofilho WM, Ribeiro A, Ferrazdepaula V, Pinheiro ML, Sakai M, Sá LRM, Ferreira AJP, Palermoneto J. 2010. Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poult Sci. 89:1905.
- Sohail MU, Hume ME, Byrd JA, Nisbet DJ, Ijaz A, Sohail A, Shabbir MZ, Rehman H. 2012. Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress. Poult Sci. 91:2235.
- Spiegel R, Shaag A, Mandel H, Reich D, Penyakov M, Hujeirat Y, Saada A, Elpeleg O, Shalev SA. 2009. Mutated NDUFS6 is the cause of fatal neonatal lactic acidemia in Caucasus Jews. Eur J Hum Genet. 17:1200–1203.
- Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, Van Baren MJ, Salzberg SL, Wold BJ, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 28:511–515.
- Ven LJF, Van De Wagenberg AV, Van Decuypere E, Kemp B, Brand H. Van Den 2013. Perinatal broiler physiology between hatching and chick collection in 2 hatching systems. Poult Sci. 92:1050–1061.
- Windhorst HW. 2006. Changes in poultry production and trade worldwide. World’s Poult Sci J. 62:585–602.
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11:1–12.
- Zhang J, Schmidt CJ, Lamont SJ. 2017. Transcriptome analysis reveals potential mechanisms underlying differential heart development in fast- and slow-growing broilers under heat stress. BMC Genomics. 18:295.
