Abstract
The aim of this study was to evaluate the differences in carcase traits, meat quality and muscle characteristics (mean fibre diameter and MyHCs levels of expression) between Mashen and Large White pigs. A total of 60 pigs (30 Mashen pigs and 30 Large White pigs, half male and half female in each breed) were used in this experiment. Results showed that Mashen pigs exhibited lower slaughter weight, reduced back-fat thickness, reduced loin eye area and lower lean meat ratio than Large White pigs (p < .05). Meat from Large White pigs had higher pH1, greater drip losses, higher Warner-Bratzler shear force values, higher lightness, higher yellowness, and greater mean diameter of muscle fibres. Also, meat from Large white pigs had lower cooked yield, lower redness, and reduced intramuscular fat content than meat from Mashen pigs (p < .05). The contents of 17 amino acids, total amino acids, essential amino acids and umami amino acids in the longissimus dorsi of Mashen pigs were higher than those in Large White pigs (p < .05). Large White pigs had higher monounsaturated fatty acids and lower polyunsaturated fatty acids than Mashen pigs (p < .05). Meanwhile, the expression of MyHCI was significantly higher and the expression of MyHCIIb was lower in Mashen than in Large White pigs (p < .05). The higher MyHCI and lower MyHCIIb found in muscles from Mashen pigs might partially explain meat quality differences found between the two breeds in the present study. These results provide valuable information for meat quality differences between Mashen and Large White pigs.
Carcase traits and meat quality between Large White and Mashen pigs exhibited significant differences.
The total amino acids, essential amino acids and umami amino acids in Mashen pigs were higher than those in Large White pigs.
Large White pigs had higher monounsaturated fatty acids and lower polyunsaturated fatty acids than Mashen pigs.
The expression of MyHCI was significantly higher and the expression of MyHCIIb was lower in Mashen than in Large White pigs.
Highlights
Introduction
Meat quality is a very important issue for consumers and it is also vital for the meat industry (Şirin et al. Citation2017). With the continuous improvement of living standards, the demand of consumers for high quality meat is constantly increasing. Consumers’ requirements for meat include to be delicious, nutritious, safe and healthy (Joo et al. Citation2013). There are many factors influencing meat quality such as breed, genotype, sex, age, nutrition and slaughter conditions. Skeletal muscle fibre composition is one of the important factors influencing meat quality (Gil et al. Citation2003; Klosowska and Fiedler Citation2003; Choi et al. Citation2007). Myosin heavy chain (MyHC) is transcriptionally regulated in pig skeletal muscle (Park et al. Citation2009). MyHCs gene expression was commonly used to define muscle fibre type composition in pigs, which was more precise and reliable than traditional methodologies (Gunawan et al. Citation2007; Choi and Kim Citation2009; Park et al. Citation2009). Four MyHC isoforms were found to be expressed in the skeletal muscle of adult pigs: type I, IIa, IIx, IIb: type I, IIa, IIx, IIb (Lefaucheur et al. Citation2002). MyHC defines the contractile nature of fibre types: MyHC type I is the slower contractile type, followed by types IIA, IIX and IIB in increasing contractile speed order. Those contractile fibre types are usually associated with different metabolic activities. Type I fibres have greater oxidative capacity to support sustained contraction, whereas type IIb fibres are predominantly glycolytic and rapidly use glycogen for short bursts of activity. The IIa and IIx fibres are intermediate to type I and IIb fibres (Chang et al. Citation2003). Muscle fibre type composition has different effects on post-mortem change of meat quality. (Ozawa et al. Citation2000; Ryu et al. Citation2005), and abundance of MyHC IIb fibres is associated with less favourable meat quality, both in terms of pH, drip loss, grain, colour, yield force and work done (Chang et al. Citation2003). Meanwhile, the presence of type I fibre was positively related to good meat quality (Wimmers et al. Citation2008). An increase in the proportion of muscle fibre type I leaded to a decrease of lightness and an improvement of water-holding capacity in pork (Gil et al. Citation2003; Ryu and Kim Citation2005; Choi et al. Citation2010) and with improved tenderness and juiciness in beef (Maltin et al. Citation1998). Previous studies have found that type IIb fibres were closely related to toughness, paleness, higher protein denaturation and lower water-holding capacity in porcine longissimus muscle (Karlsson et al. Citation1993; Larzul et al. Citation1997; Kauffman et al. Citation1998; Renand et al. Citation2001; Ryu et al. Citation2008; Choi et al. Citation2010).
The Mashen pig, one of the most important indigenous breeds in northern China, exhibits high adaptability, good meat quality, slow growth rate and low feed conversion ratio. On the other hand, the Large White pig is a common breed for its high growth rate, feed conversion ratio and lean meat percentage (Zhang et al. Citation2001; Yang et al. Citation2005; Zhao et al. Citation2015). Therefore, these two pig breeds can serve as an ideal comparison for studying meat quality. Up to now, little was known about muscle fibre composition and post-mortem muscle metabolism in Mashen pigs, nor the breed differences between Mashen and Large White pigs. In the present study, the differences in muscle fibre composition, carcase traits and meat quality between Mashen and Large White pigs were compared. The aim of this study was to evaluate the differences in carcase traits, meat quality and muscle characteristics (IMFC, mean fibre diameter and MyHCs levels of expression) between Mashen and Large White pigs.
Materials and methods
Animals
Mashen pigs (n = 30; castrated males, n = 15; females, n = 15; 72.5 ± 0.6 kg) and Large White pigs (n = 30; castrated males, n = 15; females, n = 15; 119.5 ± 1.0 kg) under the same feeding management conditions (The composition of diets were shown in Table S1) were selected at 180-days old from the Datong Pig Farm (Shanxi, China). The selected pigs all were weaned at 28-days old and males were castrated when weaning. Among them, eight Mashen pigs (4 castrated males and 4 females, 72.8 ± 0.6 kg) and eight Large White pigs (4 castrated males and 4 females, 119.9 ± 1.0 kg) were selected randomly to determain the amino acids composition, fatty acids composition and levels of MyHCs relative expression. Carcase traits and other meat quality measurements were conducted in all thirty pigs per breed. All of the animal procedures were conducted per the Code of Ethics of the World Medical Association (Declaration of Helsinki) for animal experiments (http://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm). The methods were performed in accordance with the Good Experimental Practices adopted by the College of Animal Science and Veterinary Medicine, Shanxi Agricultural University (Shanxi, China). Moreover, the local animal welfare laws, guidelines, and policies were strictly followed for the feed and use of experimental animals. The experiment was approved by the Animal Ethics Committee of Shanxi Agricultural University.
Carcase traits
Electric shock was used to stun pigs, which then were exsanguinated. After removing hair, head, hooves and internal organs, the carcases were weighed, and the carcase yields were calculated. In addition, the carcase length was measured from the midpoint of the anterior margin of the pubic symphysis to the midpoint of the anterior margin of the first cervical vertebra. The carcase bone, meat, skin and fat of the left side of carcases were separated and weighed to calculate lean meat ratio. The loin eye area was measured at the last rib level and calculated based on the loin eye height and width with 0.7 as the coefficient (Dai et al. Citation2009). The fat thickness of the first and last ribs, and the last lumbar vertebra were measured using a vernier calliper (Jiang et al. Citation2012). The average of those three measurements was used as the back-fat thickness.
Meat quality
Muscle pH (pH1) was measured at 45 min post-mortem in the longissimus dorsi (between the 13th and 14th rib) using pear-type portable pH metre (IQ-150 pH metre and PH77-SS probe, IQ Scientific Instruments, USA).
The longissimus dorsi samples at 10th to 11th thoracic were taken within 1–2 h after death and pieced to 4 cm × 4 cm × 4 cm size and weighted. Then the meat cubes were placed in inflated plastic bags and hung for 48 h at 4 °C (Honikel Citation1998). After that, meat cubes were reweighted. The drip loss is the percentage of two weight changes.
About 100 g psoas major sample was taken at 24 h post-mortem. After stripped of the outer membrane and the attached fat, the meat was placed in an aluminium pot and steamed for 30 min. After steaming, the sample was hung for 30 min before re-weighed. The difference of two weights was used to calculate the cooked meat percentage.
At 24 h post-mortem, longissimus dorsi muscle samples at 3rd to 6th lumbar vertebrae were taken and cut into 5 cm ×2 cm ×2 cm pieces along the fibres, which were used for the measurement of the Warner-Bratzler shear force (WBSF). Then those samples were packed in plastic bags and boiled about 30 min (The centre of the meat should attain 80 °C). After that, they were cooled to room temperature rapidly. The digital display muscle tenderness metre (C-LM3B, Northeast agricultural university, Heilongjiang, China) was used to measure the WBSF. Six repetitions per sample.
Muscle colour of longissimus dorsi were measured by Chroma metre (CR-300, Minolta Camera, Tokyo, Japan) at the last rib level. Muscle colour was determined on the exposed cut surface of the longissimus muscle at 24 h post-mortem. Each measurement was performed in six replicates, taking the mean value as the assay result. The results were expressed as CIE (Commission International del’Eclairage) Lightness, redness and yellowness values.
Intramuscular fat (IMF) percentage was determined in the Longissimus lumborum (between the 3rd and 4th lumbar vertebrae) by ether extraction in a Soxhlet apparatus after acid hydrolysis.
Approximately 80–100 g of longissimus dorsi tissue between the 3rd and 4th lumbar vertebrae were taken at 24 h post-mortem. About 2 mg of extracted lipid was redissolved in 2 mL of n-hexane and 1 mL of KOH (0.4 M) for saponification and methylation. The fatty acid methyl esters were analysed with a gas chromatographer (ThermoFisher Trace 1310 ISQ, Thermo Corporation, USA) equipped with a capillary column (30 m × 0.25 mm × 0.25 µm film thickness). Nitrogen was used as carrier gas, the oven temperature was initially held at 80 °C. The temperature was rose at a rate of 10 °C min−1 to 200 °C and then continue got up to 250 °C (5 °C min−1). With the rate of 2 °C min−1, the temperature finally was up to 270 °C. The ion source and transmission line temperatures were maintained at 280 °C. Each fatty acid was identified by comparing their retention times with those of authenticated standards and quantified as a percentage of total fatty acids.
Approximate 100 mg samples of longissimus dorsi tissue at the 3rd to 4th lumbar vertebrae were dissolved in a solution of water and methanol (1:1) at 4 °C for 10 min and centrifuged at 10,000 × g for 10 min. The supernatant was filtered through glass wool. The 500 μL of the supernatant was used for amino acids analysis using liquid chromatograph (ThermoFisher U3000, Thermo Corporation, USA). Chromatographic conditions: flow rate, 1.0 mL per minute; column temperature, 40 °C; wavelength, 254 nm.
Muscle characteristics
Within 45 min post-mortem, longissimus dorsi samples at the last rib were taken for histochemical analysis. Muscle samples at a size of 0.5 cm × 0.5 cm × 0.2 cm were obtained and immediately put into 4% paraformaldehyde solution for paraffin embedding, which were then cross-sectioned for HE staining. The microscopic images were captured at 100 times magnification (OLYMPUS microscope, Olympus Corporation, Japan). Muscle fibre diameters were measured on ∼100 fibres based on the smallest diameter. The diameter value was expressed as the mean value from all measurements. In addition, muscle fibre density was calculated by dividing the average number of muscle fibres in 10 randomly selected views by the view area (0.59 mm2), which was converted to the number of muscle fibre roots per mm2.
The longissimus dorsi muscles between the 11th and 12th rib of Mashen pigs and Large White pigs were taken shortly after exsanguination, immediately snapped in liquid nitrogen, and stored at −80 °C for subsequent use. Quantitative real-time polymerase chain reaction (qPCR) was used to detect the mRNA expression patterns of MyHCI, MyHCIIa, MyHCIIx and MyHCIIb. The primers of MyHCs used in present research referred to the study of Hu et al (Hu et al. Citation2008). 18S rRNA was used as the internal control. Details of primers are shown in Table . qPCR was performed using a SYBR® PrimeScriptTMII RT-PCR Kit (Takara, China) in conjunction with an ABI-7500 real-time PCR system (Applied Biosystems, USA). The reaction conditions were as follows: predenaturation at 95 °C for 30 s; 45 cycles of 95 °C for 30 s and 60 °C for 34 s; and one cycle of 95 °C for 30 s, 60 °C for 1 min, 95 °C for 30 s. To ensure robustness, each sample was analysed in triplicate. The relative expressions were quantified using the 2−ΔΔCt method.
Table 1. Primer sequences of MyHCs and 18S rRNA used in qPCR.
Statistical methods
Carcase traits, meat quality and muscle characteristics between Mashen and Large White pigs were analysed by one-way ANOVA in SPSS22.0 software. p < .05 indicates the difference is significant. Data were expressed as “means ± standard error of means (SEM)”. We are preprocessing the test data to analyse the effects of breed and sex. By building model found that gender had no significant influence on carcase traits and meat quality and muscle characteristics.
Results
Carcase traits of Mashen and large white pigs
The carcase traits in Mashen and Large White pigs are shown in Table . Compared with Large White pigs, the slaughter weight of the Mashen pigs were lower. When slaughtered at 180 days of age, the weight of the Mashen pigs were 72.5 kg, while that of Large White pigs were 119.5 kg. Mashen pigs had significantly lower back fat thickness, loin eye area and lean meat yield compared to Large White pigs (30.0 vs. 33.7, 25.6 vs. 51.6, and 48.8% vs. 63.5%, respectively, p < .05).
Table 2. Carcase traits in Mahen pigs and Large White pigs (n = 30).
Meat quality characteristics of Mashen and Large White pigs
As shown in Table , meat quality characteristics between two breeds were significantly different (p < .05). Compared with Large White pigs, Mashen pigs had lower pH1, drip loss, WBSF, lightness, yellowness and higher cooked yield, redness and IMF.
Table 3. Meat quality characteristics in Mashen and Large White pigs (n = 30).
The content of amino acids (except lysine) in Mashen pigs was significantly higher than that in Large White pigs (Table ) (p < .05). In addition, the sum of amino acids, umami amino acid and essential amino acids of Mashen pigs were significantly higher than those of Large White pigs (Table ) (p < .05). Among amino acids, the content of Glutamic acid (Glu) was the highest (Mashen pig, 4.40 g/100g; Large White pig, 3.75 g/100g) (Table ) (p < .05).
Table 4. Composition and content of amino acids in longissimus dorsi of Mashen and Large White pigs (g/100 g muscle) (n = 8).
As shown in Table , the content of monounsaturated fatty acids in Mashen pigs was significantly lower than that in Large White pigs. On the contrary, polyunsaturated fatty acids were more abundant in Mashen pigs (p < .05).
Table 5. Composition and content of fatty acids in longissimus dorsi of Mashen and Large White pigs (% of total fatty acids) (n = 8).
Muscle characteristics of Mashen and Large White pigs
As shown in Table , compared with Large White pigs, Mashen pigs had lower diameter and higher density of muscle fibres.
Table 6. Diameter and density of muscle fibres (longissimus dorsi) in Mashen and Large White pigs (n = 30).
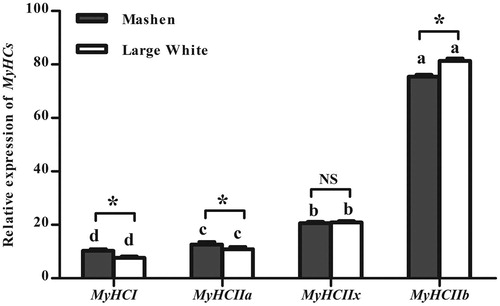
The mRNA level of MyHCI was the lowest and MyHCIIb was the highest between the 4 MyHC isoforms compared within each pig breed. The mRNA expression of MyHCI and MyHCIIa in Mashen pigs was higher than that in Large White pigs (p < .05). On the other hand, the expression of MyHCIIb in Large White pigs was higher than that in Mashen pigs (p < .05). Regarding MyHCIIX mRNA levels of expression, no significant differences were observed between the two studied breeds (p>.05).
Discussion
In the present study, the muscle fibre diameters of Mashen pigs were smaller than Large White pigs, which might partially explain the lower drip loss, WBSF and higher cooked yield in Mashen pork. Previous studies suggested that muscle fibre diameter influences meat quality, and pork with greater muscle fibre diameters have higher levels of shear force, drip loss, cooking loss (Seideman and Crouse Citation1986; Rehfeldt et al. Citation2000; Bulotiene and Jukna Citation2008). We found that the IMF of Mashen pigs was 3.73% and Large White pigs was 2.51%. Consistently, it has been reported that the IMF of European pigs is 2% to 3%, while in Chinese indigenous pigs, the IMF is around 4% or even higher (Liu et al. Citation2016; Yang et al. Citation2016). In addition, Mashen pigs exhibited reduced loin eye area, reduced lean meat rate, lower drip loss, lower WBSF, and lower lightness values than Large White pigs. This is consistent with other studies evaluating meat quality of Chinese indigenous pig (Young Citation1995; Miao et al. Citation2009; Guo et al. Citation2011; Jiang et al. Citation2011; Wu et al. Citation2013; Liu et al. Citation2015). Because of genetic selection, the Large White pigs have higher growth rate and lean ratio when compared to less selected native pig breeds (Mashen pig). Consistently, Korean native pigs exhibited a higher IMF content and backfat thickness than European meat breeds (Kim et al. Citation2007). The Lantang pig is native to the southern part of China, which had a higher IMF content and back-fat thickness than the commercial European pig Landrace, while the Landrace exhibited a higher loin eye area and ash content than Lantang pig (Dai et al. Citation2009). The Min pig (such as Heilongjiang Province), a well-known Chinese fat-type breed, is famous for its high IMF, superior meat quality, and stress resistance. Compared with Min pig, the Large White pig has a faster growth rate and higher lean meat ratio (Liu et al. Citation2017).
Figure 1. Relative expression of MyHCs mRNA in longissimus dorsi of Mashen and Large White pigs. Notes: Different letters in the same breed indicate significant differences (p < .05) among different types of MyHCs. * indicates that difference in the same gene between the Mashen and Large White pigs is significant (p < .05), and NS represents no significant difference.
Muscle fibre types were related to meat quality and carcase traits in various species, including cattle (Ozawa et al. Citation2000; Picard et al. Citation2006; Hwang et al. Citation2010), pigs (Larzul et al. Citation1997; Ryu and Kim Citation2006) and poultry (Dransfield and Sosnicki Citation1999; Kim et al. Citation2008). In adult pigs, skeletal muscles consist of four contractile fibre types (Type I, IIa, IIx, IIb), which are characterised by the expression of MyHC gene isoforms (Lefaucheur et al. Citation2002). The expression of MyHC genes was highly correlated with the relative amount of the corresponding protein and is used to classify the muscle fibre type composition (Gunawan et al. Citation2007). The positive correlation between MyHC mRNA expression and its corresponding protein level has also been shown by others (Cox and Buckingham Citation1992; Schiaffino and Reggiani Citation1996; Mckoy et al. Citation1998). Therefore, MyHCs gene expression was used to define muscle fibre type composition, which was more precise and reliable than traditional methodologies (Gunawan et al. Citation2007; Choi and Kim Citation2009; Park et al. Citation2009).
In the present study, both Mashen and Large White pigs, showed higher levels of expression of MyHCIIb than that of MyHCI. This is consistent with previous studies which generally show that the longissimus muscle of pigs has higher proportions of type IIb than type I fibres (Marita and Eero Citation2004; Ryu et al. Citation2008; Lee et al. Citation2012). The proportions of type IIb fibres were 69.7-90.3% in pig longissimus dorsi from various breeds, such as Berkshire, Yorkshire, Landrace, Duroc and their crossbred pigs (Marita and Eero Citation2004; Ryu et al. Citation2008; Lee et al. Citation2012). Importantly, the expression of MyHCI was higher in Mashen than in Large White pigs, whereas the expression of MyHCIIb was higher in Large White pigs. Each muscle fibre type has different biochemical and biophysical characteristics. The differentially expressed muscle fibre types may be the most important factors influencing meat quality, particularly IMF content and drip loss (Ozawa et al. Citation2000; Ryu et al. Citation2005; Kim et al. Citation2008; Guo et al. Citation2011). Type I fibres contain neutral lipids, whereas only 26% and 1% of types IIa and IIb fibres contain lipids (Karlsson et al. Citation1999). The type I fibres contain abundant mitochondria and greater amount of intramyofibrilar lipid content (Lefaucheur et al. Citation2002), while pig muscle with a higher proportion of fast-twitch fibres had a lower intramuscular lipid content (Fiedler et al. Citation2003). Hence, the higher IMF in Mashen pigs than Large White pigs might be partially due to a higher proportion of type I and a lower proportion of type IIb in Mashen pigs. In addition, drip loss is influenced by the characteristics of muscle fibres, especially type IIb (Larzul et al. Citation1997; Ryu and Kim Citation2006). Meat from Large White pigs had a higher drip losses and lower pH1, which might be due to a higher proportion of type IIb fibres. Type IIb fibres are predominantly glycolytic, which contribute to the rapid metabolism of glycogen during the early post-mortem period, producing higher amounts of intramuscular lactate and faster pH decline rates, resulting in poorer meat quality (Schiaffino and Reggiani Citation1996; Pette and Staron Citation2001; Choi and Kim Citation2009). Consistently, meat with a higher percentage of type IIb fibres and lower percentage of type I fibres showed lower pH, paler colour, and higher drip loss (Ryu and Kim Citation2006; Choi et al. Citation2007; Choe et al. Citation2008; Choi et al. Citation2010). With the higher expression level of MyHCI and lower expression level of MyHCIIb, Mashen pigs had a higher redness and lower lightness, and lower WBSF. In agreement, tenderness is affected by fibre type, especially type IIb fibres. Muscles with larger type IIb fibres may be tougher or may have greater hardness (Karlsson et al. Citation1993; Żochowska et al. Citation2005). In addition, due to the presence of abundant mitochondria and myoglobin in type I fibres, higher percent of type I fibres were related to lower lightness and higher redness (Ryu and Kim Citation2006; Hwang et al. Citation2010; Kim et al. Citation2013).
The soluble amino acid content correlates with the flavour of meat (Yang et al. 1994; Shi et al. 2003; Chen et al. 2004; Zhu et al. Citation2008). Six umami amino acids (Gly, Ile, Pro, Ser, Ala and Glu) are directly related to the flavour, of which Glu is the most important umami amino acid, (Hood and Allen Citation1973). Comparing the amino acid composition of Bamei, Landrace and their hybrid pigs, 17 amino acids were significantly different among these breeds, of which Bamei pigs had the highest content of umami amino acids (such as Glu) (Yang et al. 1994). In this study, the contents of Glu, Gly, Ile, Pro, Ser and Ala, were higher in Mashen pigs than that in Large White pigs, which may cause the Mashen pig has the better taste of pork.
In present study, the monounsaturated fatty acids content was lower in Mashen pigs than in Large White pigs, while the content of polyunsaturated fatty acids was higher in Mashen pigs. The content of saturated fatty acids is also related to muscle quality (Hood and Allen Citation1973; Cameron and Enser Citation1991). Unsaturated fatty acids are not only an important precursor to meat flavour, but also an indispensable nutrient for human body (Hallenstvedt et al. Citation2012). On the other hand, saturated fatty acids can cause atherosclerosis, elevated blood lipids, thrombotic disorders and other issues, while increased unsaturated fatty acids (n-3 polyunsaturated fatty acids) uptake can decrease blood cholesterol level and prevent arteriosclerosis (Bhavsar and Stonge Citation2016). In addition, a higher content of C18:3 was found in Mashen pork than Large White pork. Polyunsaturated fatty acids, especially the n-3 fatty acids, are beneficial to human health (Webb and O’Neill Citation2008; Wood et al. Citation2008). Therefore, Mashen pig meat products may have more nutritional value and may be more beneficial to human health.
Conclusions
The Mashen pigs had lower drip loss, WBSF, lightness, yellowness, pH1, diameter of muscle fibres and higher cooked yield, redness, IMF than Large White pigs. The content of MyHCI was higher and MyHCIIb was lower in Mashen pigs, which might relate to their meat quality. These data provide valuable information for meat quality difference of Mashen and Large White pigs.
Supplemental Material
Download MS Word (36.5 KB)Acknowledgements
Thanks to all the staff of the Datong pig farm.
Disclosure statement
We confirm that this manuscript has not been published in whole or in part and is not being considered for publication elsewhere. The authors declare that there is no conflict of interests.
Funding
This work was supported by the Programme for Sanjin Scholar [grant numbers 2016, 2017], the Fund for Shanxi 1331 Project [grant number 2017], the Foundation of Science and Technology Innovation Team of Shanxi Province [grant number 201705D131028-19], the National Natural Science Foundation of China [grant number 31872336] and the Programme for the Top Young Academic Leaders of Higher Learning Institutions of Shanxi.
References
- Bhavsar N, Stonge MP. 2016. The diverse nature of saturated fats and the case of medium-chain triglycerides: how one recommendation may not fit all. Curr Opin Clin Nutr Metab Care. 19:81.
- Bulotiene G, Jukna V. 2008. The influence of muscle fibre area on pork quality. Vet Zootech. 42:34–37.
- Cameron ND, Enser MB. 1991. Fatty acid composition of lipid in Longissimus dorsi muscle of Duroc and British Landrace pigs and its relationship with eating quality. Meat Sci. 29:295–307.
- Chang KC, Da CN, Blackley R, Southwood O, Evans G, Plastow G, Wood JD, Richardson RI. 2003. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 64:93–103.
- Choe JH, Choi YM, Lee SH, Shin HG, Ryu YC, Hong KC, Kim BC. 2008. The relation between glycogen, lactate content and muscle fiber type composition, and their influence on postmortem glycolytic rate and pork quality. Meat Sci. 80:355–362.
- Choi YM, Kim BC. 2009. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livestock Sci. 122:105–118.
- Choi YM, Ryu YC, Kim BC. 2007. Influence of myosin heavy- and light chain isoforms on early postmortem glycolytic rate and pork quality. Meat Sci. 76:281–288.
- Choi YM, Ryu YC, Kim BC. 2010. Effect of myosin heavy chain isoforms on muscle fiber characteristics and meat quality in porcine longissimus muscle. J Muscle Foods. 17:413–427.
- Cox RD, Buckingham ME. 1992. Actin and myosin genes are transcriptionally regulated during mouse skeletal muscle development. Dev Biol. 149:228–234.
- Dai FW, Feng DY, Cao QY, Hui Y, Zhang CM, Xia WG, Zuo JJ. 2009. Developmental differences in carcass, meat quality and muscle fibre characteristics between the Landrace and a Chinese native pig. South Afr J Anim Sci. 39:267–273.
- Dransfield E, Sosnicki AA. 1999. Relationship between muscle growth and poultry meat quality. Poultry Sci. 78:743–746.
- Fiedler I, Nürnberg K, Hardge T, Nürnberg G, Ender K. 2003. Phenotypic variations of muscle fibre and intramuscular fat traits in Longissimus muscle of F(2) population Duroc × Berlin miniature pig and relationships to meat quality. Meat Sci. 63:131–139.
- Gil M, Oliver MÀ, Gispert M, Diestre A, Sosnicki AA, Lacoste A, Carrión D. 2003. The relationship between pig genetics, myosin heavy chain I, biochemical traits and quality of M. longissimus thoracis. Meat Sci. 65:1063–1070.
- Gunawan AM, Park SK, Pleitner JM, Feliciano L, Grant AL, Gerrard DE. 2007. Contractile protein content reflects myosin heavy-chain isoform gene expression. J Anim Sci. 85:1247–1256.
- Guo J, Shan T, Wu T, Zhu LN, Ren Y, An S, Wang Y. 2011. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J Anim Sci. 89:185–191.
- Hallenstvedt E, Verland M, Rehnberg A, Kjos NP, Thomassen M. 2012. Sensory quality of short- and long-term frozen stored pork products. Influence of diets varying in polyunsaturated fatty acid (PUFA) content and iodine value. Meat Sci. 90:244–251.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49:447–457.
- Hood RL, Allen CE. 1973. Lipogenic enzyme activity in adipose tissue during the growth of swine with different propensities to fatten. J Nutr. 103:353.
- Hu HM, Wang JY, Zhu RS, Guo JF, Ying W. 2008. Effect of myosin heavy chain composition of muscles on meat quality in Laiwu pigs and Duroc. Sci China. 51:127–132.
- Hwang YH, Kim GD, Jeong JY, Hur SJ, Joo ST. 2010. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 86:456–461.
- Jiang YZ, Zhu L, Li XW, Si T. 2011. Evaluation of the Chinese indigenous pig breed Dahe and crossbred Dawu for growth and carcass characteristics, organ weight, meat quality and intramuscular fatty acid and amino acid composition. Animal Int J Anim Biosci. 5:1485–1492.
- Jiang YZ, Zhu L, Tang GQ, Li MZ, Jiang AA, Cen WM, Xing SH, Chen JN, Wen AX, He T. 2012. Carcass and meat quality traits of four commercial pig crossbreeds in China. Genet Mol Res GMR. 11:4447–4455.
- Joo ST, Kim GD, Hwang YH, Ryu YC. 2013. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 95:828–836.
- Karlsson AH, Klont RE, Fernandez X. 1999. Skeletal muscle fibres as factors for pork quality. Livestock Prod Sci. 60:255–269.
- Karlsson A, Enfält A-C, Essén-Gustavsson B, Lundström K, Rydhmer L, Stern S. 1993. Muscle histochemical and biochemical properties in relation to meat quality during selection for increased lean tissue growth rate in pigs. J Anim Sci. 71:930–938.
- Kauffman RG, van Laack RL, Russell RL, Pospiech E, Cornelius CA, Suckow CE, Greaser ML. 1998. Can pale, soft, exudative pork be prevented by postmortem sodium bicarbonate injection? J Anim Sci. 76:3010.
- Kim GD, Jeong JY, Jung EY, Yang HS, Lim HT, Joo ST. 2013. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 94:267–273.
- Kim NK, Lim JH, Song MJ, Kim OH, Park BY, Kim MJ, Hwang IH, Lee CS. 2007. Developmental proteomic profiling of porcine skeletal muscle during postnatal development. Asian Aust J Anim Sci. 20:1612–1617.
- Kim NK, Lim JH, Song MJ, Kim OH, Park BY, Kim MJ, Hwang IH, Lee CS. 2008. Comparisons of longissimus muscle metabolic enzymes and muscle fiber types in Korean and western pig breeds. Meat Sci. 78:455–460.
- Klosowska D, Fiedler I. 2003. Muscle fibre types in pigs of different genotypes in relation to meat quality. Anim Sci Pap Rep. 21:49–60.
- Larzul C, Lefaucheur L, Ecolan P, Gogué J, Talmant A, Sellier P, Le RP, Monin G. 1997. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J Anim Sci. 75:3126.
- Lee SH, Choe JH, Choi YM, Jung KC, Rhee MS, Hong KC, Lee SK, Ryu YC, Kim BC. 2012. The influence of pork quality traits and muscle fiber characteristics on the eating quality of pork from various breeds. Meat Sci. 90:284–291.
- Lefaucheur L, Ecolan P, Plantard L, Gueguen N. 2002. New insights into muscle fiber types in the pig. J Histochem Cytochem. 50:719–730.
- Liu S, Han W, Jiang S, Zhao C, Wu C. 2016. Integrative transcriptomics and proteomics analysis of longissimus dorsi muscles of Canadian double-muscled Large White pigs. Gene. 577:14–23.
- Liu Y, Yang X, Jing X, He X, Wang L, Liu Y, Liu D. 2017. Transcriptomics analysis on excellent meat quality traits of skeletal muscles of the Chinese indigenous min pig compared with the large white breed. Int J Mol Sci. 19:21.
- Liu X, Xiong X, Yang J, Zhou L, Yang B, Ai H, Ma H, Xie X, Huang Y, Fang S, et al. 2015. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc × (Landrace × Yorkshire) commercial population. Genet Select Evol. 47:44.
- Maltin CA, Sinclair KD, Warriss PD, Grant CM, Porter AD, Delday MI, Warkup CC. 1998. The effects of age at slaughter, genotype and finishing system on the biochemical properties, muscle fibre type characteristics and eating quality of bull beef from suckled calves. Anim Sci. 66:341–348.
- Marita R, Eero P. 2004. Histochemical properties of fibre types in muscles of wild and domestic pigs and the effect of growth rate on muscle fibre properties. Meat Sci. 67:533–539.
- Mckoy G, Léger ME, Bacou F, Goldspink G. 1998. Differential expression of myosin heavy chain mRNA and protein isoforms in four functionally diverse rabbit skeletal muscles during pre‐ and postnatal development. Dev Dyn. 211:193–203.
- Miao ZG, Wang LJ, Xu ZR, Huang JF, Wang YR. 2009. Developmental changes of carcass composition, meat quality and organs in the Jinhua pig and Landrace. Anim Int J Anim Biosci. 3:468–473.
- Ozawa S, Mitsuhashi T, Mitsumoto M, Matsumoto S, Itoh N, Itagaki K, Kohno Y, Dohgo T. 2000. The characteristics of muscle fiber types of longissimus thoracis muscle and their influences on the quantity and quality of meat from Japanese Black steers. Meat Sci. 54:65–70.
- Park SK, Gunawan AM, Scheffler TL, Grant AL, Gerrard DE. 2009. Myosin heavy chain isoform content and energy metabolism can be uncoupled in pig skeletal muscle. J Anim Sci. 87:522–531.
- Pette D, Staron RS. 2001. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 115:359–372.
- Picard B, Jurie C, Duris MP, Renand G. 2006. Consequences of selection for higher growth rate on muscle fibre development in cattle. Livestock Sci. 102:107–120.
- Rehfeldt C, Fiedler I, Dietl G, Ender K. 2000. Myogenesis and postnatal skeletal muscle cell growth as influenced by selection. Livestock Prod Sci. 66:177–188.
- Renand G, Picard B, Touraille C, Berge P, Lepetit J. 2001. Relationships between muscle characteristics and meat quality traits of young Charolais bulls. Meat Sci. 59:49–60.
- Ryu YC, Choi YM, Kim BC. 2005. Variations in metabolite contents and protein denaturation of the longissimus dorsi muscle in various porcine quality classifications and metabolic rates. Meat Sci. 71:522–529.
- Ryu YC, Choi YM, Lee SH, Shin HG, Choe JH, Kim JM, Hong KC, Kim BC. 2008. Comparing the histochemical characteristics and meat quality traits of different pig breeds. Meat Sci. 80:363–369.
- Ryu YC, Kim BC. 2005. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 71:351–357.
- Ryu YC, Kim BC. 2006. Comparison of histochemical characteristics in various pork groups categorized by postmortem metabolic rate and pork quality. J Anim Sci. 84:894.
- Schiaffino S, Reggiani C. 1996. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 76:371–423.
- Seideman SC, Crouse JD. 1986. The effects of sex condition, genotype and diet on bovine muscle fiber characteristics. Meat Sci. 17:55–72.
- Şirin E, Aksoy Y, Uğurlu M, Çiçek Ü, Önenç A, Ulutaş Z, Şen U, Kuran M. 2017. The relationship between muscle fiber characteristics and some meat quality parameters in Turkish native sheep breeds. Small Ruminant Res. 150:46–51.
- Webb EC, O’Neill HA. 2008. The animal fat paradox and meat quality. Meat Sci. 80:28–36, 54.
- Wimmers K, Ngu NT, Jennen DG, Tesfaye D, Murani E, Schellander K, Ponsuksili S. 2008. Relationship between myosin heavy chain isoform expression and muscling in several diverse pig breeds. J Anim Sci. 86:795.
- Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. 2008. Fat deposition, fatty acid composition and meat quality: a review. Meat Sci. 78:343–358.
- Wu T, Zhang Z, Yuan Z, Li JL, Chen J, Wang Y, Peng J. 2013. Distinctive genes determine different intramuscular fat and muscle fiber ratios of the longissimus dorsi muscles in Jinhua and Landrace pigs. PloS One. 8:e53181.
- Yang WP, Cao GQ, Shi JZ, Liu JH, Zhou ZX. 2005. Study on the finishing ability of different cross combination in pig. Chin J Anim Sci. 41:48–49.
- Yang H, Xu XL, Ma HM, Jiang J. 2016. Integrative analysis of transcriptomics and proteomics of skeletal muscles of the Chinese indigenous Shaziling pig compared with the Yorkshire breed. BMC Genet. 17:80.
- Young LD. 1995. Survival, body weights, feed efficiency, and carcass traits of 3/4 white composite and 1/4 Duroc, 1/4 Meishan, 1/4 Fengjing, or 1/4 Minzhu pigs. J Anim Sci. 73:3534–3542.
- Zhang JG, Wang X, Du MH, Zhou ZX. 2001. Species diversity and the way to protect Ma Shen Zhu. J Shanxi Agric Univ. 21:188–191.
- Zhao YY, Gao PF, Li W, Zhang YQ, Xu K, Guo XH, Li BG, Cao GQ. 2015. Study on the developmental expression of Lbx1 gene in longissimus dorsi of Mashen and large white pigs. Ital J Anim Sci. 14:3720–2087.
- Zhu L, Li XW, Shuai SR, Li MZ, Chen L. 2008. Muscle nutrient composition analysis of Dahe pigs and Dahewu pigs. Chin J Anim Sci. 44:6–9.
- Żochowska J, Lachowicz K, Gajowiecki L, Sobczak M, Kotowicz M, Żych A. 2005. Effects of carcass weight and muscle on texture, structure and myofibre characteristics of wild boar meat. Meat Sci. 71:244–248.
