Abstract
We evaluated the effects of a compost bedded-pack barn as an alternative housing system for dairy cattle in Italy. Twenty-two Fleckvieh dairy cows were randomly allocated to two housing systems: (1) a conventional freestall barn (FB) and (2) a compost bedded-pack barn (CB). Clinical data and behavioural measurements were collected. Milk quality parameters and cheese characteristics were also evaluated. Scores for hind limb cleanliness and locomotion were better in the CB-housed than in the FB-housed cows (p < .01). The incidence of standing was higher (p < .01), while that of lying in head-up position and the expression of agonistic interaction were lower (p < .001) in the CB-housed animals. An avoidance distance of 50 cm was more frequent, whereas a distance of more than 100 cm was less frequent among the CB-housed animals (p < .001). Milk fat content was greater and somatic cell count was lower, as were total bacterial count (TBC) and coliforms in cheese made from milk from the CB-housed cows (p < .05). Descriptive comparisons between CB and FB bedding materials revealed promising results for salinity, fulvic + humic acid content, ammonia concentration, and TBC, but also issues related to bacterial levels, temperature, and nutrient composition of CB bedding material. Our results suggest that cow health and welfare, as well as milk and milk product quality, may benefit from CB housing, supporting its potential as a promising alternative loose-housing system for dairy cattle in Italy.
Housing systems and management practices exert a remarkable influence on dairy cattle welfare, making the choice of environment extremely important.
Cow health and welfare, as well as milk and milk product quality, may benefit from compost bedded-pack housing system.
Issues related to bacterial levels, temperature, and nutrient composition of bedding material may be encountered in compost bedded-pack housing system.
Highlights
Introduction
Public concern about farm animal welfare continues to focus on animal functioning, feeling, and ability to live a relatively natural life and express natural behaviours. This last aspect has been extensively investigated in recent years, leading to mounting evidence that animals experience pain or distress from widely accepted management practices that subject them to being kept under apparently ‘unnatural’ conditions (Fraser Citation2008). Animal performance can also be indirectly influenced by management decisions that determine the conditions under which animals live (Adler et al. Citation2019). The choice of environment for lactating dairy cows is therefore a key decision for dairy producers (Bewley et al. Citation2017), especially because herd productivity and profitability may strictly depend on it (Villettaz Robichaud et al. Citation2019).
Housing system and management practices have been reported to widely influence animal productivity, health, milk quality, reproduction, and well-being, as well as farm profitability (Bewley et al. Citation2017). Even if less documented, also cheese characteristics – especially when local, traditional products are concerned – may vary on varying the animal rearing system (Romanzin et al. Citation2013). In this regard, the compost bedded-pack barn constitutes an alternative loose-housing system that was developed in Virginia (USA) to improve cow comfort, increase animal longevity, and reduce initial barn costs, while potentially reducing the risk of mastitis associated with conventional bedded packs (Wagner Citation2002). The bedded-pack system is composed of a large bedded-pack (resting) area separated from a feed alley by a 1.2-m-high concrete wall. Different from conventional bedded-pack barns, the bedding material (predominantly dry, fine wood shavings or sawdust) is aerated twice daily with cultivator-type equipment to dry the surface and incorporate manure into the pack (Barberg et al. Citation2007a).
Previous research on the potential of using the compost bedded-pack barn as a housing system for dairy cattle has yielded promising results for animal health, welfare, and performance. Indeed, compared to those kept in conventional housing facilities, cows housed with this system had lower hock scores (Barberg et al. Citation2007b; Fulwider et al. Citation2007; Lobeck et al. Citation2011), lower incidence of lameness (Barberg et al. Citation2007b; Petzen et al. Citation2009; Lobeck et al. Citation2011; Black et al. Citation2013), lower somatic cell count and incidence of mastitis (Barberg et al. Citation2007b), and improved reproductive performance (Barberg et al. Citation2007b). No such studies (also focussing on milk and milk product quality) are currently available for Italy.
Based on the above reported background, the aims of the present study were to (1) develop and describe a compost bedded-pack barn as a housing system for dairy cattle, (2) assess and compare the health and welfare of cows housed in this alternative system versus those kept in a conventional one (freestall barn), and (3) characterise the quality of the milk and three local, traditional products of the North-West Italy: Bra, Raschera and Toma cheeses.
Materials and methods
Animals and experimental design
All experimental procedures were conducted in accordance with current animal welfare regulations (Directive 98/58/EC and Italian Decree Law 146/2001).
The study was conducted between January 2014 and December 2014 at the Cascina Bianca farm (Cervasca, CN, Italy). A total of twenty-two 2-year-old primiparae Fleckvieh cows in early lactation were specifically bought from the same supplier for the purposes of the study. After an acclimation period of 40 days in a separate stall, the animals were randomly allotted to two housing systems: 11 were housed in a conventional freestall barn (FB) with a concrete floor in which space allowance was 10 m2/head and 11 were housed in a compost bedded-pack barn (CB) in which space allowance was at least 25 m2/head. The CB facility consisted of a large resting area open on one side supported by wooden trusses and covered with plastic sheets. About 50 cm of permanent, organic bedding material was distributed on the floor and aerated twice daily without additional bedding added. The compost used as bedding material was obtained from domestic food (60%) and vegetable (40%) wastes. Its physicochemical characteristic were: humidity 23.4%, pH 6.6, organic carbon (C) 35.9% of dry matter (DM), organic nitrogen (N) 95.3% of DM, carbon to nitrogen ratio (C/N) 19.08 and fulvic and humic acids (FA + HA) 8.48% of DM. A transportable manger with water troughs and a milking pen with places for four cows at a time were also provided, with the latter facilitating both milking operations and clinical examination. Before beginning the study, all animals were tested for bovine viral diarrhoea virus (BVDV) and paratuberculosis and underwent a complete blood count (CBC) and biochemical profile. Finally, the animals were fed twice a day with the same quantity of unifeed; water was provided ad libitum.
Clinical examination
Clinical data were obtained during farm visits every 2 weeks (for a total of 15 examinations throughout the study period). The data for each cow were collected by the same two experienced, trained observers who recorded body condition score (BCS), hygiene score, locomotion score (LS), number of hock and hoof lesions, faecal score (FS) and general physical examination findings. The BCS is derived from a 5-pointscale, from 1 (thin) to 5 (obese) (Ferguson et al. Citation1994). Animal hygiene was measured using a 5-point hygiene score system from 1 to 5, wherein 1 denotes clean cows and 5 very dirty cows (Reneau et al., Citation2005). Lameness status was evaluated using the 5-point locomotion scoring system described in Sprecher et al. (Citation1997), with additional observations suggested by O’Callaghan et al. (Citation2003), as follows:
Score 1, normal locomotion – the cow stands and walks with a level-back posture. Her gait is normal.
Score 2, imperfect locomotion – the cow stands with a level-back posture but develops an arched-back posture while walking. Her gait remains normal.
Score 3, moderately lame – an arched-back posture is evident both while standing and walking. Her gait is affected and is best described as short-striding with one or more limbs.
Score 4, lame – an arched-back posture is always evident and gait is best described as one deliberate step at a time. The cow favours one or more limbs/feet.
Score 5, severely lame – the cow additionally demonstrates an inability or extreme reluctance to bear weight on one or more of her limbs/feet.
The presence of hock lesions was scored as follows: no lesions, mild lesion (hair loss), moderate lesion (skin wounds) or severe lesion (swollen hocks). Hoof lesions involving white line, interdigital area, horn, sole, and toe were also diagnosed and recorded by a veterinary podiatrist. Faecal scores based on a 4-point scale were assigned as follows: 1 normal-firm but not hard, 2 soft-does not hold firm, 3 runny-spreads easily, and 4 devoid of solid matter (Larson et al. Citation1977). Episodes of coughing, nasal, ocular and vulvar discharges, dyspnoea, diarrhoea, ruminal tympanism, dystocia, downer cow syndrome, and mammary gland alterations were also recorded. To avoid any potential bias, the two observers switched over to evaluate both housing facilities.
Behaviour assessment
Behaviour measurements were collectively recorded from each housing system before the clinical procedures (for a total of 15 measurements throughout the study period) by the same observers. The cows were continuously monitored for 4-h-periods, with observations taking place at the same time of day (starting at approximately 2 h after the morning milking) and from a standardised perched point. Every 15 min, the number of animals standing, lying (with type of lying position), ruminating, or eating, and expressing social and agonistic interactions was recorded. The lying positions were: flat on the side, head on the ground and head up (Krohn and Munksgaard Citation1993). The social interactions were allogrooming (licking another cow) and mutual sniffing, while chasing away (the actor cow coming within 0.5 m of the reactor cow and causing her to move away without any physical contact), pushing (hard push by the actor cow against the body of the reactor cow, causing her to move 1 or 2 steps), and head butting (fast blow with the head by the actor cow, rather than a hard push), to the reactor cow, generally not causing the reactor cow to retreat from the actor cow) were considered as agonistic behaviours (Endres and Barberg Citation2007). Lying down time was also recorded individually and summarised as the mean lying down time per 4 h-period for each housing system. An avoidance distance test at the feeding place was also individually recorded (Waiblinger et al. Citation2003). To assess the avoidance distance, the experimenter positioned himself in the feeding aisle (FB) or milking pen (CB) 1.5 m in front of a standing animal. He held his arms out (with the backside of the hand pointing at the animal’s muzzle) at a 45° angle in front of him, gazed at the muzzle and waited for the cow to focus attention. He then slowly approached the cow at a constant speed of 1 step/s from the front until the animal withdrew or allowed touching. The distance between the animal’s muzzle and the experimenter’s hand was estimated in steps of 10 cm at the moment of withdrawal. Four situations were recorded: (1) touching (an avoidance distance of 0 cm), (2) withdrawal at an avoidance distance of 50 cm, (3) withdrawal at an avoidance distance between 50 and 100 cm, and (4) withdrawal at an avoidance distance of more than 100 cm. Avoidance distance tests were conducted when the cows were locked in the feeding rack (FB) or milking pen (CB) before clinical examination. Avoidance distance was measured three times per cow in order to ensure robustness of data. The two observers periodically evaluated both housing facilities.
Milk quality evaluation
Milk samples were taken from each animal every month during the lactation period (for a total of eight samples throughout the study period). The milk volume was individually measured using a bucket during milking. After milking, standardised samples were collected, immediately put under ice, transported to the Provincial Farmers’ Association (APA) laboratory (Cuneo, Italy) and refrigerated at 4 °C until processing. Centesimal composition of fat, protein, lactose, casein, and urea in milk samples was analysed using the infra-red spectroscopy method (ISO 9622 - IDF Standart 141C: 2000); somatic cell count (SCC) was measured using the flow cytometer method (13366-2 - IDF Standard 148-2: 2006).
Bedding analysis
Bedding samples from the CB housing system were collected for physicochemical and bacterial analysis in May, July and October (for a total of three analyses throughout the study period). The CB was divided into 12 equally sized regions and bedding temperature was recorded for each. A composite measure of four bedding surface samples (top 5 cm) was collected in each of the 12 areas within the barn (Barberg et al. Citation2007b). Bedding samples were similarly collected from the FB in May. All bedding samples were immediately cooled and later frozen until analysis in a local specialised laboratory (Medilabor, Cavallermaggiore, CN, Italy). The physicochemical parameters were pH, humidity (%), organic C and N (% of DM), C/N, salinity (µS/cm2), ammonia (mg/kg), and FA + HA (% p/p of DM). Salmonella spp., faecal streptococci, Enterobacteriaceae and vital microorganisms were also determined (colony-forming units per gram of bedding sample, CFU/g). The pH, humidity, organic C and N and ammonia parameters, as well as Salmonella spp., faecal streptococci, Enterobacteriaceae and vital microorganisms, were determined according to IRSA-CNR (Citation1985). The C/N was directly calculated, while salinity and FA + HA parameters were determined according to an internal method and ANPA (Citation2001), respectively.
Cheese characteristics
From April to November, the milk obtained from all the cows from each housing system was made into cheese every 2 weeks (for a total of 16 examinations throughout the study period). The coagulation time and curd pH and consistency were evaluated for (1) milk (independently of the produced cheese) and (2) three local cheeses (Bra, Raschera and Toma). Total bacterial count (TBC) and coliforms were also measured in the pasteurised milk before the cheesemaking and in the three different cheeses according to Harrigan (Citation1998). Finally, cheese ripening defects were recorded.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software (version 5.0, GraphPad Software, Inc., La Jolla, CA, USA). Shapiro–Wilk’s test was applied to establish normality or non-normality of data distribution. The Mann–Whitney U test was used to test for differences in the three scale scores (BCS, LS and FS) between the CB-housed and the FB-housed cows, as well as data on milk production, fat, protein, lactose, casein, urea and SCC (mean of total examinations and analyses per cow). Two-way ANOVA for repeated measures (post-hoc Bonferroni) test was used to evaluate the influence of housing system (CB and FB) on hygiene scores, number of animals observed standing, lying, ruminating, eating, and cow lying positions (mean of the total number of animals per observation), as well as avoidance test (total number of cows per observation). The Mann–Whitney U test was used to determine differences in social and agonistic interactions (total number of events per observation), as well as lying down time (mean of the total number of animals per observation) between the CB-housed and the FB-housed cows. Spearman rank correlation and Mann–Whitney U tests were performed to evaluate the relationship and to compare, respectively, between compost bedding (mean of the 12 examined areas) and environmental (mean of monthly records) temperature data. Finally, the Wilcoxon rank sum test was used to determine differences in coagulation time, curd pH, TBC and coliforms count (single values per group per each examination) for both the milk (independently on the produced cheese) and the cheeses (differentiating the three cheese types) between the CB-housed and the FB-housed cows.
The results are presented as the mean and standard error of the mean (SEM, data in the text) and the median and interquartile range (IQR, 25–75%, data in the Tables).
Results
Clinical examination
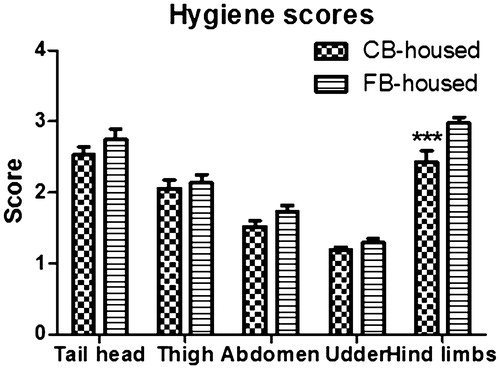
All animals were negative for both BVDV and paratuberculosis; CBC and clinical biochemistry showed no significant alterations. The BCS did not differ (p > .05) between the CB-housed (3.05 ± 0.03) and the FB-housed (3.05 ± 0.03) cows. Hygiene scores for lower hind limb cleanliness were better for the CB-housed animals (p < .001) (Figure ), as were the LS (p < .01) (1.00 ± 0.00 versus 1.11 ± 0.03). No hock or hoof lesions were observed in the CB-housed animals, whereas 25% of the FB-housed cows presented hock and 5% presented hoof lesions at each visit. Furthermore, among the 14 episodes of altered locomotion recorded in the FB-housed animals, 12/14 (85.7%) were scored with 2 and 2/14 (14.3%) with 3. The majority of the cows scoring 2 (11/12, 92%) were diagnosed with sole haemorrhages, while the animals scoring 3 were diagnosed with sole abscess (1/2, 50%) and heel traumatic injuries (1/2, 50%), respectively. One of the two FB-housed cows scoring 3 also remained chronically lame throughout the experimental period, reducing its LS from 3 to 2. No difference in FS was noted between the CB-housed (p > .05) and the FB-housed animals (1.41 ± 0.10 versus 1.45 ± 0.09). Among the 46 alterations observed at general physical examination, 15/46 (33%) were noted in the CB-housed cows and 31/46 (66%) in the FB-housed cows. In detail: coughing with nasal discharge was seen in 10/15 (66%,) of the CB-housed animals, ocular discharge in 3/15 (20%), vulvar discharge in 1/15 (7%) and skin alopecia in 1/15 (7%), whereas ocular discharge developed in 10/31 (32%) of the FB-housed cows, skin abscesses in 8/31 (26%), alopecia in 5/31 (16%), mycosis in 1/31 (3%), coughing with nasal discharge in 5/31 (16%) and flea infestation in 2/31 (7%). No macroscopic mammary gland alterations or episodes of clinical or subclinical mastitis occurred in the CB-housed animals. Similarly, no mammary gland alterations or episodes of clinical mastitis occurred in the FB-housed cows. However, subclinical mastitis was noted at each SCC evaluation in 21% of these animals. Finally, no cows received pharmacological treatment for disease.
Behaviour assessment
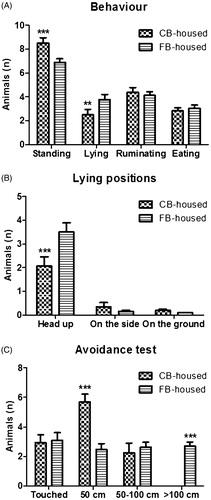
The incidence of standing was higher and the incidence of lying was lower among the CB-housed animals (p < .001 and p < .01, respectively) (Figure ). Animals in head up lying position (p < .001) were more frequently observed among the CB-housed cows (Figure ). Social positive interactions did not differ between the two groups (4.46 ± 0.74 for the CB-housed versus 6.09 ± 0.89 for the FB-housed; p > .05); agonistic interactions were less frequent among the CB-housed than the FB-housed (6.36 ± 1.08 versus 18.55 ± 2.93; p < .001). Lying down time did not differ between the CB-housed and the FB-housed animals (5.25 ± 0.11 and 5.14 ± 0.09, respectively; p > .05). Compared to the FB-housed animals, more CB-housed cows withdrew at an avoidance distance of 50 cm (p< .001) and fewer at an avoidance of more than 100 cm (p < .001) (Figure ).
Milk quality evaluation
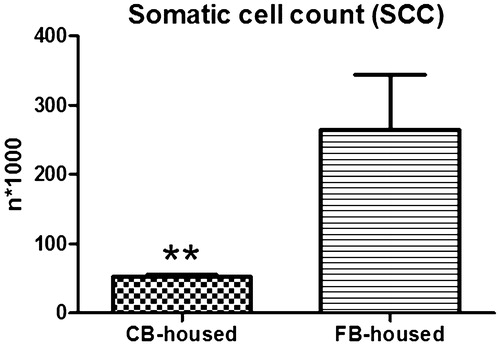
The milk volume did not differ between the two groups (CB-housed 20.98 ± 0.64 kg and FB-housed 19.49 ± 0.66 kg; p > .05). Fat content was higher in the milk from the CB-housed than in that from the FB-housed cows (4.04 ± 0.11% versus 3.54 ± 0.12%; p < .05), whereas no differences between the two groups was observed for milk protein (CB-housed 3.48 ± 0.06% versus FB-housed 3.28 ± 0.06%; p > .05), lactose (CB-housed 4.97 ± 0.02% versus FB-housed 4.99 ± 0.02%; p > .05), casein (CB-housed 2.78 ± 0.05% versus FB-housed 2.61 ± 0.04%; p > .05) and urea (CB-housed 27.44 ± 3.21% versus FB-housed 24.32 ± 1.60%; p > .05). The SCC was lower (p < .01) in the CB-housed cows (Figure ).
Bedding analysis
Table presents the physicochemical and bacteriological findings for the CB and FB bedding materials. Compared to environmental temperature, the CB bedding material temperature was higher (31.02 ± 1.57 °C versus 15.75 ± 3.18 °C; p < .05). However, no significant relationship was observed between CB bedding material and environmental temperature (p > .05).
Table 1. Physicochemical and bacterial analyses of CB and FB bedding materials.
Cheese characteristics
The milk and cheese characteristics are presented in Tables and , respectively. There were no differences in the milk coagulation time and curd pH for the two groups (Table , p > .05). The TBC in the pasteurised milk from CB-housed animals was lower (p < .01) when compared to that from FB, while no significant differences between the two groups (p > .05) were observed for coliforms. As far as the cheese type is concerned, there were no differences in the milk coagulation time and curd pH for the two housing systems (Table , p > .05). The TBC in the Bra and Toma cheeses from the CB-housed cows were lower (p = .001) than those obtained from FB, as were the coliforms (p < .05). On the contrary, the coliforms in the Raschera cheese from the CB-housed animals were higher (p < .05) when compared to that from FB, with the TBC still being lower (p < .05). Finally, no signs of late swelling, moulds or yeasts were noted in the cheese from either group.
Table 2. Characteristics and bacteriological findings of the pasteurised milk obtained from CB-housed and FB-housed cows.
Table 3. Characteristics and bacteriological findings of cheese made from the pasteurised milk obtained from CB-housed and FB-housed cows.
Discussion
Clinical examination
The average BCS (3.05 ± 0.03) was similar for the two groups, indicating a normal nutritional status (Ferguson et al. Citation1994). The overall hygiene scores were better for the CB-housed animals (average 1.95 ± 0.09 for the CB-housed versus 2.18 ± 0.06 for the FB-housed), with a significant difference in lower hind limb cleanliness, owing to the different bedding material, which is drier and less apt to adhere to the animal’s coat. The hygiene scores we observed are in line with the better hygiene scores Fulwider et al. (Citation2007) reported for CB-housed cows than for animals housed with sand FB or rubber-filled mattresses. In contrast, similar hygiene scores were observed between CB-housed and FB-housed animals on waterbeds (Fulwider et al. Citation2007) or sand-bedded FB (Eckelkamp et al. Citation2016). Lobeck et al. (Citation2011) even reported that overall hygiene scores were worst for CB-housed cows than animals in sand-bedded cross-ventilated and naturally ventilated FB. The mean hygiene score we recorded for the CB-housed (1.95) was remarkably better than the scores reported in previous studies (2.66, Barberg et al. Citation2007b; 3.1, Shane et al. Citation2010; and 3.18, Lobeck et al. Citation2011). This difference could be attributed to the space allowance per cow, since overstocking the CB may result in more dirty cows (Black et al. Citation2014). Although pack density was in line with the range of 6.0–7.4 m2/cow recommended by Janni et al. (Citation2007), there was a huge difference between the space allowance in our study (25 m2/cow) and the previous studies mentioned above. Indeed, the space allowance therein reported ranged from 7.6 ± 1.1 (Barberg et al. Citation2007b) to 8.6 ± 2.6 m2/cow Lobeck et al. Citation2011). Also important is that hind limb cleanliness is closely related to scraping frequency, ease of movement and manure management (Schreiner and Ruegg Citation2003). In a partial confirmation, dirty lower hind limbs were more often observed among the FB-housed cows, which also had less space allowance (10 m2/head versus 25 m2/head).
The lameness score was better among the CB-housed cows, as previously observed by Barberg et al. (Citation2007b), Black et al. (Citation2013), Lobeck et al. (Citation2011) and Petzen et al. (Citation2009), who reported less lameness in CB-housed than in FB-housed animals. Furthermore, hock and hoof lesions developed only in the FB-housed cows. This may have been related to the softer bedding material used for the CB, since concrete flooring and/or uncomfortable FB have been reported to increase the incidence of lameness and hock lesions (Cook et al. Citation2004).
Overall, the occurrence of pathological events was lower among the CB animals, suggesting their better health status. This could be attributed to the greater promiscuity, since greater space allowance may reduce the spread of contagious pathogens.
Behaviour assessment
The CB-housed cows were more often observed standing than lying as compared to the FB-housed cows. Haley et al. (Citation2001) reported that cows kept in an environment with softer flooring lay down for longer times over the entire day when compared to animals housed in barns with concrete floors. The authors also hypothesised that cows are more reluctant to stand up and lie down on hard surfaces because of the discomfort related to changing position of standing up and lying down. Endres and Barberg (Citation2007) observed that CB systems in general have a soft, cushioned lying surface that allows cows to stand up and lie down without apparent discomfort. Therefore, our results suggest that the CB-housed animals were comfortable and capable of expressing a normal lying behaviour. The CB-housed cows were noted to show lower head-up lying positions as compared to the FB-housed cows. This observation contrasts with Endres and Barberg (Citation2007), who found that the majority of the CB-housed animals they observed lying down assumed the head-up position. However, cows have been reported to spend more time lying with their heads resting on the ground or back when housed on pasture in comparison with tie-stall barns (Ketelaar-de Lauwere et al. Citation1999). Since CB is a loose-housing system, it is reasonable to hypothesise that animals can behave in a similar way. Finally, the lying down time was the same for the CB-housed and the FB-housed cows. This observation is shared by a previous study, in which the lying down time did not differ between cows housed in tie-stalls and those in loose housing systems (Krohn and Munksgaard Citation1993).
Similar social positive interactions were observed for the CB-housed and the FB-housed cows, except that the CB-housed animals exhibited less agonistic interactions. This difference may be related to the greater space allowance per cow. This hypothesis seems to be supported by Miller and Wood-Gush (Citation1991), who reported that the number of agonistic behaviours of animals housed in indoor cubicles was greater than those of cows on pasture. Similarly, agonistic interactions have been reported to occur 5–6 times less often in cows on pasture than in those kept in tie-stalls, concrete yards or bedded packs (Krohn Citation1994).
Avoidance test results for the CB-housed cows may also be related to the greater amount of space allowed per animal, consistent with the negative association between avoidance distance and herd size (Waiblinger et al. Citation2003).
Milk quality evaluation
Milk production volume was similar for both groups. Fat content was higher in the milk from the CB-housed cows. Barberg et al. (Citation2007b) similarly reported an increase in milk fat content after moving their cows to CB, but gave no specific explanation for this finding. Furthermore, the SCC was lower for the CB-housed animals, which may be related to their better coat cleanliness, since individual cow SCC has been reported to be positively associated with dirty lower hind limbs and udder (Schreiner and Ruegg Citation2003; Reneau et al. Citation2005). Interestingly, a lower SCC could explain the greater milk fat content. Indeed, milk fat content has been reported to decrease with increasing SSC as a result of cell detachment damage due to mammary gland oedema (Garcia et al. Citation2015). Although SCC values below the state average were observed in Minnesota cows after transitioning to CB (Barberg et al. Citation2007b), no differences in relation to SCC or bulk tank SCC were found between CB-housed animals and those in sand FB (Eckelkamp et al. Citation2016). Furthermore, the average SCC in the milk from the CB-housed cows of our study (51,510 cells/mL) was remarkably lower than those reported previously (325,000 cells/mL, Barberg et al. Citation2007b; 133,000 cells/mL, 214,000 cells/mL, and 229,000 cells/mL, Klaas et al. Citation2010).
Bedding analysis
The mean CB bedding material temperature (31.02 ± 1.57 °C) was lower than that reported previously (42.5 ± 7.6 °C, Barberg et al. Citation2007a; 33.5 ± 8.5 °C, Klaas et al. Citation2010; and 36.1 ± 11.0 °C, Black et al. Citation2014). This difference could be attributed to the difference in the number and depth of the CB regions sampled. However, none of the temperatures reported in our and in previous studies reached the level necessary (55–65 °C) for material sanitisation. As suggested by Black et al. (Citation2014), the lack of material sanitisation during microbial processes in the CB indicates that the system is more like a semi-composting system that does not fully cycle through the entire composting process. This represents a potential drawback that needs to be investigated in future studies, since the microbial population is much more diverse and not as efficient at degrading CB bedding material when temperatures are between 35 and 40 °C (Stentiford Citation1996). However, the average temperature we measured in the CB bedding material was higher than that of the environment, indicating that periodical aeration increases metabolic heat production by aerobic microbes and bacteria (Black et al. Citation2014). Comparison across months shows that the lowest CB bedding material temperature was recorded in July, suggesting a reduction in fermentation during the summer. This reduction may also be reflected by the concomitant decrease in FA + HA content. Furthermore, the average CB bedding material moisture was 48.12 ± 5.85%, which was quite similar to the 56.1 ± 12.4% reported by Black et al. (Citation2014). Given that the optimal moisture content for composting is between 40% and 60% (Stentiford Citation1996), this is a promising result. Indeed, excessive moisture content may inhibit aerobic activity (NRAES Citation1992) and increase the ease with which material can adhere to teat ends (Black et al. Citation2014).
The mean pH of the CB bedding material was 8.72 ± 0.20, which is quite similar to the range commonly recommended for matured compost (7.0–8.5, Yang et al. Citation2013). Furthermore, the average CB bedding material C/N was 2.77 ± 0.36. This contrasts with earlier studies that reported an average C/N of 19.5 ± 7.5 (Barberg et al. Citation2007a) and 26.7 ± 7.8 (Black et al. Citation2014). These higher values may be related to the greater availability of bedding material in the United States than in Italy and to the differences in CB dimensions. Since the recommended range for optimal composting has been reported to be 25:1 to 30:1 (NRAES Citation1992), further studies are needed to improve C/N.
The average TBC we calculated for the CB bedding material was 11.46 ± 0.12 log10 CFU/g of DM. This contrasts with previous studies that reported average bacterial levels of 7.0 ± 6.8 (Barberg et al. Citation2007a) and 8.2 ± 0.4 (Black et al. Citation2014) log10 CFU/g, respectively The higher bacterial counts we found may be related to environmental differences between the United States and Italy, farm management practices or bedding materials, as suggested by Black et al. (Citation2014). Irrespective of the cause, the higher bacterial levels could pose a potential drawback. Bacterial counts in bedding are known to be directly related to bacterial counts on teat ends and clinical mastitis rates: bedding containing more than 106 CFU of total bacteria/g is, indeed, associated with increased risk of intramammary infection (Black et al. Citation2014). However, the CB-housed cows showed no mammary gland or teat alterations, along with remarkably low SCC, thus suggesting no negative influence of bedding bacteria on cow health in the present study.
In the final comparison between CB and FB bedding materials, some results appear particularly relevant. First, the mean CB bedding material salinity (15,483 ± 12,327 µS/cm2) was remarkably higher than the FB bedding. This may be related to the biodegradation of organic matter typical of composting process, which is characterised by the release of mineral salts, such as ammonium and sulphur ions. Furthermore, water generally evaporates during composting (as seen by the reduced moisture content in CB bedding) and thus concentrates the composting matrix and raises the salinity (Zhang et al. Citation2018). Another aspect to consider is that the average material ammonia content in the CB bedding (312.6 ± 297.0 mg/kg) was lower than in the FB. Aside from the reduced moisture content, this could be explained by the fact that most of the accessible nitrogen is usually bound in microorganisms in the compost (Klaas et al. Citation2010). Also, the mean FA + HA content (16.33 ± 4.16% p/p dry matter) was higher in the CB bedding material. During composting, organic matter is gradually transformed into humic substances (i.e. humic and fulvic acids), with the degree of humification of organic matter considered as an agronomic criterion of compost quality (Zhang et al. Citation2018). Finally, the average TBC, faecal streptococci (5.62 ± 0.48 log10 CFU/g DM) and enterobacteriaceae (7.31 ± 0.69 log10 CFU/g DM) levels were lower in the CB bedding material. This finding is relevant for animal health, since environmental mastitis is generally caused by Gram-negative bacteria of the Enterobacteriaceae family (i.e. coliforms, Klebsiella spp., Enterobacter spp. and non-coliform Enterobacteriaceae such as Serratia spp.) and Gram-positive catalase-negative cocci (i.e. streptococci, enterococci and lactococci) (Klaas and Zadoks Citation2018).
Cheese characteristics
This is the first study to characterise cheese made from the milk of CB-housed cows. The milk coagulation time and curd pH (either considering or not considering the different cheese types) were similar for both groups, suggesting that the CB housing system does not negatively affect the transformation of milk into cheese. The TBC and coliform levels in the milk from the CB-housed cows, as well as in Bra and Toma cheeses, were also lower when compared to those observed for FB. This suggests the potential for improvement in food quality and safety. The identification of higher coliform levels in Raschera cheese obtained from CB-housed animals than FB appears, however, difficult to explain. This cheese type is characterised by a greater fat content (43–53%) than Bra (35%) and Toma (45%). High cheese fat content has also been reported to increase the populations of non-starter lactic acid bacteria in dairy cows (Fenelon et al. Citation2000). Since fat content was higher in the milk from the CB-housed cows when compared to that from FB, a potential relationship between the fat content and other bacteria (such as coliforms) cannot be excluded. Furthermore, another factor potentially determining the differences in Raschera coliforms between the two housing systems may be represented by the peculiar seasoning characteristics of this cheese. Indeed, only the Raschera has a milk pre-maturation period of 1.30 h that can significantly affect the bacterial proliferation. Considering that we hypothesised that coliforms may be associated with increasing cheese fat content, and we observed that milk obtained from CB-housed cows had a higher fat content than that from FB, it is reasonable that the coliform proliferation could have been more pronounced in Raschera from CB-housed cows during the milk pre-maturation period. However, the overall findings related to the milk and cheese microbiology are consistent with the lower SCC and overall better lower hind limb and udder cleanliness observed in the CB-housed animals. The average TBC measured in the milk from the CB-housed animals was higher than those previously reported (15,392 CFU/mL, Rodrigues et al. Citation2005; and 3420 CFU/mL, Barberg et al. Citation2007b). This was probably related to the higher mean TBC detected in the CB bedding material. However, it is important to underline that the TBC in milk and cheese was lower compared to the bacterial levels found in the CB bedding material. Since the type and number of bacteria in bedding material are generally related to the total bacterial load on the teat ends and rates of clinical mastitis in lactating dairy cows (Black et al. Citation2014), our results in terms of TBC reduction suggest that cow preparation procedures at milking time were effective in achieving high milk quality. The two limitations of the present study are the small sample size and the single study site. Further studies involving a larger sample size and multiple sites are strongly desired.
Conclusions
Dairy cow health and welfare and milk and milk product quality may benefit from use of a CB housing system, which holds potential for becoming a promising, alternative loose-housing system for dairy cattle also in Italy. However, additional research is needed to investigate and solve the issues related to bacterial levels, temperature and nutrient composition of bedding material.
Ethical Approval
The study was approved by the Animal Welfare and Ethical Committee of the Department of Veterinary Sciences of the University of Turin (Italy).
Acknowledgements
The authors gratefully acknowledge Mr Bima Livio (Cascina Bianca farm) for technical support and animal care.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adler F, Christley R, Campe A. 2019. Invited review: examining farmers' personalities and attitudes as possible risk factors for dairy cattle health, welfare, productivity, and farm management: a systematic scoping review. J Dairy Sci. 100:1–16.
- ANPA. 2001. Metodi di analisi del compost. Capitolo 11. In: ANPA, editor. Manuale e linee guida ANPA 3/2001. Rome (Italy): ANPA.
- Barberg AE, Endres MI, Janni KA. 2007a. Compost dairy barns in Minnesota: a descriptive study. Appl Eng Agric. 23:231–238.
- Barberg AE, Endres MI, Salfer JA, Reneau JK. 2007b. Performance and welfare of dairy cows in an alternative housing system in Minnesota. J Dairy Sci. 90:1575–1583.
- Bewley JM, Robertson LM, Eckelkamp EA. 2017. A 100-year review: lactating dairy cattle housing management. J Dairy Sci. 100:10418–10431.
- Black RA, Taraba JL, Day GB, Damasceno FA, Bewley JM. 2013. Compost bedded pack dairy barn management, performance, and producer satisfaction. J Dairy Sci. 96:8060–8074.
- Black RA, Taraba JL, Day GB, Damasceno FA, Newman MC, Akers KA, Wood CL, McQuerry KJ, Bewley JM. 2014. The relationship between compost bedded pack performance, management, and bacterial counts. J Dairy Sci. 97:2669–2679.
- Cook NB, Bennett TB, Nordlund KV. 2004. Effect of free stall surface on daily activity patterns in dairy cows with relevance to lameness prevalence. J Dairy Sci. 87:2912–2922.
- Eckelkamp EA, Taraba JL, Akers KA, Harmon RJ, Bewley JM. 2016. Sand bedded freestall and compost bedded pack effects on cow hygiene, locomotion, and mastitis indicators. Livest Sci. 190:48–57.
- Endres MI, Barberg AE. 2007. Behavior of dairy cows in an alternative bedded-pack housing system. J Dairy Sci. 90:4192–4200.
- Fenelon MA, O’Connor P, Guinee TP. 2000. The effect of fat content on the microbiology and proteolysis in cheddar cheese during ripening dairy foods. J Dairy Sci. 83:2173–2183.
- Ferguson JD, Galligan DT, Thomsen N. 1994. Principal descriptors of body condition score in Holstein cows. J Dairy Sci. 77:2695–2703.
- Fraser D. 2008. Understanding animal welfare: the science in its cultural context. Oxford (UK): Wiley-Blackwell.
- Fulwider WK, Grandin T, Garrick DJ, Engle TE, Lamm WD, Dalsted NL, Rollin BE. 2007. Influence of free-stall base on tarsal joint lesions and hygiene in dairy cows. J Dairy Sci. 90:3559–3566.
- Garcia RR, Maioni VB, de Almeida KM, de Santana EHW, Costa MR, Fagnani R, Ludovico A. 2015. Relationship between somatic cell counts and milk production and composition in Jersey cows. Rev Salud Anim. 37:137–142.
- Haley DB, De Passille AM, Rushen J. 2001. Assessing cow comfort: effects of two floor types and two tie stall designs on the behaviour of lactating dairy cows. Appl Anim Behav Sci. 71:105–117.
- Harrigan WF. 1998. Laboratory methods in food microbiology. 3rd ed. San Diego (USA): Academic Press.
- IRSA-CNR. 1985. Metodi analitici per i fanghi. Parametri chimico-fisici. In: IRSA-CNR, editor. Quaderni IRSA-CNR 64. Rome (Italy): Edizioni CNR.
- Janni KA, Endres MI, Reneau JK, Schoper WW. 2007. Compost dairy barn layout and management recommendations. Appl Eng Agric. 23:97–102.
- Ketelaar-de Lauwere CC, Ipema AH, Ouwerkerk ENJ, Hendriks M, Metz JHM, Noordhuizen J, Schouten W. 1999. Voluntary automatic milking in combination with grazing of dairy cows: milking frequency and effects on behaviour. Appl Anim Behav Sci. 64:91–109.
- Klaas IC, Zadoks RN. 2018. An update on environmental mastitis: challenging perceptions. Transbound Emerg Dis. 65:166–185.
- Klaas IC, Bjerg BS, Friedmann S, Bar D. 2010. Cultivated barns for dairy cows: an option to promote cattle welfare and environmental protection in Denmark? Dansk Veterinaertidsskrift. 93:20–29.
- Krohn CC. 1994. Behaviour of dairy cows kept in extensive (loose housing/pasture) or intensive (tie stall) environments. III. Grooming, exploration and abnormal behaviour. Appl Anim Behav Sci. 42:73–86.
- Krohn CC, Munksgaard L. 1993. Behaviour of dairy cows kept in extensive (loose housing/pasture) or intensive (tie stall) environments. II. Lying and lying-down behaviour. Appl Anim Behav Sci. 37:1–16.
- Larson LL, Owen FG, Albright JL, Appleman RD, Lamb RC, Muller LD. 1977. Guidelines toward more uniformity in measuring and reporting calf experimental data. J Dairy Sci. 60:989–991.
- Lobeck KM, Endres MI, Shane EM, Godden SM, Fetrow J. 2011. Animal welfare in cross-ventilated, compost-bedded pack, and naturally ventilated dairy barns in the upper Midwest. J Dairy Sci. 94:5469–5479.
- Miller K, Wood-Gush D. 1991. Some effects of housing on the social behavior of dairy cows. Anim Prod. 53:271–278.
- NRAES. 1992. On-farm composting handbook. NRAES-54. Ithaca (NY): Northeast Regional Agricultural Engineering Service.
- O’Callaghan KA, Cripps PJ, Downham DY, Murray RD. 2003. Subjective and objective assessment of pain and discomfort due to lameness in dairy cattle. Anim Welf. 12:605–610.
- Petzen J, Wolfanger C, Bonhotal J, Schwarz M, Terry T, Youngers N. 2009. Case study: Eagleview compost dairy barn. Warsaw (NY): Cornell Cooperative Extension of Wyoming County [accessed 2018 October 24]. https://ecommons.cornell.edu/handle/1813/44658.
- Romanzin A, Corazzin M, Piasentier E, Bovolenta S. 2013. Effect of rearing system (mountain pasture vs. indoor) of Simmental cows on milk composition and Montasio cheese characteristics. J Dairy Res. 80:390–399.
- Reneau JK, Seykora AJ, Heins BJ, Endres MI, Farnsworth RJ, Bey RF. 2005. Association between hygiene scores and somatic cell scores in dairy cattle. J Am Vet Med Assoc. 227:1297–1301.
- Rodrigues ACO, Caraviello DZ, Ruegg PL. 2005. Management of Wisconsin dairy herds enrolled in milk quality teams. J Dairy Sci. 88:2660–2671.
- Schreiner DA, Ruegg PL. 2003. Relationship between udder and leg hygiene scores and subclinical mastitis. J Dairy Sci. 86:3460–3465.
- Shane EM, Endres MI, Janni KA. 2010. Alternative bedding materials for compost bedded pack barns in Minnesota: a descriptive study. Appl Eng Agric. 26:465–473.
- Sprecher DJ, Hostetler DE, Kaneene JB. 1997. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology. 47:1179–1187.
- Stentiford EI. 1996. Composting control: principles and practice. In: de Bertoldi M, Sequi P, Lemmes B, Papi T, editors. The science of composting. Part 1. London (UK): Blackie Academic and Professional; p. 49–59.
- Villettaz Robichaud M, Rushen J, de Passillé AM, Vasseur E, Orsel K, Pellerin D. 2019. Associations between on-farm animal welfare indicators and productivity and profitability on Canadian dairies: I. On freestall farms. J Dairy Sci. 102:4341–4351.
- Wagner PE. 2002. Bedded pack shelters. Lancaster Farming. 47:36.
- Waiblinger S, Menke C, Fölsch DW. 2003. Influences on the avoidance and approach behaviour of dairy cows towards humans on 35 farms. Appl Anim Behav Sci. 84:23–39.
- Yang F, Li GX, Yang QY, Luo WH. 2013. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere. 93:1393–1399.
- Zhang D, Luo W, Li Y, Wang G, Li G. 2018. Performance of co-composting sewage sludge and organic fraction of municipal solid waste at different proportions. Bioresour Technol. 250:853–859.