 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To determine the protein profile of rabbit meat, the protein composition in meat of rabbits with different breeds and ages was investigated. In this article, M. Longissimus thoracis et lumborum (LTL) samples were obtained from six groups (ten rabbits in each group) of 60 female rabbits (Ira and Sichuan white; 35, 70, and 105 days old). Their nutritional characteristics, amino acid profile, protein quantity, and protein profile were analysed. The results showed that rabbit meat contained high content of essential amino acids, especially for Lys (2.26%). The analysis of amino acid profile in rabbit meat indicated that it varied significantly with rabbit breeds and ages. The proportion of stromal protein to total protein in rabbit meat was significantly influenced by age (p < .05), and the mean proportions of sarcoplasmic, myofibrillar, and stromal protein to total protein in rabbit meat were 32.57%, 57.20% and 10.23%, respectively. A total of 835 proteins were identified from the samples using shotgun proteomics. These proteins were mainly involved in energy metabolism, material transport and biochemical reactions, and the highest number of proteins was observed in meat of 35-day-old rabbit (p < .05). Although breed had a limited influence on the number of proteins in rabbit meat (p > .05), 69 and 35 unique proteins were identified in Ira and Sichuan white, respectively. In conclusion, both breed and age can affect the protein profile of rabbit meat, and the difference in protein profile may be the reason for the quality variation of different rabbit meat samples.
Comparing the differences between two rabbit breeds from the perspective of proteome.
Elucidate proteome in rabbit muscle during its growth. The outcome means that a total of 835 proteins were identified from the samples using shotgun proteomics, and the highest number of unique proteins was observed in meat of 35-day-old rabbit.
Highlights
Introduction
Rabbit meat is an excellent nutritional source of healthy food. It comprises high contents of proteins, phospholipid, and polyunsaturated fatty acids. Meanwhile, low cholesterol, low-fat levels, and moderately high energy values are conducive to its desirable composition and sensory properties (Dalle Zotte and Szendro Citation2011). Such nutritional values can fulfil consumers’ desire for new and healthy meat; thus, rabbit meat has become increasingly popular in Asia during the last three decades (Petracci et al. Citation2018). The global production of rabbit meat has steadily increased from 1,107,323 to 1,482,441 tons during 2008 to 2017 (FAO Citation2019), among which China alone is accounted for more than 55% of the total production (Li et al. Citation2018).
Rabbit meat is a good source of protein that has higher digestibility compared with those from grains, plant pulses, or other meats such as beef or pork (Combes and Dalle Zotte Citation2005; Pereira and Vicente Citation2013). Unfortunately, rabbit meat protein has not been fully utilised due to lack of understanding (Petracci et al. Citation2018). In general, meat proteins are classified into 3 categories, which include myofibrillar, sarcoplasmic, and stromal protein, and the protein quantity of meat has a critical impact on meat nutritional values, as well as meat processing and sensory quality traits (Ito et al. Citation2003). Particularly, meat proteins confer lots of the processing properties by imparting specific functionalities. The appearance, mouthfeel, and texture properties of meat products are determined by protein functionality (Mudalal et al. Citation2014). However, few studies are available on the protein quantity of rabbit meat.
To have a deeper understanding of protein, proteomics, which has attracted increasing attention, can be used. Proteome can describe the total proteins expressed by cell, tissue, or organ of living organisms under various survival and developmental conditions (Altelaar et al. Citation2013). Proteomics is a subject that studies the dynamic changes of protein characteristic, expression, and function in the proteome (Graves and Haystead Citation2002). In recent years, proteomics technologies, particularly for mass spectrometry (MS)-based proteomics, have immensely matured with the cumulative technological advances (Cox and Mann Citation2011). As a type of MS-based proteomics, shotgun proteomics has been widely used, owing to its large-scale, high-throughput, and systematic analysis. Yu et al. (Citation2016) utilised shotgun proteomics to examine the changes of protein profile in cooked lamb and demonstrated that roasting could result in reduced protein truncation and protein extractability. Furthermore, shotgun proteomics was employed to detect trace contaminants in meat and identify biomarker peptides in adulterated meat (Bargen et al. Citation2013; Yuswan et al. Citation2018). Although Liu et al. (Citation2013) have analysed the protein profile of sarcoplasmic reticulum in rabbit skeletal muscle using shotgun proteomics, and Almeida et al. (Citation2009) have established the first proteomic reference map for gastrocnemius muscle in rabbit, studies concerning the proteome in rabbit muscle during its growth remain to be elucidated.
Therefore, the present study aimed at investigating the effect of breed and age on the protein composition of rabbit meat, and evaluating the nutritional characteristics, amino acid profile, protein quantity (myofibrillar, sarcoplasmic, and stromal protein), and protein profile of rabbit meat. This research may lay the foundation for wider utilisation of rabbit meat protein while indirectly promoting the development of rabbit industry.
Materials and methods
Animals and sample preparation
The experiment was approved by the Animal Research Committee of Southwest University. A total of 60 rabbits were used in this experiment. Thirty commercial female Ira rabbits and thirty female Sichuan white rabbits of three different ages (35, 70 and 105 days; ten rabbits in each age group) were obtained from Chongqing Amamji Food Co., Ltd (Chongqing, China) and Chengdu Dingxin Rabbit Industry Development Co. Ltd. (Sichuan, China), respectively. All the rabbits were fed the same brand of commercial feed (30.1% alfalfa, 21.2% corn, 12.4% soybean meal, and 8.7% wheat bran). The rabbits were divided into six groups and slaughtered according to the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China. The carcases were bled, skinned, and eviscerated within 2 h. After that, they were transported at 0–4 °C to the laboratory, and the carcase was immediately cut between the 6th and 7th lumbar vertebrae and between the 7th and 8th thoracic vertebrae as M. longissimus thoracis et lumborum (LTL). The LTL samples for shotgun proteomics analysis were pallet packed and stored at −80 °C, and other samples were stored at −20 °C until subsequent analysis.
Proximate composition analysis
The proximate composition analysis was carried out in accordance with the AOAC (Citation2000) methods. Moisture content was determined by oven drying at 105 °C to a constant weight. Contents of crude protein and lipid were analysed by the Kjeldahl method and by the Soxhlet method, respectively. Ash content was determined by igniting sample in a muffle furnace at 550 °C until no black ash was further produced.
Amino acid profile analysis
Amino acid contents in rabbit meat were determined according to the method of Luo et al. (Citation2017) with minor modifications. Rabbit meat samples were hydrolysed with 10 mL of 6 mol L−1 hydrochloric acid (HCl) in a vacuum at 110 °C for 24 h. The hydrolysates were taken out of the oven, cooled down, and then diluted with ultrapure water in a 50-mL volumetric flask. One millilitre of the mixed liquor was evaporated in a rotary evaporator at 55 °C until it was completely dry. After that, the residue was diluted with 50 mL of 0.02 mol L−1 HCl and 1 mL of the diluted solution was subjected to an amino acid analyser (L-8900; Hitachi, Tokyo, Japan). Seventeen amino acids, including valine (Val), lysine (Lys), threonine (Thr), histidine (His), tyrosine (Tyr), phenylalanine (Phe), methionine (Met), leucine (Leu), proline (Pro), isoleucine (Ile), cysteine (Cys), aspartic acid (Asp), alanine (Ala), arginine (Arg), serine (Ser), glycine (Gly) and glutamic acid (Glu) were identified and quantified by standard curves constructed using a mixed standard (Sigma Aldrich, St Louis, MO, USA). Because tryptophan (Trp) can be dissociated by acid hydrolysis, its content was determined by the method of Dawood and Alkanhal (Citation1995).
Protein quantity (sarcoplasmic, myofibrillar, and stromal protein) analysis
Sarcoplasmic, myofibrillar, and stromal protein was extracted from different rabbit meat samples using the methods described by Hashimoto et al. (Citation1979). The contents of extracted proteins were determined by the Kjeldahl method. The percentages of the three proteins were calculated by the following equations:
(1)
(1)
(2)
(2)
(3)
(3)
Where P1, P2, and P3 are the percentage of sarcoplasmic, myofibrillar, and stromal protein, respectively; and Psarcoplasmic, Pmyofibrillar, and Ptotal are the contents of sarcoplasmic protein, myofibrillar protein, and total protein in rabbit meat sample, respectively.
Shotgun proteomics analysis
Sample preparation
The samples for shotgun proteomics analysis were prepared following the method of Zhu et al. (Citation2014). Briefly, SDT buffer (4% sodium dodecyl sulphate (SDS), 150 mM Tris- hydrochloric acid (HCl), 100 mM dithiothreitol (DTT), pH 8.0) was added to rabbit LTL, and then transferred to 2 mL tubes containing a 1/4 inch ceramic bead and some quartz sands. The lysate was then homogenised twice (24 × 2, 6.0M/S, 60s; MP Fastprep-24, MP Biomedicals, CA, USA). The homogenate was further sonicated by an ultrasonic liquid processor (JY92-II; Scientz Biological Polytron Technologies Inc., Ningbo, China) and then boiled for 15 min. Subsequently, the sample was centrifuged at 14000 × g for 40 min at 4 °C. The supernatant was filtered through a 0.22-µm filter, and the filtrate was quantified using the BCA Protein Assay Kit (Bio-Rad, USA). All samples were stored at −80 °C.
SDS-polyacrylamide gel electrophoresis (SDS-PAGE)
Twenty micrograms of protein sample was mixed with 5× loading buffer (10% SDS, 0.5% Bromophenol Blue, 50% Glycerol, 500 mM DTT, 250 mM Tris-HCl, pH 6.8) and then boiled for 5 min. Then, the protein was separated on a 12.5% SDS-PAGE gel (EPS601; GE Healthcare, MA, USA) for 90 min at a constant current of 14 mA. The protein bands were observed by Coomassie Blue R-250 staining.
Filter-aided sample preparation (FASP digestion)
FASP digestion was prepared following the methods of Wiśniewski et al. (Citation2009) with minor modifications. Briefly, 200 μg of protein sample was added into 30 μL of SDT buffer. Superfluous low-molecular-weight components, DTT, and detergent were then removed by repeated ultrafiltration using UA buffer (8 M Urea, 150 mM Tris-HCl, pH 8.0). The filter was added with 100 μL of iodoacetamide and then incubated in darkness for 30 min. After that, it was washed three times with 100 μL of UA buffer and then washed twice with 100 μL of 25 mM NH4HCO3 buffer. The resultant protein suspension was digested with 4 μg trypsin in 40 μL of 25 mM NH4HCO3 buffer at 37 °C overnight, and the digested peptides were filtered with 10 kD ultrafiltration tube, and the filtrate was collected. After that, the peptides were desalted on a C18 Cartridges, concentrated in a vacuum centrifuge (Eppendorf Concentrator Plus, Hamburg, Germany), and finally stored at −80 °C until subsequent analysis.
Mass spectrometry
Samples were analysed for 60 min on a Q Exactive mass spectrometer (Thermo Scientific, MA, USA), coupled with an Easy nLC (Thermo Fisher Scientific, MA, USA). The mass spectrometer was worked in positive ion mode. Resolution for higher energy C-trap dissociation spectra was set to 17,500 with 200 mz−1, and the isolation width was set at 2 mz−1. The underfill ratio was set at 0.1%, and the normalised collision energy was defined as 30 eV (Zhu et al. Citation2014).
Statistical analysis
The measurements were replicated thrice, and the results from proximate composition and amino acid profile were analysed based on a general linear model of analysis of variance (ANOVA) with breed and age in the model using SPSS 22.0 (IBM Corp., NY, USA). The significant difference between means was analysed by Duncan’s multiple range test (p < .05). The MS data were analysed by MaxQuant software version 1.5.3.17 (Max Planck Institute of Biochemistry, Martinsried, Germany), in which the cut-off global false discovery rate for protein and peptide identification was set at 0.01.
Results and discussion
Proximate composition
The analysis of the nutritional characteristic of LTL from rabbit with different breeds and ages are summarised in Table . In general, the moisture content in fresh rabbit meat can vary from 70% to 77%. Moisture and fat content in meat are usually inversely correlated to a certain degree; meat with high moisture content has lower fat content and visa versa (Hoffman et al. Citation2009). In this study, we found that mean moisture content in 35-day-old rabbits (76.03%) was higher (p < .05) than that in 105-day-old rabbits (74.89%). As predicted, the fat content in 35-day-old rabbits (1.25%) was lower (p < .05) than that in 105-day-old rabbits (1.76%). This is consistent with the findings by Metzger et al. (Citation2011), in which the moisture and fat content in rabbit meat was found to be influenced by age. Furthermore, ash content of the meat was significantly influenced by the age (p < .05), but not the breed (p > .05).
Table 1. Proximate composition (% as basis) in LTL samples of rabbits with different breeds and ages.
As a key component in meat, protein plays an important role in the quality of meat because it can greatly influence colour, juiciness and tenderness of meat (Petracci and Cavani Citation2013; Zhang et al. Citation2013). A number of studies have demonstrated that protein can influence consumers’ overall acceptability of meat products (Conti-Silva et al. Citation2011; Petrescu and Petrescu-mag Citation2018; Spencer et al. Citation2018). In the present study, protein content was significantly increased with age (p < .05), and it was not influenced by breed (p > .05). However, this is inconsistent with the results by Maj et al. (Citation2012), who reported that the protein content in meat of New Zealand White rabbit, slaughtered at the 12th, 21st, and 31st week, was not significantly different (p > .05). This may be due to the difference in rabbit breed and age at which it was slaughtered, as well as the quality and composition of the feed. No interaction effects of age and breed on the proximate composition of rabbit meat has been found in this study.
Amino acid profile in rabbit meat
The amino acid profile in rabbit LTL muscle is shown in Table . All amino acids tested, except for Val, Lys, Phe, Tyr, and Arg, were not significantly (p > .05) influenced by breed. Meat from 105-day-old rabbit contained higher (p < .05) levels of Leu, Tyr, Pro, Asp, Arg, and Gly, but lower (p < .05) levels of Thr and Ser compared with that from 35-day-old rabbit with the same breed. This is because amino acid composition is affected by different synthesis of amino acid as related to different biological stage of the animals. And the difference in diets of the two rabbits may also result in the difference in amino acid composition; while the 35-day-old rabbit, which has just been weaned, had breast milk as the main source of diet, the 105-day-old had rabbit feed as the main source of diet. Such difference may result in different amino acid profile of rabbit meat. The result is in agreement with that the study by Bivolarski et al. (Citation2011), who reported that weaning age can not only affect the amino acid profile in rabbit meat, but also its biological value. Moreover, numerous other studies have also reported the effect of diet on the characteristics of rabbit meat (except for amino acid profile), such as carcase characteristics, fatty acid metabolism and sensory qualities (Dalle Zotte Citation2002; Liu et al. Citation2009; Peiretti et al. Citation2013; Volek et al. Citation2018).
Table 2. Amino acids (% as basis) in LTL samples of rabbits with different breeds and ages.
It is important for individuals to meet the demands for essential amino acids, and the contents of essential amino acids are often used to evaluate the biological value of protein (Bohrer Citation2017). In this study, the mean content of total essential amino acids in all groups was 9.37%, and the content of Lys (2.26%) was the highest among all essential amino acids. This is in consistence with the report by Hernàndez and Dalle Zotte (Citation2010), who found that rabbit meat contained the high Lys content (2.12%), as well as other essential amino acids, e.g. Ile (0.91%), Val (1.08%), and Leu (1.72%). Furthermore, Glu, Pro and Asp were the main amino acids found in the non-essential fraction for all groups studied representing mean contents of 2.62%, 2.17%, and 2.03%, respectively. Nasr et al. (Citation2017) also found that Glu and Asp content was high in New Zealand White longissimus thoracis et lumborum muscles, but Pro content was much lower than the present study. This is likely due to the difference in breeds and muscle parts. Simply put, rabbit meat protein has high biological value, owing to its elevated and balanced essential amino acids contents.
Protein quantity
The protein quantity in rabbit LTL is presented in Figure . Generally, there is a strong correlation between the quality of meat and the contents of sarcoplasmic, myofibrillar, and stromal proteins. Such correlation is especially strong for myofibrillar protein, which is responsible for muscle contraction, therefore has a significant effect on water-holding capacity, tenderness, and texture parameters of meat (Choi and Kim Citation2009; Goll et al. Citation2008). In the present study, the mean percentages of myofibrillar protein and sarcoplasmic protein were 57.20% and 32.57%, respectively, which is in agreement with the data reported by Tornberg (Citation1996). In this study, the sarcoplasmic protein and myofibrillar protein were found to constitute 30 to 35% and 55 to 60% of the total muscle protein, respectively. However, the contents of sarcoplasmic and myofibrillar protein were not significantly influenced by breed and age (p > .05).
Figure 1. Percentage of sarcoplasmic, myofibrillar, and stromal protein in rabbit meat with different breeds and ages.
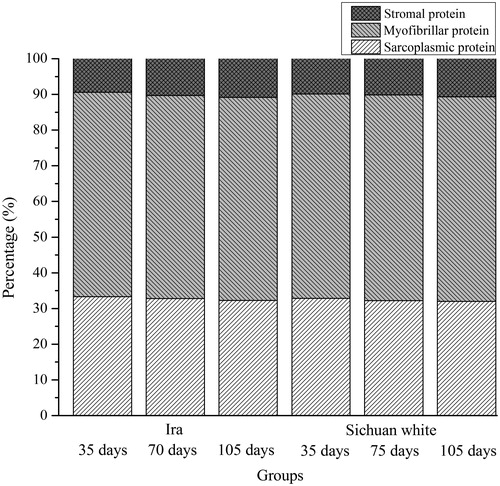
Stromal protein, which mainly consists of collagen, has a significant influence on meat tenderness; it is considered as the ‘background toughness’ of meat (Veiseth et al. Citation2004). In this study, the 105-day-old rabbit had higher (p < .05) percentage of stromal protein compared to the 35-day-old rabbit with the same gender; however, the effect of breed on the percentage of stromal protein was not significant (p > .05). Furthermore, the mean percentage of stromal protein was 10.23%, which is much higher than that of fish muscle of 3–5% (Klont et al. Citation1998). The difference may be due to the difference in species. As noted by Venugopal and Shahidi (Citation1996) both animal species and age can affect the content and type of collagen, which is a component of stromal protein.
Shotgun proteomics analysis
Relationship between rabbit breed and meat proteome
The relationship between rabbit breed and its meat proteome is shown in Figure . A total of 835 proteins were identified from the samples of two breeds. Although the breed had a limited influence on the number of proteins in rabbit meat (p > .05), 69 and 35 unique proteins were identified in Ira and Sichuan white, respectively. This is likely due to the difference in gene expression of the two rabbits (Yang et al. Citation2015). And 731 common proteins were identified in meat of rabbits with two breeds, and these proteins were mainly involved in energy metabolism, material transport and biochemical reactions. No interaction effects of breed and age on the number of protein has been found in this study.
Figure 2. Protein profiles in meat of rabbits with different breeds. (a) Number of proteins and unique proteins (presented by protein ID) identified in meat samples of rabbits with different breeds. (b) Molecular function of the 731 common proteins identified in meat of rabbits with two breeds.
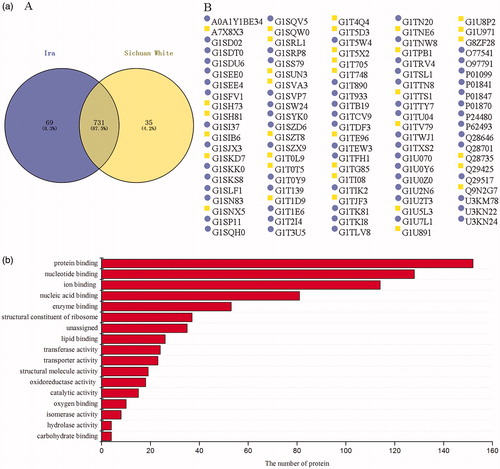
Nine unique proteins involving in adenosine triphosphate (ATP) binding were found in female Ira rabbits, including G1SKK0 (trifunctional purine biosynthetic protein adenosine-3), G1TK81 (nucleoside diphosphate kinase), and Q28701 (embryonic/neonatal myosin heavy chain), etc. These ATP-binding proteins are important for body to regulate metabolic process. For example, G1TK81 or nucleoside diphosphate kinase, which catalyses the phosphorylation of nucleoside diphosphates and consumes ATP to produce nucleoside triphosphates (Takács-Vellai et al. Citation2015). Additionally, other unique proteins found in female Ira rabbits were ion binding proteins, such as calcium ion binding protein Q29517 (phosphoinositide phospholipase C), zinc ion binding protein G1TCV9 (tripartite motif containing 54), and copper ion binding protein G1SJX3 (ceruloplasmin). Proteins, including antigen binding protein or lg kappa-b5 chain C region, chaperone binding protein or dnaj heat shock protein family (Hsp40) member B4, and calmodulin-binding protein or smoothelin like 1, were also found in female Ira rabbits.
Three unique metabolic regulating proteins, including G1SIB6, G1TPB1, and G1UB91, were identified from female Sichuan white rabbit. G1SIB6 or biliverdin reductase B, which is a newly identified cellular redox regulator in mature red blood cells (Paukovich et al. Citation2018), plays an important role in haem catabolic process due to its ability to interfere with biliverdin reductase and riboflavin reductase activity. G1TPB1 or carnitine O-acetyltransferase regulates carnitine metabolic process and is involved in acetyl coenzyme A metabolic process, by affecting the shuttling of acetyl units (Hynes et al. Citation2011). Furthermore, proteins involving in other biological processes, such as immune response (interleukin enhancer binding factor 2), protein deneddylation (COP9 signalosome subunit 7A), and circadian regulation of gene expression (nicotinamide phosphoribosyltransferase), were also identified in female Sichuan white rabbit.
Relationship between rabbit age and meat proteome
The relationship between rabbit age and its meat proteome is shown in Figure . The number of proteins identified in meat of 35-day-old rabbit was higher (p < 0.05) than that in meat of 105-day-old rabbit. The result is in agreement with that reported by Teltathum and Mekchay (Citation2009), in which a total of 259, 120, and 107 proteins were found in chicken pectoralis muscles at the ages of 0, 6 and 18 weeks, respectively. The difference is likely due to that different protein expression which varies with growth period (Hollung et al. Citation2009). And the molecular function of the common proteins identified in meat of rabbits with three ages was involved in protein binding, ion binding, and nucleotide-binding.
Figure 3. Protein profiles in meat of rabbits with different ages. (a) Number of proteins and unique proteins (presented by protein ID) identified in meat samples of rabbits with different ages. (b) Molecular function of the 601 common proteins identified in meat of rabbits with three ages.
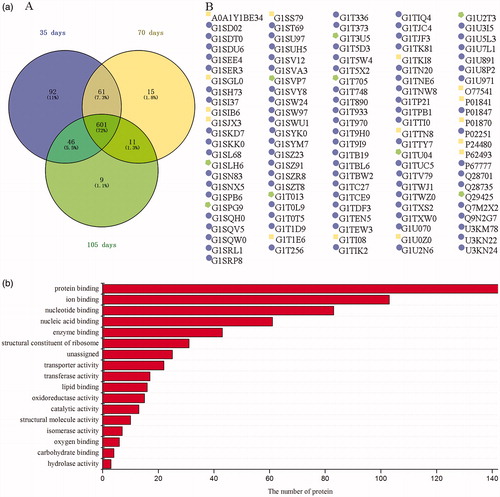
Ninety-two unique proteins were identified in 35-day-old rabbit. Some proteins such as G1SD02 (COP9 signalosome subunit 6), G1SVA3 (proteasome 26S subunit, non-ATPase 11), and U3KN24 (26S proteasome non-ATPase regulatory subunit 1) are involved in protein catabolic process, and other proteins such as G1SEE4 (peptidase M20 domain-containing protein 2), G1TPB1 (carnitine O-acetyltransferase), G1U3I5 (enoyl-CoA hydratase 1), and U3KN22 (glutathione S-transferase kappa) regulate the metabolic process. However, other 27 unique proteins, which have special molecular function, but are not involved in biological process, were also identified. These proteins were, such as phospholipase binding protein G1ST69 (lamin B1), actin filament binding protein G1TI08 (transgelin), and RNA binding protein G1TEW3 (uncharacterized protein). Nonetheless, both the proteins regulating the biological process and those having a special molecular function are crucial in maintaining the regular function of the body.
Four unique proteins possessing enzymatic activity were found in 70-day-old rabbit. These proteins were G1SIB6 (biliverdin reductase B) – an enzyme possessing riboflavin reductase activity, G1SJX3 (ceruloplasmin) – an enzyme possessing ferroxidase activity, G1T1E6 (secernin 3) – an enzyme possessing dipeptidase activity, and P62493 (ras-related protein Rab-11A) – an enzyme possessing guanosine triphosphatase activity. And some proteins involving in haem catabolic process, cellular iron ion homeostasis, and the regulation of cell proliferation were also found in 70-day-old rabbit. Furthermore, only nine unique proteins were identified in 105-day-old rabbit, and most of these proteins were metabolic regulating proteins. For example, G1SLH6 or 5'-nucleotidase cytosolic IA is related to the nucleotide metabolic process, Q29425 or Cullin-5 is involve in the ubiquitin-dependent protein catabolic process, and G1T013 or malic enzyme regulates malate metabolic process.
Conclusions
Rabbit meat is rich in protein, thus is a good source of essential amino acids. We found that the meat of female rabbits with varying breed and age had significantly different amino acid profile. The contents of sarcoplasmic, myofibrillar, and stromal protein in female rabbit meat were also found to vary with breed and age of rabbits. Furthermore, a total of 835 proteins were identified from the samples. Most of these proteins are involved in biological process, and they are crucial in maintaining the regular function of the body. Both breed and age can affect the protein profile of female rabbit meat, and the difference in protein profile may be the reason for the quality variation of different rabbit meat samples. More detailed research is needed to study the effect of gender on the protein profile of rabbit meat and research the relationship between protein composition and the unique proteins in meat samples from rabbits with different breeds and ages.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Almeida AM, Campos A, Harten S, Cardoso LA, Coelho AV. 2009. Establishment of a proteomic reference map for the gastrocnemius muscle in the rabbit (Oryctolagus cuniculus). Res Vet Sci. 87:196–199.
- Altelaar AF, Munoz J, Heck AJ. 2013. Next-generation proteomics: towards an integrative view of proteome dynamics. Nat Rev Genet. 14:35–48.
- AOAC (Association of Official Analytical Chemists). 2000. Official methods of analysis. 17th edition. Gaithersburg, MD: AOAC International.
- Bargen C, Dojahn J, Waidelich D, Humpf H, Brockmeyer J. 2013. New sensitive high-performance liquid chromatography − tandem mass spectrometry method for the detection of horse and pork in halal beef. J Agri Food Chem. 61:11986–11994.
- Bivolarski B, Vachkova E, Ribarski S, Uzunova K, Pavlov D. 2011. Amino acid content and biological value of rabbit meat proteins, depending on weaning age. Bulg J Vet Med. 14:94–102.
- Bohrer BM. 2017. Review: nutrient density and nutritional value of meat products and non-meat foods high in protein. Trend Food Sci Technol. 65:103–112.
- Choi YM, Kim BC. 2009. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livestock Sci. 122:105–118.
- Combes S, Dalle Zotte A. 2005. La viande de lapin: valeur nutritionnelle et particularités technologiques. Presented at 11. Journées de la Recherche Cunicole, Paris, FRA (29 Sep–30 Nov). p 167–180. Paris, FRA: ITAVI–Institut Technique de l'Aviculture.
- Conti-Silva AC, Pinto ME, Arêas JA. 2011. Sensory acceptability of raw and extruded bovine rumen protein in processed meat products. Meat Science. 88:652–656.
- Cox J, Mann M. 2011. Quantitative, high-resolution proteomics for data-driven systems biology. Ann Rev Biochem. 80:273–299.
- Dalle Zotte A. 2002. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livestock Prod Sci. 75:11–32.
- Dalle Zotte A, Szendro Z. 2011. The role of rabbit meat as functional food. Meat Sci. 88:319–331.
- Dawood AA, Alkanhal MA. 1995. Nutrient composition of Najdi-camel meat. Meat Sci. 39:71–78.
- FAO (Food and Agriculture Organization). 2019. Online statistical database: Food balance. FAOSTAT. [2019-03-17]. http://www.fao.org/faostat/en/#data/QL
- Goll DE, Neti G, Mares SW, Thompson VF. 2008. Myofibrillar protein turnover: the proteasome and the calpains. J Anim Sci. 86:E19–35.
- Graves PR, Haystead TA. 2002. Molecular biologist’s guide to proteomics. Microbiol Mol Biol Rev. 66:39–63.
- Hashimoto K, Watabe S, Kono M, Shiro K. 1979. Muscle protein-composition of sardine and mackerel. Bull Japan Soc Sci Fish. 45:1435–1441.
- Hernàndez P, Dalle Zotte A. 2010. Influence of diet on rabbit meat quality. In: de Blas C, Wiseman J, editors. Nutrition of the rabbit. Wallingford: CABI Publishing. pp. 163–178.
- Hoffman LC, Mostert AC, Kidd M, Laubscher LL. 2009. Meat quality of kudu (Tragelaphus sterpsiceros) and impala (Aepyceros melampus): carcass yield, physical quality and chemical composition of kudu and impala Longissimus doris muscle as affected by gender and age. Meat Sci. 83:788–795.
- Hollung K, Grove H, Faergestad EM, Sidhu MS, Berg P. 2009. Comparison of muscle proteome profiles in pure breeds of Norwegian Landrace and Duroc at three different ages. Meat Sci. 81:487–492.
- Hynes MJ, Murray SL, Andrianopoulos A, Davis MA. 2011. Role of carnitine acetyltransferases in acetyl coenzyme A metabolism in Aspergillus nidulans. Eukaryotic Cell. 10:547–555.
- Ito Y, Tatsumi R, Wakamatsu J, Nishimura T, Hattori A. 2003. The solubilization of myofibrillar proteins of vertebrate skeletal muscle in water. Anim Sci J. 74:417–425.
- Klont RE, Brocks L, Eikelenboom G. 1998. Muscle fibre type and meat quality. Meat Sci. 49:S219–S229.
- Li S, Zeng W, Li R, Hoffman LC, He Z, Sun Q, Li H. 2018. Rabbit meat production and processing in China. Meat Sci. 145:320–328.
- Liu H, Gai F, Gasco L, Brugiapaglia A, Lussiana C, Guo K, Tong J, Zoccarato L. 2009. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 83:678–683.
- Liu Z, Du X, Yin C, Chang Z. 2013. Shotgun proteomic analysis of sarcoplasmic reticulum preparations from rabbit skeletal muscle. Proteomics. 13:2335–2338.
- Luo F, Xing R, Wang X, Peng Q, Li P. 2017. Proximate composition, amino acid and fatty acid profiles of marine snail Rapana venosa meat, visceral mass and operculum. J Sci Food Agri. 97:5361–5368.
- Maj D, Bieniek J, Bekas Z. 2012. Effect of age and gender of rabbits on indices of their meat quality. Zywnosc-Nauka Technol Jakosc. 19:142–153.
- Metzger S, Odermatt M, Szabo A, Radnai I, Biro-Nemeth E, Nagy T, Szendro Z. 2011. Effect of age and body weight on carcass traits and meat composition of rabbits. Arch Anim Breed. 54:406–418.
- Mudalal S, Babini E, Cavani C, Petracci M. 2014. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poultry Sci. 93:2108–2116.
- Nasr MA, Abd-Elhamid T, Hussein MA. 2017. Growth performance, carcass characteristics, meat quality and muscle amino-acid profile of different rabbits breeds and their crosses. Meat Science. 134:150–157.
- Paukovich N, Xue M, Elder JR, Redzic JS, Blue A, Pike H, Miller BG, Pitts TM, Pollock DD, Hansen K, et al. 2018. Biliverdin reductase B dynamics are coupled to coenzyme binding. J Mol Biol. 430:3234–3250.
- Peiretti PG, Gai F, Rotolo L, Brugiapaglia A, Gasco L. 2013. Effects of tomato pomace supplementation on carcass characteristics and meat quality of fattening rabbits. Meat Sci. 95:345–351.
- Pereira PM, Vicente AF. 2013. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 93:586–592.
- Petracci M, Cavani C. 2013. Rabbit meat processing: historical perspective to future directions. World Rabbit Sci. 21:217–226.
- Petracci M, Soglia F, Leroy F. 2018. Rabbit meat in need of a hat-trick: from tradition to innovation (and back). Meat Sci. 146:93–100.
- Petrescu DC, Petrescu-mag RM. 2018. Consumer behaviour related to rabbit meat as functional food. World Rabbit Sci. 26:321–333.
- Spencer M, Cienfuegos C, Guinard J. 2018. The flexitarian flipTM in university dining venues: student and adult consumer acceptance of mixed dishes in which animal protein has been partially replaced with plant protein. Food Qual Prefer. 68:50–63.
- Takács-Vellai K, Vellai T, Farkas Z, Mehta A. 2015. Nucleoside diphosphate kinases (NDPKs) in animal development. Cell Mol Life Sci. 72:1447–1462.
- Teltathum T, Mekchay S. 2009. Proteome changes in Thai Indigenous chicken muscle during growth period. Int J Biol Sci. 5:679–685.
- Tornberg E. 1996. Biophysical aspects of meat tenderness. Meat Sci. 43:175–191.
- Veiseth E, Shackelford S, Wheeler T, Koohmaraie M. 2004. Factors regulating lamb longissimus tenderness are affected by age at slaughter. Meat Sci. 68:635–640.
- Venugopal V, Shahidi F. 1996. Structure and composition of fish muscle. Food Res Int. 12:175–197.
- Volek Z, Bureš D, Uhlířová L. 2018. Effect of dietary dehulled white lupine seed supplementation on the growth, carcass traits and chemical, physical and sensory meat quality parameters of growing-fattening rabbits. Meat Sci. 141:50–56.
- Wiśniewski JR, Zougman A, Nagaraj N, Mann M. 2009. Universal sample preparation method for proteome analysis. Nat Method. 6:359–362.
- Yang CJ, Ge J, Chen SJ, Liu YJ, Chen BJ, Gu ZL. 2015. Sequence and gene expression analysis of the agouti signalling protein gene in Rex rabbits with different coat colours. Italian J Anim Sci. 14:563–565.
- Yuswan MH, Aizat WM, Lokman AA, Desa M, Mustafa S, Junoh NM, Yusof ZNB, Mohamed R, Mohmad Z, Lamasudin DU. 2018. Chemometrics-assisted shotgun proteomics for establishment of potential peptide markers of non-halal pork (sus scrofa) among halal beef and chicken. Food Anal Method. 11:3505–3515.
- Yu TY, Morton JD, Clerens S, Dyer JM. 2016. Proteomic investigation of protein profile changes and amino acid residue-level modification in cooked lamb longissimus thoracis et lumborum: the effect of roasting. Meat Sci. 119:80–88.
- Zhang W, Xiao S, Ahn DU. 2013. Protein oxidation: basic principles and implications for meat quality. Crit Rev Food Sci Nutr. 53:1191–1201.
- Zhu Y, Xu H, Chen H, Xie J, Shi M, Shen B, Deng X, Liu C, Zhan X, Peng C. 2014. Proteomic analysis of solid pseudopapillary tumor of the pancreas reveals dysfunction of the endoplasmic reticulum protein processing pathway. Mol Cell Proteomics. 13:2593–2603.
