Abstract
The aim of this study was to evaluate the effects of fresh chicory forage in chicken diets on growth performance, carcass characteristics, meat and egg quality, as well as intestinal microbiota. A total of 600 healthy 16 weeks female Beijing-you chickens were randomly assigned to four dietary treatments containing 0%, 5%, 8% and 10% chicory forage with six replicate pens and 25 chickens in each replicate. Chickens were raised in a free-range system for 13 weeks after seven days of adaptation. High-throughput sequencing was used to characterise the microbiota in three different intestinal sections (duodenum, ileum and caecum). The results showed that dietary supplementation of chicory forage increased (p < .05) egg weight, yolk colour, total amino acids and delicious amino acids in muscles, and decreased (p < .05) abdominal fat yield compared to the control. However, increased (p < .05) average daily feed intake, increased (p < .05) feed conversion ratio, increased (p < .05) mortality and decreased (p < .05) eggshell strength were observed in chickens fed chicory forage than in those fed a basal diet. Dietary supplementation of chicory forage stimulated the proliferation of the Lactobacillus in ileum and the Bacteroides in caecum, and depressed pathogenic microbes including the Rikenella, thereby further improved intestinal health and nutrient utilisation. It was concluded that 8% chicory forage could be considered as a beneficial feed additive in Beijing-you chicken diets. In addition to the good nutritional profile, medicinal properties were associated with the beneficial effects of chicory forage on chickens.
Dietary supplementation of chicory forage improved muscle nutritional value and flavour.
Dietary supplementation of chicory forage affected egg quality.
Dietary supplementation of chicory forage regulated the intestinal microbiota.
Highlights
Introduction
The demand for animal protein for human nutrition in the developing world is still increasing, especially for poultry products, and the cost of feed concentrates for poultry is increasing. In this regard, there is a growing interest in using novel feed resources, and the utilisation of forage for poultry diet could be a sustainable and natural alternative (Tufarelli et al. Citation2018). Forage and forage meal could be valuable alternative sources of protein for poultry where they are easily available and not expensive (Mourao et al. Citation2008). Forage available to poultry showed a relatively high level of protein with a desirable amino acids profile, especially lysine, methionine and other sulphur-amino acids, which for poultry adequately balances the limitations of cereal proteins (Luscher et al. Citation2014). The primary benefit of forage consumption is that dietary fibre in plant matter can stimulate the development of the gastrointestinal tract (GIT), gut motility and nutrient utilisation (Liu et al. Citation2011). In addition, some medicinal plants were found to have prebiotic effects to modulate intestinal microbiota through favouring a quick proliferation of beneficial strains and inhibiting the growth of pathogenic microbes (Ali Citation2011). It is well established that a large range of forages, such as alfalfa, ryegrass, clover and grass meals, can be successfully used in poultry diets (Almeida et al. Citation2012; Zhang et al. Citation2013; Zheng et al. Citation2019).
Chicory (Cichorium intybus L.), which is native to Europe, many parts of Asia, Africa and America, is a perennial herb that can produce nutritious and high-quality forage (Li and Kemp Citation2005; Liu et al. Citation2018a). The nutritional profile of chicory is good because it has certain amount of protein, metabolic energy, vitamins, minerals and different types of bioactive compounds (Saeed et al. Citation2017). In addition, all parts of this plant possess great medicinal importance due to the presence of a number of medicinally important compounds such as alkaloids, inulin, sesquiterpene lactones, coumarins, chlorophyll pigments, unsaturated sterols, flavonoids, saponins and tannins (Shad et al. Citation2013). Chicory has been used as a forage for livestock in many parts of the world, and the results showed that animal performance on chicory is similar to that on legumes and superior to grass-based pastures (Li and Kemp Citation2005). Recently, chicory was reported as a beneficial feed ingredient for poultry. Liu et al. (Citation2011) suggested that chicory forage had potential to be used as a valuable fibre source for broiler chickens and could be included at 6% without any major negative effects on diets digestibility and growth performance. In one of our previous studies, Beijing-you chickens had a better growth performance, meat and egg quality when grazed on chicory pasture rather than on bare land (Meng et al. Citation2016).
Microbial community in the GIT plays an important role in overall health and function of host, be it in human or in animals (Shaufi et al. Citation2015). During the processes of nutrition, metabolism, physiology and immunity, the intestinal microbiota can promote digestion and absorption of nutrients, stimulate the immune response of the host and enhance resistance to infection (Wang et al. Citation2017). In addition to animal age, hygiene and environmental factors, diet is a major factor that can influence the microbial population in the intestinal tract (Dong et al. Citation2017). Previous studies suggested that dietary supplementation of forage had an impact on poultry performance by modifying the gut microbial structure to a bacterial community that is more conducive to host growth (Jiang et al. Citation2014; Saeed et al. Citation2017). Extensive studies have been conducted to disclose the most abundant microbial community in the chicken GIT by using culture-based approach, DNA fingerprinting method and polymerase chain reaction (PCR)-based sequencing (Xiao et al. Citation2017). Although these studies have expanded our knowledge of the intestinal microbiota, they only identified a few of the most abundant operational taxonomic units (OTUs) present, because of their poor limits of detection (Zheng et al. Citation2017).
Recent advancements in molecular tools, especially for high-throughput sequencing, have enabled us to perform a comprehensive assessment of the intestinal microbiota. One of our former studies showed that dietary supplementation of alfalfa meal stimulated the proliferation of beneficial bacteria, such as the Lactobacillus and Bacteroides, and inhibited potential pathogens including the Clostridium (Zheng et al. Citation2019). However, limited information is available regarding the metagenomic analysis of the intestinal microbiota affected by dietary chicory forage supplementation. In this study, therefore, we aim to perform a comprehensive assessment of the intestinal microbiota in chickens with and without dietary supplementation of chicory forage by high-throughput sequencing. We also provide data on growth performance, carcass characteristics, meat and egg quality of chickens. This approach enables us to obtain information on intestinal microbiota correlated with performance measures and provides base information for designing high-efficiency feed formula with chicory forage supplementation.
Materials and methods
Experimental design and dietary treatments
Beijing-you chicken is one of the most famous Chinese local breeds with superior meat and egg quality (Fu et al. Citation2015). A total of 600 healthy 16 weeks female Beijing-you chickens were selected and randomly allocated into four dietary treatments for a period of 13 weeks. As shown in , the chickens received isoenergetic and isonitrogenic diets supplemented with 0% (control group), 5%, 8% and 10% fresh chicory forage (on dry matter base, treatment groups) that were formulated to meet or exceed nutrient requirement estimates (NRC Citation1994). Chicory (Cichorium intybus cv. Grassland Puna) forage that was used in this study contained 10.3% dry matter (DM), 23.5% crude protein (CP), 22.3% crude fibre, 3.2% crude fat and 10.7% crude ash. Chicory forage was chopped to about 1 cm using a forage cutter and mixed with the basal diet thoroughly. Each dietary treatment consisted of six replicates with 25 chickens per replicate. Chickens were raised in a free-range system, in which chickens were fed in an indoor house (20 m2) with free access to an outdoor paddock as a playground (30 m2). Chickens were allowed ad libitum access to feed and water throughout the experiment, and fed daily at 7:00 and 16:00. This study was carried out at Shunyi District (Beijing, China) during the period from 13 July to 11 October 2017, with ambient temperature from 8.9 to 32.3 °C.
Table 1. Ingredients and nutrient compositions of experimental diets fed to 16–28 weeks Beijing-you chickens.
Data collection and sampling
Residual feed was weighed daily to determine average daily feed intake (ADFI). Mortalities and health status were visually observed and recorded daily, and ADFI was adjusted for dead chickens. Egg numbers and weight per pen were recorded daily. Feed conversion ratio (FCR) was calculated as grams of total feed consumption per grams of total egg mass. Individual body weight (BW) was recorded after 12 h of feed deprivation at the start and the end of the trial to determine average daily gain (ADG). Three healthy chickens of average BW from each replicate pen were chosen and slaughtered at the end of the trial. After exsanguination by cutting the carotid arteries and jugular veins, chickens were scalded in water at 60 °C for 45 s before defeathering, eviscerating, tissue and intestinal content sample collection. Intestinal contents were scrapped aseptically from duodenum, ileum (2 cm from Merkel’s diverticulum and 2 cm from caecum junction) and caecum (both pairs) by sterile glass slides, and pooled into sterile centrifuge tubes to reduce individual variation. Intestinal contents were then flash-frozen in liquid nitrogen and stored at –80 °C until DNA isolation. The de-feathered carcass, including head and feet, was eviscerated manually and weighed as eviscerated weight (EW). Eviscerated yield was calculated as the percentages of BW. Breast muscle, thigh muscle and abdominal fat pad including leaf fat surrounding the cloaca and gizzard were separated and weighed. Breast and thigh muscle yields were calculated as the percentages of EW. Abdominal fat percentage was calculated by abdominal fat weight/(abdominal fat weight + EW). Muscle samples were minced using a food mixer and then freeze-dried for further analysis.
Meat quality measurements
The DM, CP, crude fat and ash contents of breast and thigh muscle samples were determined by the standard procedures of the Association of Official Analytical Chemists (AOAC Citation1990). The inosine monophosphate (IMP) concentration in breast and thigh muscles was determined by high-performance liquid chromatography (LC-10A, Shimadzu Corporation, Tokyo, Japan) according to the method of Jung et al. (Citation2013). Free amino acids were determined by automatic amino acid analyser (L-8800, Hitachi Ltd., Tokyo, Japan) as described by Yan et al. (Citation2018).
Egg quality measurements
At the end of the experiment, 36 saleable eggs (no shell defects, cracks or double yolks) per treatment (six eggs per replicate) were collected and used to determine egg quality within 24 h after collection. The egg shape index was calculated as the ratio of vertical and horizontal diameter of the egg using Egg Form Coefficient Measurement Instrument (FHK, Fujihira Industry Co., Ltd., Tokyo, Japan). Eggshell thickness was measured with Eggshell Thickness Gauge (ETG-1061, Robotmation Co., Ltd., Tokyo, Japan). Eggshell strength was measured using Egg Force Reader (Orka Food Technology Co., Ltd., Israel). Also, egg weight, albumen height, Haugh unit and yolk colour were measured automatically using Egg Multi Tester (EMT-5200, Robotmation Co., Ltd., Tokyo, Japan). Yolk was separated from albumen to determine yolk weight. Yolk and albumen samples were dried using a freeze dryer (Modulyod-230, Thermo Fisher Scientific Inc., MA) and then ground for protein, lecithin and cholesterol determination (Trupia et al. Citation2016; Liu et al. Citation2018b).
DNA extraction and 16S rRNA gene amplification
Genomic DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen Inc., Valencia, CA) by following the manufacturer instructions. The normalised concentration of purified genomic DNA was used as a template to analyse microbial communities. The V3-V4 region of the 16S rRNA gene was amplified using eubacterial primers (338F: 5′-ACTCCTACGGGAGGCAGCA-3′ and 806R: 5′-GGACTACHVGGGTWTCTAAT-3′). The forward primer contained 12-bp barcodes unique to each sample, in order to enable the pooling of all PCR products for sequencing and the subsequent assignation of sequence reads to their respective samples. PCR reactions were performed in 25 μL volumes containing 30 ng of template DNA, 1 μL of each primer (5 μmol/L), 3 μL of BSA (2 ng/μL), 12.5 μL of 2 × Taq PCR Master Mix (Takara Bio Inc., Shiga, Japan), and double-distilled water was added to obtain a final volume of 25 μL. Thermocycling conditions were as follows: 95 °C for 5 min to denature the DNA, with amplification proceeding for 28 cycles at 95 °C for 45 s, 55 °C for 50 s and 72 °C for 45 s; a final extension of 10 min at 72 °C was added to ensure complete amplification.
High-throughput sequencing and data processing
PCR products were purified with GeneJET Gel Extraction Kit (Thermo Fisher Scientific Inc., Carlsbad, CA) and mixed in equidensity ratios. Sequencing libraries were generated using NEB Next Ultra DNA Library Prep Kit for Illumina (New England Biolabs Inc., Ipswich, MA) and sequenced on an Illumina MiSeq PE300 platform (Illumina Corporation, San Diego, CA). Quality control and assignment of Illumina MiSeq sequences to samples based on their barcodes were done following the standard QIIME pipeline (ver. 1.7.0, http://qiime.org/index.html). The sequence reads were then clustered into OTUs by de novo OTU picking at a 97% level of sequence similarity. A single representative sequence from each clustered OTU was assigned to different taxonomic levels (phylum and genus) at a cut-off of 97% comparing with the SILVA bacteria reference database (ver. 1.8, http://www.arb-silva.de). Alpha diversity and Good’s coverage analyses consist of community diversity (Shannon) and richness (OTUs number observed and Chao1) were performed using Mothur software (ver. 1.30.1, http://www.mothur.org/wiki/Classify.seqs) based on summary single command. Principal coordinate analysis (PCoA) was performed at the genus level using Mothur software.
Statistical analysis
Data on the growth performance, carcass, meat and egg traits were subjected to one-way ANOVA to test the effect of dietary chicory forage content as a completely randomised design using the GLM procedure of SAS (SAS Institute Inc., Cary, NC). Significant differences among the means were determined using Tukey’s multiple range test. Treatment differences were considered significant at p < .05.
Results
Growth performance
The effects of dietary supplementation of chicory forage on growth performance, egg production and FCR of Beijing-you chickens are presented in . An increase (p < .05) in ADFI, FCR and mortality was observed with increasing supplementation of chicory forage in the diet. The egg weight increased (p < .05) by 10.87%, 10.89% and 7.94% in chickens fed 5%, 8% and 10% chicory forage diets compared to those given no chicory forage. However, no diet effects (p > .05) on BW, ADG or egg-laying rate were detected.
Table 2. Effects of dietary treatments on performance and feed efficiency of Beijing-you chickens.
Carcass characteristics and meat quality
Yields of eviscerated carcass, breast muscle and thigh muscle were increased because of dietary supplementation of chicory forage compared to the control, but no significant differences (p > .05) were observed among treatments (). However, dietary supplementation of 5%, 8% and 10% chicory forage decreased (p < .05) abdominal fat yield by 75.30%, 65.38% and 73.12% compared to the control.
Table 3. Effects of dietary treatments on carcass characteristics and meat quality of Beijing-you chickens.
Dietary supplementation of 5% and 8% chicory forage increased (p > .05) DM, CP, IMP and essential amino acids contents of breast muscle compared to the control. Irrespective of the amount of chicory forage added, there was an increase (p < .05) in crude fat, total amino acids, non-essential amino acids and delicious amino acids contents of breast muscle in chickens fed chicory forage diets compared to the control. The highest DM, CP, crude fat, IMP, total amino acids, non-essential amino acids and delicious amino acids contents of breast muscle were observed in chickens fed 8% chicory forage diet, which increased by 1.08%, 1.41%, 79.46%, 4.03%, 12.46%, 16.84% and 22.77% compared to the control. An increase (p < .05) in total amino acids, non-essential amino acids and delicious amino acids contents of thigh muscle was also observed because of dietary supplementation of chicory forage. Dietary supplementation of 8% chicory forage resulted in the highest thigh muscle contents of CP, crude ash, total amino acids, essential amino acids, non-essential amino acids and delicious amino acids, which increased by 0.41%, 2.38%, 12.42%, 4.32%, 18.52% and 22.88% compared to the control.
Egg quality
A slight improvement in albumen height, Haugh unit, yolk weight, eggshell weight, yolk protein, yolk lecithin and albumen protein was observed in chickens fed diets containing chicory forage compared to those fed a basal diet, although no statistically significant differences (p > .05) were observed among treatments (). In terms of eggshell thickness and egg shape index, the values were also statistically similar (p > .05) for all chickens on experimental diets. Eggshell strength decreased (p < .05) by 14.02%, 13.08% and 13.55%; yolk colour increased (p < .05) by 17.55%, 37.05% and 14.21%; yolk cholesterol decreased (p > .05) by 7.75%, 4.80% and 6.64%, respectively, in chickens receiving 5%, 8% and 10% chicory forage diets than in those given no chicory forage.
Table 4. Effects of dietary treatments on egg quality of Beijing-you chickens.
Intestinal microbiota
A total of 233,592 valid sequences with an average length of 422 base pairs were obtained from all samples after filtering for quality (). The sequences were further clustered into 238 to 768 OTUs for each sample, resulting in a total of 1,236 OTUs based on 97% sequence identity. Compared to the control, dietary supplementation of 5%, 8% and 10% chicory forage decreased microbial community richness in duodenum and ileum, while an increase in microbial community richness in caecum was observed in chickens fed 8% and 10% chicory forage diets. The microbial community diversity decreased in duodenum and ileum, while it increased in caecum because of dietary supplementation of chicory forage compared to the control. Good’s coverage was around 0.99 in all samples, indicating that the sampling depth had adequately captured most of the microbial community.
Table 5. Effects of dietary treatments on alpha diversity of the intestinal microbiota of Beijing-you chickens.
The Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria were the major phyla common to the three GIT sections of Beijing-you chickens (). Regardless of the dietary treatments, the Firmicutes was the most abundant phylum in both duodenum (with the relative abundance of 76.20% to 90.28%) and ileum (40.44% to 70.72%), while the dominant phylum in caecum was identified as the Bacteroidetes (50.71% to 59.61%). The Firmicutes (26.42% to 30.31%) and Bacteroidetes (22.91% to 41.83%) were also commonly found in caecum and ileum, respectively. Compared to the microbial community in the control, the relative abundance of the Bacteroidetes increased in caecum, while it decreased with a concomitant increase of the Firmicutes in ileum because of dietary supplementation of chicory forage.
Figure 1. Effects of dietary treatments on the intestinal microbiota of Beijing-you chickens at the phylum and genus levels. C0: control; C1, C2 and C3: dietary supplementation of 5%, 8% and 10% fresh chicory forage (on dry matter base), respectively.
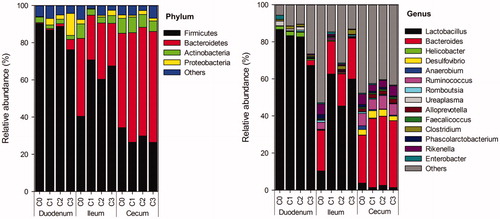
The genus Lactobacillus accounted for more than 67% of the sequences obtained from duodenum of chickens with all dietary treatments, while the relative abundance of other detected genera was less than 3%. The Lactobacillus (10.32% to 62.59%) was also observed as the dominant genus in ileum, followed by the genus Bacteroides (17.42% to 22.04%). However, in caecum, the dominant genus was replaced by the Bacteroides (25.81% to 37.20%), the genera Desulfovibrio (2.45% to 3.93%), Ruminococcus (5.56% to 7.31%), Alloprevotella (1.67% to 2.35%) and Rikenella (3.44% to 5.52%) were also commonly identified. For the microbial community in ileum, the relative abundance of the Lactobacillus increased by more than 35%, while the Ruminococcus, Romboutsia, Phascolarctobacterium and Rikenella decreased because of dietary supplementation of chicory forage compared to the control. In caecum, dietary supplementation of chicory forage resulted in an increase of more than 10% in the relative abundance of the Bacteroides, and a decrease of the Anaerobium and Rikenella compared to the control.
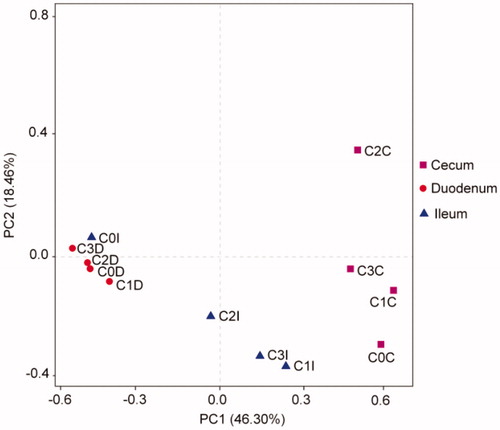
The similarity and difference of microbial community composition in the three GIT sections of chickens with different dietary treatments are shown in the PCoA plot (). The microbial community of caecum formed a distinct cluster separated from that of duodenum and ileum, and there were overlaps among duodenum and ileum groups. In addition, the microbial community in ileum and caecum of chickens fed 8% chicory forage diet separated from that of chickens given other three diets.
Figure 2. Principal coordinate analysis (PCoA) of the dissimilarity between the microbial samples: PCoA plotted against the PC1 vs. PC2 axes. The percentages indicate the relative contribution of the two principal coordinates (PC1-PC2). C0: control; C1, C2 and C3: dietary supplementation of 5%, 8% and 10% fresh chicory forage (on dry matter base), respectively.
Discussion
Various dietary strategies have been used to improve the poultry growth performance and feed efficiency, such as probiotics including Bacillus subtilis and Lactobacillus sp. (Guo et al. Citation2017; Fathi et al. Citation2018), prebiotics including fructooligosaccharides and galactooligosaccharides (Park et al. Citation2017), forage products including alfalfa meal (Zheng et al. Citation2019) and other natural products including green tea (Xia et al. Citation2018). In this study, however, chickens fed diets containing chicory forage had a lower feed efficiency compared to those fed a basal diet. The increased FCR because of dietary supplementation of chicory forage was associated with the increased ADFI, which was in agreement with Almeida et al. (Citation2012). The increased ADFI because of dietary supplementation of chicory forage might be due to the following reasons: (1) feed intake was weighed based on fresh chicory forage rather than dried sample; and (2) chickens have compensated for a comparatively smaller availability of the commercial feed by consuming more chicory forage. The prebiotic effects of chicory forage or its extracts (such as inulin and oligofructose) on chicken health have been reported previously (Swiatkiewicz et al. Citation2011; Shad et al. Citation2013; Liu et al. Citation2018a). In this study, in addition to high moisture content of fresh chicory forage, low temperature of the rearing system might contribute to the increased mortality and decreased egg-laying rate of chickens fed chicory forage diets compared to the control.
Carcass yield is one of the most important indicators of the overall performance of modern broiler chicken lines (Kwiatkowska et al. Citation2017). In this study, however, dietary supplementation of chicory forage had no effect on some carcass traits, such as yields of eviscerated carcass, breast muscle and thigh muscle. Lee et al. (Citation2003) suggested that the use of highly digestible feed ingredients in the diet and hygienic conditions in research studies could mask the beneficial effects of forage on growth performance and carcass traits. A series of animal studies demonstrated that chicory fructans could affect the metabolism of lipids primarily by decreasing triglyceridemia (Van Loo Citation2007; Lin et al. Citation2014). In this study, dietary supplementation of chicory forage significantly decreased abdominal fat yield compared to the control, which confirmed other reports (Yusrizal and Chen Citation2003; Ali Citation2011; Saeed et al. Citation2017).
In this study, the nutritional value of breast and thigh muscles was improved by dietary supplementation of chicory forage, as indicated by the higher contents of total amino acids and CP in chickens receiving chicory forage diets than in those fed a basal diet. In addition to the abundant essential amino acids, the low concentration of condensed tannins in chicory forage might be partly responsible for the improved efficiency of protein utilisation (Li and Kemp Citation2005; Saeed et al. Citation2017). Flavour is another important factor that determines meat quality, and the active flavour components in chicken meat are mainly composed of free glutamic acid, 5′-inosinic acid and potassium ion. In this study, delicious amino acids (Yan et al. Citation2018) contents in breast and thigh muscles increased significantly because of dietary supplementation of chicory forage compared to the control. Therefore, dietary supplementation of chicory forage could improve the taste of meat by increasing some of the delicate flavours produced by amino acids. In agreement with our findings, improved muscle flavour was obtained when chickens were grazed on chicory pasture (Azcona et al. Citation2008; Meng et al. Citation2016).
Measurements on eggshell parameters showed that forage crops were sufficient to meet the calcium and phosphorus requirements of hens (Horsted et al. Citation2006). It seems surprising, however, the results of this study showed that dietary supplementation of chicory forage decreased eggshell strength. It could not be excluded that chicory forage supplementation changed the balance of feed nutrition, which resulted in insufficient absorption and utilisation of calcium and phosphorus. In agreement with previous findings (Van Loo Citation2007; Saeed et al. Citation2017), dietary supplementation of chicory forage had good effects on laying hen health status, reducing eggs cholesterol levels. These beneficial effects might be due to the inulin and oligofructose amount provided by chicory forage. Chen et al. (Citation2005) suggested that inulin and oligofructose prebiotic dietary supplementations increased concentrations of unabsorbable cholesterol in the jejunum contents and cholesterol excretion, therefore resulting in lowered concentrations of cholesterol in the yolk and in serum. Large amount of cholesterol in egg yolk once has been a primary health concern for consumers (Xia et al. Citation2018). However, recent epidemiologic data have clearly demonstrated that cholesterol is an essential constituent of animal cells and increasing concentrations of dietary cholesterol are not correlated with increased risk for coronary heart disease (Secci et al. Citation2018). Yolk colour is an another important egg quality parameter for the consumer, and it has been demonstrated to become dark with increasing dietary supplementation of forage crops (Hammershoj and Steenfeldt Citation2005). Quantity of xanthophyll contained in chicory forage might be related to the dark egg yolk from hens with access to chicory forage (Horsted et al. Citation2006).
Previous studies demonstrated that feed additives (prebiotics and probiotics) modulate the microbial ecology of the poultry GIT, and improve gut functionality and consequentially the health status (Jiang et al. Citation2014; Borrelli et al. Citation2017). In this study, pyrosequencing results indicated that dietary supplementation of chicory forage might have benefited intestinal health by increasing the microbial community diversity in caecum of chickens. Several studies, in fact, revealed that a rich microbial community is associated with a healthy status as a more diverse microbial community shows stronger homeostasis of the intestinal microbial community and resistance to pathogens (Borrelli et al. Citation2017; Li et al. Citation2017). On the contrary, the stimulation of predominant bacteria might have led to a fall in the microbial diversity in duodenum and ileum of chickens fed chicory forage diets compared to the control. In agreement with Liu et al. (Citation2018a), the microbial richness in duodenum and ileum was decreased because of dietary supplementation of chicory forage compared to the control. The results of PCoA suggested that microbial community in caecum formed a distinct cluster and separated from that in duodenum and ileum, confirming that microbial compositions in caecum were different from those in duodenum and ileum. Xiao et al. (Citation2017) suggested that variance in the microbiota among different GIT sections might be attributed to different GIT functions. As reported, the small intestine (duodenum, jejunum and ileum) is the important site for nutrient digestion and absorption, while intestinal microbiota in caecum carries many important roles such as fermentation and breaking undigested substrates (Shaufi et al. Citation2015).
In agreement with our findings, plenty of studies indicated that the Firmicutes was the dominant phylum in duodenum and ileum of chickens, while caecum was inhabited mostly by the Bacteroidetes (Li et al. Citation2017; Xiao et al. Citation2017; Zheng et al. Citation2019). At the genus level, the Firmicutes and Bacteroidetes were primarily composed of the Lactobacillus and Bacteroides, respectively. Our findings showed that the Lactobacillus was more common in duodenum and ileum, indicating the Lactobacillus contributes to the intestinal functions related to nutrient digestion and absorption. The Lactobacillus has also been widely used as probiotics in poultry feed to reduce egg yolk cholesterol concentration (Ramasamy et al. Citation2009), improve eggshell thickness (Gallazzi et al. Citation2008), stimulate the immune system (Fong et al. Citation2015), improve growth performance (Peng et al. Citation2016) and regulate the intestinal microbiota (Li et al. Citation2017) . In this study, compared to the control, dietary supplementation of chicory forage significantly increased the relative abundance of the Lactobacillus in ileum, and thereby further improved intestinal health and nutrient utilisation.
The Bacteroides plays an important role in breaking down complex molecules to simpler compounds which are essential for the growth of host and gut microbiota (Shaufi et al. Citation2015). Therefore, the Bacteroides showed significant negative correlations with FCR and feed intake, and a significant positive correlation with body weight gain (Crisol-Martinez et al. Citation2017). In this study, the increased populations of the Bacteroides in caecum of chickens fed chicory forage diets are further indications for a chicory-induced stimulation of intestinal fermentative activity. As we suggested previously, dietary fibre in diet might stimulate the Bacteroides proliferation (Zheng et al. Citation2019). In addition, pathogenic microbes, such as the Rikenella, were depressed because of dietary supplementation of chicory forage. This study showed that dietary supplementation of chicory forage promoted the growth of the Lactobacillus and Bacteroides, whereas it could inhibit the growth of the Rikenella. The inulin content in chicory forage might serve as substrate for the intestinal microbiota, influencing the composition and the microbial fermentation metabolites (Liu et al. Citation2018a). Our findings strongly corroborate the hypothesis of possible beneficial effects of chicory forage-based diet on global health of hens, even though further studies are necessary to decipher its impact on bacterial metabolites production.
Conclusions
Fresh chicory forage appeared to be palatable and could be supplemented in Beijing-you chicken diets at 8% (on dry matter base) with some beneficial effects on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota. Dietary supplementation of chicory forage increased the relative abundance of the Lactobacillus in ileum and the Bacteroides in caecum, while depressed pathogenic microbes including the Rikenella. Therefore, productive performance increased with shifts in the intestinal microbiota. In addition to the good nutritional profile, medicinal properties were associated with the beneficial effects of chicory forage on chickens.
Ethical approval
All study procedures were approved by the Animal Care and Use Committee of Beijing Academy of Agriculture and Forestry Sciences and were in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China). All efforts were made to minimize the suffering of the animals.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Additional information
Funding
References
- Ali N. 2011. Effects of different levels of chicory (Cichorium intybus L.), zizaphora (Zizaphora tenuior L.), nettle (Urtica dioica L.) and savoury (Satureja hortensis L.) medicinal plants on carcass characteristics of male broilers. J Med Plants Res. 5:4354–4359.
- Almeida GFD, Hinrichsen LK, Horsted K, Thamsborg SM, Hermansen JE. 2012. Feed intake and activity level of two broiler genotypes foraging different types of vegetation in the finishing period. Poult Sci. 91:2105–2113.
- AOAC. 1990. Official methods of analysis. 15th ed. Washington (DC): Association of Official Analytical Chemists.
- Azcona JO, Garcia PT, Cossu ME, Iglesias BF, Picallo A, Perez C, Gallinger CI, Schang MJ, Canet ZE. 2008. Meat quality of Argentinean “Camperos” chicken enhanced in omega-3 and omega-9 fatty acids. Meat Sci. 79:437–443.
- Borrelli L, Coretti L, Dipineto L, Bovera F, Menna F, Chiariotti L, Nizza A, Lembo F, Fioretti A. 2017. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci Rep. 7:16269.
- Chen YC, Nakthong C, Chen TC. 2005. Effects of chicory fructans on egg cholesterol in commercial laying hens. Int J Poult Sci. 4:109–114.
- Crisol-Martinez E, Stanley D, Geier MS, Hughes RJ, Moore RJ. 2017. Understanding the mechanisms of zinc bacitracin and avilamycin on animal production: linking gut microbiota and growth performance in chickens. Appl Microbiol Biotechnol. 101:4547–4559.
- Dong XY, Azzam MMM, Zou XT. 2017. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult Sci. 96:3654–3663.
- Fathi M, Al-Homidan I, Al-Dokhail A, Ebeid T, Abou-Emera O, Alsagan A. 2018. Effects of dietary probiotic (Bacillus subtilis) supplementation on productive performance, immune response and egg quality characteristics in laying hens under high ambient temperature. Ital J Anim Sci. 17:804–814.
- Fong FLY, Kirjavainen P, Wong VHY, El-Nezami H. 2015. Immunomodulatory effects of Lactobacillus rhamnosus GG on dendritic cells, macrophages and monocytes from healthy donors. J Funct Foods. 13:71–79.
- Fu DZ, Zhang DX, Xu GY, Li KY, Wang Q, Zhang ZB, Li JY, Chen Y, Jia YX, Qu LJ. 2015. Effects of different rearing systems on meat production traits and meat fiber microstructure of Beijing-you chicken. Anim Sci J. 86:729–735.
- Gallazzi D, Giardini A, Mangiagalli MG, Marelli S, Ferrazzi V, Orsi C, Cavalchini LG. 2008. Effects of Lactobacillus addophilus D2/CSL on laying hen performance. Ital J Anim Sci. 7:27–37.
- Guo SS, Liu D, Zhang BB, Li Z, Li YH, Ding BY, Guo YM. 2017. Two Lactobacillus species inhibit the growth and alpha-toxin production of Clostridium perfringens and induced proinflammatory factors in chicken intestinal epithelial cells in vitro. Front Microbiol. 8:2081.
- Hammershoj M, Steenfeldt S. 2005. Effects of blue lupin (Lupinus angustifolius) in organic layer diets and supplementation with foraging material on egg production and some egg quality parameters. Poult Sci. 84:723–733.
- Horsted K, Hammershoj M, Hermansen JE. 2006. Short-term effects on productivity and egg quality in nutrient-restricted versus non-restricted organic layers with access to different forage crops. Acta Agric Scand Sect A-Anim Sci. 56:42–54.
- Jiang JF, Song XM, Wu JL, Jiang YQ. 2014. Effects of alfalfa meal on the intestinal microbial diversity and immunity of growing ducks. J Anim Physiol Anim Nutr (Berl). 98:1039–1046.
- Jung S, Bae YS, Kim HJ, Jayasena DD, Lee JH, Park HB, Heo KN, Jo C. 2013. Carnosine, anserine, creatine, and inosine 5'-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult Sci. 92:3275–3282.
- Kwiatkowska K, Kwiecień M, Winiarska-Mieczan A. 2017. Fast-growing chickens fed with lucerne protein-xanthophyll concentrate: growth performance, slaughter yield and bone quality. J Anim Feed Sci. 26:131–140.
- Lee KW, Everts H, Kappert HJ, Frehner M, Losa R, Beynen AC. 2003. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br Poult Sci. 44:450–457.
- Li GD, Kemp PD. 2005. Forage chicory (Cichorium intybus L.): a review of its agronomy and animal production. In: Sparks DL, editors. Advances in agronomy. San Diego (CA): Elsevier Academic Press Inc.
- Li Z, Wang WW, Liu D, Guo YM. 2017. Effects of Lactobacillus acidophilus on gut microbiota composition in broilers challenged with Clostridium perfringens. PLoS One. 12:e0188634.
- Lin ZJ, Zhang B, Liu XQ, Jin R, Zhu WJ. 2014. Effects of chicory inulin on serum metabolites of uric acid, lipids, glucose, and abdominal fat deposition in quails induced by purine-rich diets. J Med Food. 17:1214–1221.
- Liu HY, Hou R, Yang GQ, Zhao F, Dong WG. 2018. In vitro effects of inulin and soya bean oligosaccharide on skatole production and the intestinal microbiota in broilers. J Anim Physiol Anim Nutr. 102:706–716.
- Liu HY, Ivarsson E, Jonsson L, Holm L, Lundh T, Lindberg JE. 2011. Growth performance, digestibility, and gut development of broiler chickens on diets with inclusion of chicory (Cichorium intybus L.). Poult Sci. 90:815–823.
- Liu K, Xin HW, Sekhon J, Wang T. 2018. Effect of fluorescent vs. poultry-specific light-emitting diode lights on production performance and egg quality of W-36 laying hens. Poult Sci. 97:834–844.
- Luscher A, Mueller-Harvey I, Soussana JF, Rees RM, Peyraud JL. 2014. Potential of legume-based grassland-livestock systems in Europe: a review. Grass Forage Sci. 69:206–228.
- Meng L, Mao PC, Guo Q, Tian XX. 2016. Evaluation of meat and egg traits of Beijing-you chickens rotationally grazing on chicory pasture in a chestnut forest. Rev Bras Cienc Avic. 18:1–6.
- Mourao JL, Pinheiro VM, Prates JAM, Bessa RJB, Ferreira LMA, Fontes C, Ponte P. 2008. Effect of dietary dehydrated pasture and citrus pulp on the performance and meat quality of broiler chickens. Poult Sci. 87:733–743.
- NRC. 1994. Nutrient requirements of poultry. 9th ed. Washington (DC): National Academy Press.
- Park SH, Perrotta A, Hanning I, Diaz-Sanchez S, Pendleton S, Alm E, Ricke SC. 2017. Pasture flock chicken cecal microbiome responses to prebiotics and plum fiber feed amendments. Poult Sci. 96:1820–1830.
- Peng Q, Zeng XF, Zhu JL, Wang S, Liu XT, Hou CL, Thacker PA, Qiao SY. 2016. Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult Sci. 95:893–900.
- Ramasamy K, Abdullah N, Jalaludin S, Wong M, Ho YW. 2009. Effects of Lactobacillus cultures on performance of laying hens, and total cholesterol, lipid and fatty acid composition of egg yolk. J Sci Food Agric. 89:482–486.
- Saeed M, Abd El-Hack ME, Alagawany M, Arain MA, Arif M, Mirza MA, Naveed M, Chao S, Sarwar M, Sayab M. 2017. Chicory (Cichorium intybus) herb: chemical composition, pharmacology, nutritional and healthical applications. Int J Pharmacol. 13:351–360.
- Secci G, Moniello G, Gasco L, Bovera F, Parisi G. 2018. Barbary partridge meat quality as affected by Hermetia illucens and Tenebrio molitor larva meals in feeds. Food Res Int. 112:291–298.
- Shad MA, Nawaz H, Rehman T, Ikram N. 2013. Determination of some biochemicals, phytochemicals and antioxidant properties of different parts of Cichorium intybus L.: a comparative study. J Anim Plant Sci. 23:1060–1066.
- Shaufi MAM, Sieo CC, Chong CW, Gan HM, Ho YW. 2015. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathogens. 7:4.
- Swiatkiewicz S, Koreleski J, Arczewska WA. 2011. Effect of inulin and oligofructose on performance and bone characteristics of broiler chickens fed on diets with different concentrations of calcium and phosphorus. Br Poult Sci. 52:483–491.
- Trupia S, Winkler-Moser JK, Guney AC, Beckstead R, Chen C. 2016. Nutritional quality of eggs from hens fed distillers dried grains with solubles. Poult Sci. 95:2592–2601.
- Tufarelli V, Ragni M, Laudadio V. 2018. Feeding forage in poultry: a promising alternative for the future of production systems. Agriculture. 8:81.
- Van Loo J. 2007. How chicory fructans contribute to zootechnical performance and well-being in livestock and companion animals. J Nutr. 137:2594S–2597S.
- Wang J, Fan H, Han Y, Wei JP, Zhao JZ, Zhou ZJ. 2017. Pyrosequencing of the broiler chicken gastrointestinal tract reveals the regional similarity and dissimilarity of microbial community. Can J Anim Sci. 97:302–313.
- Xia B, Liu YL, Sun D, Liu J, Zhu YJ, Lu LZ. 2018. Effects of green tea powder supplementation on egg production and egg quality in laying hens. J Appl Anim Res. 46:927–931.
- Xiao YP, Xiang Y, Zhou WD, Chen JG, Li KF, Yang H. 2017. Microbial community mapping in intestinal tract of broiler chicken. Poult Sci. 96:1387–1393.
- Yan JS, Liu PF, Xu LM, Huan HL, Zhou WR, Xu XM, Shi ZD. 2018. Effects of exogenous inosine monophosphate on growth performance, flavor compounds, enzyme activity, and gene expression of muscle tissues in chicken. Poult Sci. 97:1229–1237.
- Yusrizal, Chen TC. 2003. Effect of adding chicory fructans in feed on broiler growth performance, serum cholesterol and intestinal length. Int J Poult Sci. 2:214–219.
- Zhang SJ, Zhu CH, Guo J, Tang QP, Li HF, Zou JM. 2013. Metabolizable energy and fiber digestibility of uncommon feedstuffs for geese. Poult Sci. 92:1812–1817.
- Zheng ML, Mao PC, Tian XX, Guo Q, Meng L. 2019. Effects of dietary supplementation of alfalfa meal on growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken. Poult Sci. 98:2250–2259.
- Zheng ML, Niu DZ, Jiang D, Zuo SS, Xu CC. 2017. Dynamics of microbial community during ensiling direct-cut alfalfa with and without LAB inoculant and sugar. J Appl Microbiol. 122:1456–1470.
