Abstract
Wine grape pomace (WGP) contains polyphenols that possess antioxidative ability, and its potential as feed ingredients in the lamb industry remains undefined. The objective of this study was to investigate the effect of dietary WGP supplementation on jejunum epithelial morphology, apoptosis and antioxidative activity in lambs. A total of 18 lambs (25.0 ± 0.2 kg) were randomly selected and equally divided into three groups, and offered diet containing different levels of WGP (0%, 5% and 10%). The results showed that dietary WGP supplementation decreased reactive oxygen species concentration (p < .05). Supplementation with 10% dietary WGP reduced villus height (p < .05), while 5% WGP supplementation decreased crypt depth (p < .05), and increased villus height:crypt depth ratio (p < .05). Interestingly, apoptotic cell numbers were declined in WGP feeding lambs (p < .05). Supportively, 10% WGP supplementation decreased both phosphorylated ATM (p < .05) and Bax abundance (p < .05). Meanwhile, both 5% and 10% dietary WGP supplementation increased Bcl-2 abundance (p < .05) and reduced both active form of caspase 8 and caspase 9 protein levels (p < .05). As expected, the cleaved-caspase 3 content was reduced in WGP feeding lambs (p < .05). The total antioxidative capacity, catalase, glutathione peroxidase 4 and superoxide dismutase activity were increased when lambs were fed the WGP-containing diet (p < .05). Taken together, these results suggest that WGP can be used as a feed ingredient in lamb production to relieve jejunum epithelial apoptosis, possibly due to the enhanced of antioxidative capacity.
Dietary wine grape pomace (WGP) supplementation alleviates ROS induced apoptosis and modifies the jejunum epithelium structure in ram lambs.
WGP has great potential as a source of functional feed or additive in lamb industry.
Highlights
Introduction
Phenolic compounds from grape are currently receiving much attention because of their putative health effects, including antimutagenic and anticarcinogenic activity, anti-inflammatory activities and antimicrobial activities (Yu and Ahmedna Citation2013). In the animal industry, wine grape pomace (WGP) and its extract have been used to improve growth performance and meat quality (Brenes et al. Citation2016). In pigs, supplementation of a grape seed and grape marc meal extract improves the gain:feed ratio (Gessner et al. Citation2013) and pork meat quality (Zhang et al. Citation2015), and alters fatty acid composition in the subcutaneous fat (Yan and Kim Citation2011). In broiler chicken, dietary WGP supplementation has been demonstrated to improve the gain:feed ratio, which may be attributed to the modification of the gut morphology and intestinal microflora (Hogan et al. Citation2010).
Intestinal epithelium forms a physiochemical barrier that separates the intestinal lumen from the host's internal milieu and is critical for electrolyte passage, nutrient absorption, and interaction with commensal microbiota (Negroni et al. Citation2015). Apoptosis is recognised as an important process responsible for maintenance of the cellular balance between proliferation and death. In the gastrointestinal tract, apoptosis is especially relevant, as the mammalian intestinal mucosa undergoes a process of continual cell turnover that is essential for maintenance of normal function (Ramachandran et al. Citation2000). In the present study, we investigated the hypothesis that dietary WGP supplementation has the potential to mediate jejunum epithelial apoptosis in small intestine by modulating the antioxidative capacity.
Materials and methods
Feed preparation
Red WGP (obtained from a winery) was dried and milled. Nutrient composition of WGP, dietary ingredients and nutrient level of diets are shown in and , respectively, which have been reported in previous paper (Zhao et al. Citation2018). The feed was formulated based on the National Research Council’s (NRC Citation2007) recommendation as a pelleted mixed diet.
Table 1. Nutrient composition of wine grape pomace (air-dry matter basis, %).
Table 2. Dietary ingredients and chemical composition of diets (air-dry matter basis, %).
Care and use of animals
A total of 18 1/2 Dorper (♂) × 1/2 small thin-tailed (♀) crossed ram lambs with similar body weight (BW, 25.0 ± 0.2 kg) were randomly selected and housed in individual stalls (3 m × 0.8 m) equipped with feeders and a water source. Animals were equally assigned to three groups in a completely randomised design and individually fed with either control (0% WGP) or WGP containing (5% and 10%, air-dry matter basis) diet. Before formal trial, all animals were fed an experimental diet for 10 d for adaptation. Feeding trial lasted for 74 d, and all animals were fed twice daily at 8:00 a.m. and 18:00 p.m., and had free access to clean water and a salt block throughout the experiment.
Before harvest, lambs were fasted for 16 h, and anesthetised and exsanguinated. The whole length of the small intestine was removed and a length of approximately 5 cm of the jejunum was sampled. A 5 mm segment of jejunum was rinsed in phosphate buffered solution (PBS) and fixed in 4% paraformaldehyde (PFA) for paraffin embedding. The remaining were cut open longitudinally and snap-frozen in liquid nitrogen for further biochemical analysis.
Histological analysis of jejunum tissue sections
Paraffin embedded jejunum tissues were sectioned at 7 µm thickness, deparaffinised and subjected to haematoxylin and eosin (H&E) staining as previous described (Zhao et al. Citation2016). Histological examination and imaging were done under microscope (BX53F, Olympus, Tokyo, Japan), and 10 of fields each sample were selected randomly for observation. Villus length and crypt depth were determined using a computer-supported imaging system connected to microscope (Imag-Pro plus 7.0, Media Cybernetics, Silver Spring, MD). Villus length was measured from the tip of the villus to the villus crypt junction, and crypt depth was defined as the depth of the invagination between adjacent villi.
Tunnel staining
Tunnel staining assay was performed in dewaxed sections with an Apoptosis Detection Kit (G001-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the instruction. Briefly, rehydrated slices were incubated in a humidified chamber at room temperature for 20 min with proteinase K. Then, the specimens washed with PBS was covered with 3% H2O2 and incubated at room temperature for 10 min. After washing with PBS, TUNEL enzyme and label mixed solution were applied to slices, and incubated again in the humidified chamber for 1 h at 37 °C. After rinsing with PBS, anti-fluorescein antibody was applied for 30 min at 37 °C. Finally, the specimens washed with PBS were incubated with DAB solution for 10 min at room temperature. Haematoxylin solution was applied to stain the nuclei. Image was obtained under microscope (BX53F, Olympus, Tokyo, Japan), and 10 fields of each sample were selected randomly for observation.
Enzyme-linked immunosorbent assay
The enzyme-linked immunosorbent assay was performed as previous described (Wan et al. Citation2017). Briefly, the jejunum powder (500 mg) was weighed out and homogenised on ice in 4.5 mL of 0.9% saline, and then centrifuged at 2500×g for 10 min at 4 °C. The supernatant was used for analysis of enzyme activity, reactive oxygen species (ROS) and malondialdehyde (MDA) contents with ELISA kits. Kits information was as follows: total antioxidant capacity (T-AOC, no. HY-60021), catalase (no. HY-60015), glutathione peroxidase 4 (GPx4, no. HY-60005), superoxide dismutase (SOD, no. HY-60001) and MDA (HY-60003) were from Beijing SINO-UK Institute of Biological Technology (Beijing, China). ROS (E004) was from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Western blotting
Western blotting was performed based on the established protocol in our lab (Zhao et al. Citation2010). Briefly, the jejunum samples (100 mg) were homogenised in 500 μL of ice-cold lysis buffer containing 20 mM Tris–HCl (pH 7.4 at 4 °C), 2% SDS, 1% Triton X-100, 5.0 mM EDTA, 5.0 mM EGTA, 1 mM DTT, 100 mM NaF, 2 mM sodium vanadate and 0.5 mM phenylmethylsulfonyl fluoride. Homogenates were centrifuged for 15 min at 12,000×g, 4 °C, for soluble protein separation. Then, supernatant was mixed with an equal amount of sample loading buffer containing 150 mM Tris–HCl (pH 6.8), 20% glycerol, 2 mM 2-mercaptoethanol, 0.004% (wt/vol) bromophenol blue and boiled for 3 min for electrophoresis. Samples were subjected to SDS-PAGE and immunoblotting with the use of an Odyssey Infra-red Imaging System (LI-COR Biosciences, Lincoln, NE). Band density was normalised according to the β-tubulin content.
Antibodies
Antibodies against SOD (no. sc-8637), catalase (no. sc-34281) and GPx-4 (no. sc-50497) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Nrf2 (no. LS-C31637) was from Lifespan Biosciences Inc. (Seattle, WA) and β-tubulin (no. 2146) was from Cell Signalling (Danvers, MA). Caspase 3 (bs-0081R), caspase 8 (bs-0052R), caspase 9 (bs-20773R), Bax (bs-0127M), Bcl-2 (bs-20351R) and p-ATM (bs-11707R) were from Biosynthesis Biotechnology (Beijing, China). Goat anti-mouse secondary antibody (926-68070) and anti-rabbit secondary antibody (926-32211) were from LI-COR Biosciences (Lincoln, NE).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software package (Monrovia, CA). Six lambs were used in each group and each animal was considered as an experimental unit. Data were expressed as mean ± SEM, tested for normality, and analysed using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test. p < .05 was considered as statistical significance for all data.
Results
The ROS and MDA contents
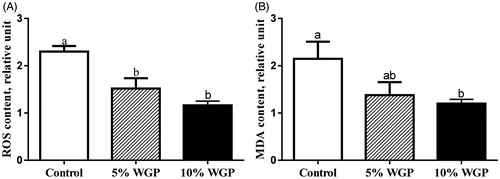
Both 5% and 10% dietary WGP supplementation decreased ROS concentration in jejunum (p < .05, ). As expected, a decreased MDA content was observed in the 10% dietary WGP supplementation ram lambs (p < .05, ).
Figure 1. Reactive oxygen species (ROS) and malondialdehyde (MDA) concentration in jejunum epithelium of ram lambs fed with the control (□), 5% WGP (
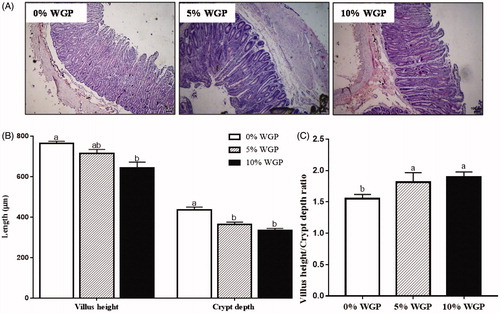
Dietary WGP supplementation affects jejunum morphology
As shown in , compared with the control lambs, the villus length was reduced in 10% WGP supplementation lambs (p < .05). Both 5% and 10% dietary WGP supplementation reduced crypt depth (p < .05), and no difference was observed between 5% and 10% WGP groups. Moreover, the ratio of the villus length to crypt depth was increased in lambs fed both 5% and 10% WGP containing diet compared with those in control group (, p < .05).
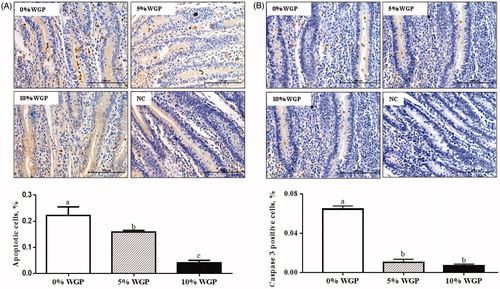
Dietary WGP supplementation altered jejunum epithelial apoptosis
Tunnel staining was used to determine epithelial apoptosis in jejunum. As shown in , both 5% and 10% of WGP supplementation decreased apoptotic cell numbers (p < .05). Accordingly, immunohistochemistry staining showed that caspase 3 positive cell numbers were decreased in jejunum of lambs fed with WGP containing diet (p < .05, ).
Figure 3. Jejunum epithelial apoptosis among control (□), 5% WGP (
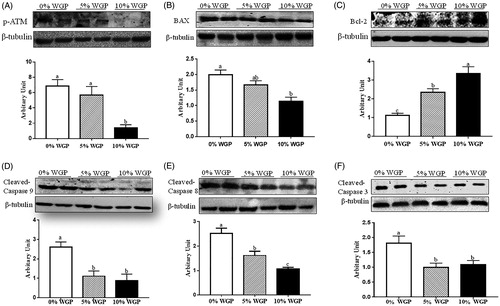
The apoptotic related protein abundance
As shown in , 10% WGP effectively decreased both phosphorylated ATM (, p < .05) and Bax abundance (, p < .05). The lambs fed the WGP containing diet exhibited greater Bcl-2 content in a dose-dependent manner (, p < .05). Both 5% and 10% dietary WGP supplementation reduced cleaved-caspase 9 protein content (, p < .05), and no difference was observed between these two groups. Meanwhile, 5% WGP supplementation was sufficient to reduce the cleaved-caspase 8 protein abundance (, p < .05). As expected, the cleaved-caspase 3 content was decreased in lambs fed both 5% and 10% diet (, p < .05).
Figure 4. The apoptotic related protein abundance among different groups. (A) Phosphorylated ATM content. (B) Bax abundance. (C) Bcl-2 content. (D) Cleaved-caspase 9 content. (E) Cleaved-caspase 8 content. (F) Cleaved-caspase 3 content. Data show that WGP supplementation altered apoptotic related protein abundance (mean ± SEM; n = 6). WGP: wine grape pomace
Antioxidative enzyme activities
As shown in , the T-AOC activity was increased when lambs were fed diet containing 10% WGP compared with lambs in both control and 5% supplementation groups (p < .05). Both 5% and 10% of WGP supplementation improved catalase and GPx4 activity (p < .05), and no differences were observed between 5% and 10% groups. Compared with control group, supplementation with 10% WGP effectively increased SOD activity (p < .05).
Table 3. Effect of dietary wine grape pomace (WGP) supplementation on antioxidative enzyme activities in jejunum epithelium of ram lambs.
Antioxidative enzyme protein abundance
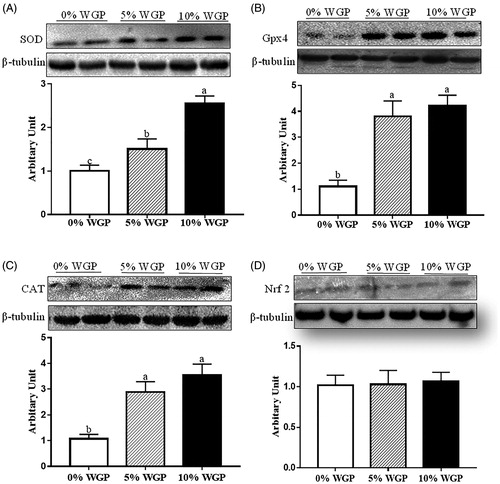
As shown in , SOD protein abundance was increased in WGP supplementation lambs in a dose-dependent manner (p < .05). GPx4 and catalase protein contents were increased in both 5% and 10% WGP supplementation groups (, p < .05), and no difference was observed between 5% and 10% groups. In addition, nuclear factor-like-2 factor (Nrf2) content was not altered by the WGP supplementation (, p < .05).
Figure 5. Antioxidative enzymes abundance in jejunum epithelium of ram lambs fed with control (□), 5% WGP (
Discussion
Lamb production has been converted to penned raising to facilitate the restoration of ecosystem in many areas of the world. However, intensive production elevates animal’s exposure to oxidative stress conditions (Casamassima et al. Citation2012), and affects production efficiency. The ROS, including hydroxyl radicals, superoxide anions, hydrogen peroxide and singlet oxygen is mainly generated by the mitochondria in most mammalian cells and has important roles in cell signalling and homeostasis (Devasagayam et al. Citation2004). ROS contents increase dramatically during environmental stress, and excess ROS further harms the health of humans and animals by triggering oxidative damage and dysfunction in cells (Schieber and Chandel Citation2014). The WGP is rich in extractable polyphenols, including phenolic acid, flavonoids, procyanidins, resveratrol and anthocyanins (Xia et al. Citation2010), and their biological functions have been extensively studied (Cottart et al. Citation2014). Importantly, WGP and its extract have even more effective antioxidative effects than vitamin C and vitamin E (Carpenter et al. Citation2007), suggesting that WGP is a promising feed ingredient for improving the oxidative stability of lambs. The present results suggested that dietary WGP supplementation effectively reduced ROS contents in jejunum, suggesting a reduced oxidative stress. Moreover, a reduced MDA content, a secondary product of lipid oxidation (Terevinto et al. Citation2015), was observed in WGP supplementation lambs, further indirectly indicating the decreased oxidative stress. The decreased oxidative stress may be attributed to the flavonoids presented in red wine by-products behaving as free radical scavengers and metal chelators (Bertelli and Das Citation2009).
The small intestinal epithelium is a self-renewing monolayer arising from stem cells located at or near the base of crypts, and regulates the uptake of nutrients and fluid while at the same time acts as a barrier to the permeation of potentially harmful substances present in the intestinal lumen (Potten et al. Citation1997). In the present study, dietary WGP supplementation reduced both villus height and crypt depth, which were in accordance with previous reports showing that supplementation of grape by-products modifies the intestinal morphology in both broiler chicks (Viveros et al. Citation2011) and piglets (Sehm et al. Citation2007). Interestingly, an increase in villus height:crypt depth ratio was observed in WGP feeding lambs, which may lead to better nutrient absorption, and attribute to greater overall performance. These data may partly explain the mechanism of our previous observation, which showed that dietary WGP supplementation in lambs increased BW, average daily gain, and reduced the feed to gain ratio (Zhao et al. Citation2018).
Apoptosis plays an important role in determining the architecture of intestinal epithelia and is also a part of the stress response of intestinal epithelial cells to toxic stimuli (Watson and Pritchard Citation2000). It is well established that oxidative stress and apoptosis are closely linked physiological phenomena, and excess ROS initiates apoptosis. As the ROS contents in jejunum were altered when lambs fed the WGP containing feed, we determined jejunum epithelium apoptosis using TUNEL staining. The current data indicated that dietary WGP effectively decreased apoptotic cell numbers, suggesting that declined ROS contents desensitises jejunum epithelium to apoptosis. Caspase 3 enzyme is known as an executioner caspase in apoptosis by coordinating the destruction of cellular structures, such as DNA fragmentation or degradation of cytoskeletal proteins (Shalini et al. Citation2015), and is therefore an important marker of the cell’s entry point into the apoptosis. In the present study, declined caspase 3 positive cell numbers further confirmed the apoptosis in jejunum epithelium was alleviated by WGP supplementation.
Apoptosis can be divided into the extrinsic pathway and the intrinsic pathway (Pileczki et al. Citation2016). Previous study has shown that oxidative stress can cause cellular apoptosis via both the extrinsic receptor pathway and the intrinsic mitochondrial pathway (Sinha et al. Citation2013). Elevated ROS concentration causes DNA double-strand breaks (Sallmyr et al. Citation2008) and further triggers DNA damage response involving checkpoint kinases ATM. Activation of ATM results in the activation of cell cycle checkpoints that lead to DNA damage-induced arrest at G1/S, S and G2/M (Maréchal and Zou Citation2013). In the present study, phosphorylated ATM contents were dramatically decreased by 10% WGP supplementation. Consequently, the protein levels of the proteolytically cleaved form of caspase 8 were decreased, confirming the involvement of caspase 8 in the ROS induced apoptosis of jejunum epithelium.
The intrinsic pathway involves a mitochondrion-centred control mechanism (Wan et al. Citation2018). Bcl-2 is known as an important gatekeeper to the apoptotic response by inhibiting cytochrome C release, while Bax functions as an apoptotic activator by increasing the opening of the mitochondrial voltage-dependent anion channel, which leads to the loss in membrane potential and the release of cytochrome C (Dai et al. Citation2016). The leaked cytochrome C forms the apoptosome with Apaf-1, and then further activates caspase 9 (Shakeri et al. Citation2017). In the present study, dietary WGP supplementation elevated Bcl-2 and decreased Bax protein content in jejunum epithelium, which reduced the cleaved form of caspase 9. As caspase 3 is activated by the upstream caspase 8 and caspase 9, we also detected cleaved-caspase 3 protein abundance. As expected, 5% dietary WGP was sufficient to decrease cleaved-caspase 3 protein level. These data suggested that that dietary WGP supplementation could alleviate ROS induced apoptosis in jejunum epithelium.
To further explore the mechanism of anti-apoptotic effect of WGP in jejunum epithelium, we determined the antioxidative enzyme activities. In the present study, 10% WGP supplementation effectively enhanced the T-AOC activity, suggesting that WGP can be used as a feed ingredient to improve oxidative stability in jejunum epithelium. Moreover, dietary WGP supplementation enhanced the activity of catalase, GPx4 and SOD, which further confirmed the antioxidative capacity. Accordingly, protein contents of catalase, GPx4 and SOD were elevated when lambs were fed with WGP containing diet. The transcription factor Nrf2 is essential for the antioxidant responsive element mediated induction of phase II detoxifying (Itoh et al. Citation1999). Upon exposure to electrophiles or oxidative stress, Nrf2 is stabilised and enters the nucleus to activate transcription of targets bearing an antioxidant response element in the promoter. In the present study, dietary WGP supplementation did not affect Nrf2 protein abundance, suggesting that dietary WGP-mediated oxidation alleviation may not be via the Nfr2 transcription factor.
Conclusions
Dietary WGP supplementation to lambs relieves jejunum epithelial apoptosis, and modifies the jejunum epithelium structure, possibly due to the enhanced antioxidative capacity.
Ethical Approval
All animal procedures were approved by the Shanxi Agricultural University Animal Care and Ethical Committee.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bertelli AA, Das DK. 2009. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 54:468–476.
- Brenes A, Viveros A, Chamorro S, Arija I. 2016. Use of polyphenol-rich grape by-products in monogastric nutrition. A review. Anim Feed Sci Technol. 211:1–17.
- Carpenter R, O'Grady MN, O'Callaghan YC, O'Brien NM, Kerry JP. 2007. Evaluation of the antioxidant potential of grape seed and bearberry extracts in raw and cooked pork. Meat Sci. 76:604–610.
- Casamassima D, Palazzo M, Martemucci G, Vizzarri F, Corino C. 2012. Effects of verbascoside on plasma oxidative status and blood and milk production parameters during the peripartum period in Lacaune ewes. Small Rumin Res. 105:1–8.
- Cottart CH, Nivet-Antoine V, Beaudeux JL. 2014. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol Nutr Food Res. 58:7–21.
- Dai H, Meng XW, Kaufmann SH. 2016. BCL2 Family, mitochondrial apoptosis, and beyond. Cancer Transl Med. 2:7–20.
- Devasagayam T, Tilak J, Boloor K, Sane KS, Ghaskadbi SS, Lele R. 2004. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 52:794–804.
- Gessner DK, Fiesel A, Most E, Dinges J, Wen G, Ringseis R, Eder K. 2013. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet Scand. 55:1–10.
- Hogan S, Canning C, Sun S, Sun X, Zhou K. 2010. Effects of grape pomace antioxidant extract on oxidative stress and inflammation in diet induced obese mice. J Agric Food Chem. 58:11250–11256.
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13:76–86.
- Maréchal A, Zou L. 2013. DNA damage sensing by the ATM and ATR kinases. Perspect Biol. 5:1–17.
- Negroni A, Cucchiara S, Stronati L. 2015. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediat Inflamm. 2015(2):1–10.
- NRC. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington (DC): Natl. Acad. Press.
- Pileczki V, Cojocneanu-Petric R, Maralani M, Neagoe IB, Sandulescu R. 2016. MicroRNAs as regulators of apoptosis mechanisms in cancer. Clujul Med. 89:50–55.
- Potten CS, Booth C, Pritchard DM. 1997. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 78:219–243.
- Ramachandran A, Madesh M, Balasubramanian KA. 2000. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol. 15:109–120.
- Sallmyr A, Fan J, Rassool FV. 2008. Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 270:8762–8771.
- Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol. 24:R453–R462.
- Sehm J, Lindermayer H, Dummer C, Treutter D, Pfaffl MW. 2007. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. J Anim Physiol Anim Nutr (Berl). 91:289–296.
- Shakeri R, Kheirollahi A, Davoodi J. 2017. Apaf-1: regulation and function in cell death. Biochimie. 135:111–125.
- Shalini S, Dorstyn L, Dawar S, Kumar S. 2015. Old, new and emerging functions of caspases. Cell Death Differ. 22:526–539.
- Sinha K, Das J, Pal PB, Sil PC. 2013. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 87:1157–1180.
- Terevinto A, Cabrera M, Saadoun A. 2015. Influence of feeding system on lipids and proteins oxidation, and antioxidant enzymes activities of meat from Aberdeen Angus steers. J Food Nutr Res. 3:581–586.
- Viveros A, Chamorro S, Pizarro M, Arija I, Centeno C, Brenes A. 2011. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult Sci. 90:566–578.
- Wan J, Zhang J, Chen DW, Yu B, He J. 2017. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs. Anim Feed Sci Technol. 234:118–127.
- Wan J, Zhang J, Chen DW, Yu B, Mao XB, Zheng P, Yu J, Luo J, He J. 2018. Alginate oligosaccharide-induced intestinal morphology, barrier function and epithelium apoptosis modifications have beneficial effects on the growth performance of weaned pigs. J Anim Sci Biotechnol. 9:58.
- Watson AJ, Pritchard DM. 2000. Lessons from genetically engineered animal models. VII. Apoptosis in intestinal epithelium: lessons from transgenic and knockout mice. Am J Physiol Gastrointest Liver Physiol. 278:G1–G5.
- Xia EQ, Deng GF, Guo YJ, Li HB. 2010. Biological activities of polyphenols from grapes. Int J Mol Sci. 11:622–646.
- Yan L, Kim I. 2011. Effect of dietary grape pomace fermented by Saccharomyces boulardii on the growth performance, nutrient digestibility and meat quality in finishing pigs. Asian-Australas J Anim Sci. 24:1763–1770.
- Yu JM, Ahmedna M. 2013. Functional components of grape pomace: their composition, biological properties and potential applications. Int J Food Sci Technol. 48:221–237.
- Zhang C, Luo JQ, Yu B, Zheng P, Huang ZQ, Mao XB, He J, Yu J, Chen JL, Chen DW. 2015. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fibre characteristics and antioxidative status. Meat Sci. 102:15–21.
- Zhao JX, Li Q, Zhang RX, Liu WZ, Ren YS, Zhang CX, Zhang JX. 2018. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim Feed Sci Technol. 236:76–85.
- Zhao JX, Liu XD, Li K, Liu WZ, Ren YS, Zhang JX. 2016. Different dietary energy intake affects skeletal muscle development through an Akt-dependent pathway in Dorper × small thin-tailed crossbred ewe lambs. Domest Anim Endocrinol. 57:63–70.
- Zhao JX, Yan X, Tong JF, Means WJ, McCormick RJ, Zhu MJ, Du M. 2010. Mouse AMP-activated protein kinase gamma3 subunit R225Q mutation affecting mouse growth performance when fed a high-energy diet. J Anim Sci. 88:1332–1340.
