Abstarct
Magnetic field affects many living organisms, fishes included. The fish reactions are observed from the early stage of development, during embryogenesis and larval period, and into adult stage. Magnetic field affects, among other processes, water exchange in the eggs, embryo heart rate and respiration, directional reactions and spatial orientation. The few papers in this field published so far present melanophores morphology and development. Melanophores developing in embryos and in newly hatched sea trout (Salmo trutta m. trutta L.) larvae which had been incubated and kept under optimal conditions constituted the material for the study. The effect of static, generated magnetic field of intensity of 1, 3 and 5 mT on melanophores of sea trout embryos and larvae was studied. The control setting was kept under geomagnetic field. The appearance of melanophores in the trout embryos exposed to magnetic field was delayed. The number of melanophores was smaller, and the movement of melanin within the melanophores, visible as aggregation of pigment in the cells. Static magnetic field of relatively low intensity influences the reaction of melanophores of developing trout embryos and newly hatched larvae. Most probably, magnetite (Fe3O4) compounds, detected in many fish species, mainly migratory fishes such as trout, are responsible for such behaviour of melanophores.
Static magnetic field delays the appearance of melanophores in the trout embryos and larvae.
The largest aggregation of melanin in melanophores was found in larvae incubated in a 5 mT magnetic field.
The largest number of melanophores was found in the embryos incubated in the geomagnetic field.
Highlights
Introduction
Living organisms, fishes included, respond to various environmental stimuli such as water chemistry, light, thermal conditions and also the magnetic field (Ogura et al. Citation1992; Diebel et al. Citation2000). Magnetic field has been observed to induce changes in the behaviour and migrations of migratory fish, e.g. the European and American eel (Anguilla anguilla and Anguilla rostrata, respectively), rainbow trout (Oncorhynchus mykiss), yellow fin tuna (Thunnus albacares) and Atlantic salmon (Salmo salar) (Tesch et al. Citation1992; Walker et al. Citation2002; Formicki et al. Citation2004). Magnetic field effects have also been found in the embryonic development of fish as well as in fish spermatozoa motility (Formicki Citation2008, Formicki et al. Citation2015). Studies on magnetic field effects at different stages of ontogeny in fish and other aquatic organisms are particularly important in view of the growing number of magnetic fields in the natural environment. The increase is due to the intensifying deployment of electric cables in marine and freshwater environments, the cables generating magnetic field which affects organisms, including fish, staying in the cable vicinity (Fey, Greszkiewicz et al. Citation2019; Fey, Jakubowska et al. Citation2019).
Relatively few studies have dealt with the effect of magnetic field on fish pigmentation. It has been shown that magnetic field affects the behaviour of rainbow trout (Oncorchynchus mykiss) melanophores, as well as melanophores of the sea trout (Salmo trutta) and eel (Anguilla anguilla) in vitro and in vivo. Exposure to static magnetic field of several hundred militesla (mT) causes a significant expansion of melanin in pigment cells (Wannitikul et al. Citation1993). In the black tetra (Gymnocorymbus ternetzi), magnetic field of 14 T causes aggregation of pigment in the cells; no significant differences, compared to the control setting, have been observed at magnetic field of 8 T (Testorf et al. Citation2002). In addition to melanophores, other pigment cells were studied in other organisms, e.g. cattle (Nicoloso et al. Citation2008). The effect of magnetic field on colour changes and amount of urine in the body of “goldfish” has also been studied. There is a decrease of urine production under the influence of 62 mT, 50 Hz magnetic field, and changes in the body colour after a 20-hour exposure have been observed (Sawaguchi et al. Citation2003). Our previous experiments using magnetic field of lower intensity showed differences in the time of appearance of melanophores, their number and appearance, resulting from the degree of expansion of pigment in melanophores of embryos and larvae of whitefish (Coregonus lavaretus L.) and vendace (Coregonus albula L.) (Brysiewicz et al. Citation2017).
Here we describe the effect of static magnetic field on melanophores during embryonic and larval development of the sea trout (Salmo trutta m. trutta L.). As opposed to the previously studied fish species, we observed differences in the timing and development of embryos incubated in magnetic field, among others thickness and permeability of egg envelopes (Sadowski et al. Citation2007). The sea trout is of great economic importance and all studies contributing to better knowledge of its complicated embryonic development may prove useful in practice of protection and restitution of the species in the Polish rivers. In this paper we describe the effect of magnetic field on the duration of embryonic development in different intensities (variants). Hypothesis 0 was that magnetic field has no effect on melanophores of early development stages of the sea trout as opposed to the hypothesis assuming differences between the melanophores exposed to magnetic field and the control. We studied the effect of magnetic field on the time of appearance of melanophores in developing embryos, we compared the numbers of melanophores for particular variants and the degree of movement of melanin in embryos incubated in magnetic field from fertilisation till hatching and in newly hatched larvae.
Material and methods
All procedures were approved by the Local Ethical Committee for Experiments on Animals in Szczecin (Resolution No. 09/2007).
The research was conducted in the isothermal laboratory of the Department of Hydrobiology, Ichthyology and Biotechnology of Reproduction. Trout eggs and sperm were obtained from several males (n = 7) and females (n = 7) from the Rega River (Baltic Sea) - 54°06′09″N, 15°27′57″E. The gametes were collected by gently pressing the abdomen of fish on the site of capture, and then transported in Eppendorf resolls (2 h) separately for each individual in containers in isothermal conditions in constant temperature (3 ± 0.2 °C). Fertilisation was carried out using dry method following the methodology commonly used in hatcheries. The male and female gametes were thoroughly mixed to obtain a uniform structure of the material for further studies.
Static magnetic field of intensity: 1 mT, 3 mT and 5 mT (militesla), generated using magnets with expanders, were used in the experiments. The control was placed between plastic dummy magnets (sham exposure). The direction of each experimental field was concomitant with the Earth’s magnetic field lines.
Embryos developing inside the eggs and freshly hatched larvae were incubated in 600 ml glass crystallizers, with mounted aerators. Every two days water was partially replaced with fresh water (200 ml). Constant temperature (5 ± 0.2 °C) and illumination 30 lux (for 12 h/day) were maintained in the laboratory.
The degree of movement of melanin in the melanophores of the embryos and larvae was analysed under a microscope (Nikon ECLIPSE TE-2000 S equipped with a camera Sony DSLR-A330).
The effect of magnetic field on the melanophores in developing embryos and newly hatched larvae was studied by analysing responses of melanophores during the embryonic development, from fertilisation to hatching. Responses of melanophores in the larvae were also studied. In all variants the value of magnetic field was kept constant.
Besides, the duration of embryonic development was examined in the control setting and in the setting under magnetic field.
Reaction of embryo melanophores to magnetic field
In the first stage of the experiments, trout eggs (400 pieces per variant) were incubated (5 ± 0.2 °C) under generated magnetic field as well as under control conditions. The eggs were randomly selected and the moment of appearance of developing melanophores in the embryos in the magnetic field and in the control was recorded. In addition, the average number of melanophores in the newly hatched embryos was counted.
Then, eleven newly hatched embryos (1-day larva) were selected randomly from each experimental variant; the degree of expansion of pigment in the melanophores were recorded. In order to estimate the degree of expansion of melanin, 25 melanophores from the cephalic region were randomly selected, and then, using the melanophore index that allows for cells to be assigned to one of five stages of melanin aggregation and expansion (Patil and Jain Citation1993; Daiwile et al. Citation2015), selected as suitable for identification was documented and assessed. Cells with the greatest expansion of melanin were assigned to stage 5, while those with the greatest aggregation, and the least expansion to stage 1.
Reaction of larval melanophores to magnetic field
The second stage involved the observation of behaviour of melanophores in larvae exposed to magnetic field for a period of 14 days. Just after hatching, 25 individuals incubated under geomagnetic field were randomly selected and placed in a water-filled crystallizer with continuous aeration and constant temperature (5 ± 0.2 °C). For a period of 14 days the larvae were kept under generated magnetic field of the same intensity as that used for the incubated embryos.
Twenty five cells from the head and trunk region of 11 individuals from each experimental variant were randomly selected. Following the exposure of larvae to magnetic field, the degree of movement of melanin in individual melanophores was documented and assessed, using the same procedures as in the case of embryos.
The study on the embryos and larvae was carried out over a two-year period (two replicates). The results were statistically analysed with Statistica 12.0 PL, using analysis of variance (ANOVA). In addition, post-hoc multiple comparison tests were applied to compare results of the replicates.
Results and discussion
Differences were observed between the variants exposed to generated magnetic field and the control, both in the embryos incubated under magnetic field throughout embryonic development and in the larvae exposed to magnetic field for a period of 14 days.
Reaction of embryo melanophores to magnetic field
There were differences in the time of appearance of melanophores in the embryos (ANOVA p < .05). In the control series, the first melanophores were observed after 184 D°, and for variants with 1 mT and 3 mT magnetic field – 192 D°, for 5 mT – 198 D°. Static magnetic field slowed down the embryonic development. In the control setting, this time amounted to 245 D°, while in the magnetic field the delay was directly proportional to the intensity of the field: 1 mT – 260 D°, 3 mT – 268 D° and 5 mT – 288 D°.
Differences in the number of melanophores in the embryos incubated under geomagnetic field and those under generated, static magnetic field were also observed.
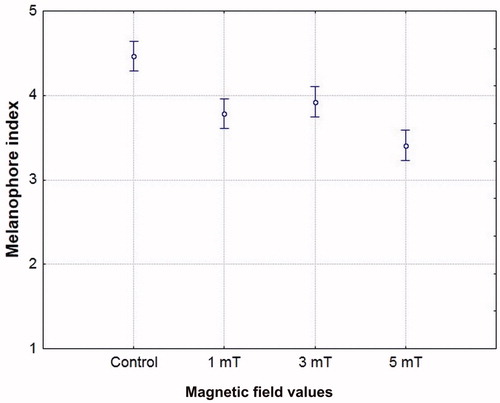
The average number of melanophores in the body and yolk sac of newly hatched embryos was the highest in the control series, and the smallest in the field of 5 mT. The difference between the control setting and the 5 mT variant was statistically significant ().
Figure 1. Average number of melanophores in the sea trout (Salmo trutta trutta) newly hatched embryos in geomagnetic field (control) and in static magnetic field of 1, 3 and 5 mT; ANOVA F = 3.9468, p = .01478.
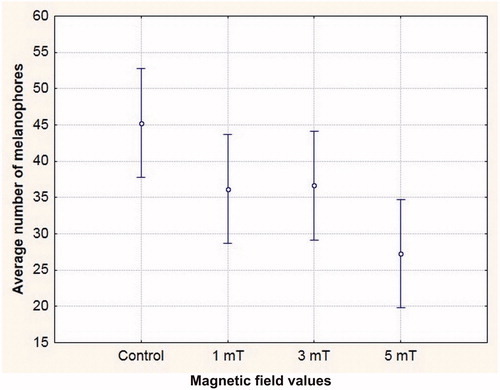
There were also differences in the movement of pigment in the melanophores of the newly hatched embryos (melanophore index). The greatest expansion of melanin was observed in the control setting, and the smallest in the 5 mT variant. The difference between the control setting and the latter magnetic field variant was statistically significant (). Similar results were obtained in the studies on the appearance of pigmentation in the whitefish and vendace embryos (Brysiewicz et al. Citation2017); magnetic field significantly affected the pigment aggregation in the central areas of melanophores compared to the control.
Reactions of larval melanophores to magnetic field
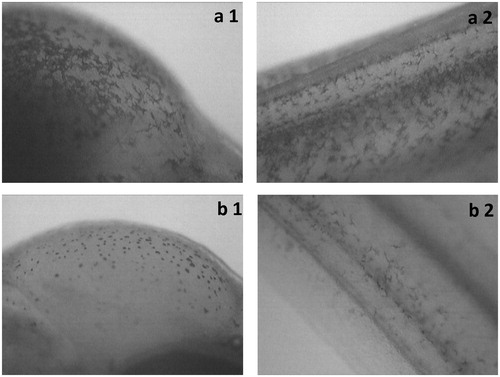
We observed changes in the behaviour of melanophores in the head and trunk region ().
Figure 3. Melanophores in sea trout larvae (Salmo trutta m. trutta) (a – geomagnetic field, b – static, generated magnetic field of 1 mT; 1 – head region, 2 – trunk region).
Static magnetic field was also found to affect the degree of expansion of pigment in the larval melanophores. The greatest expansion of pigment in melanophores in the head region was observed in the control setting, while the highest aggregation was recorded for magnetic field of 1 mT. Statistically significant differences between the control series and all the experimental variants were also observed. The difference between the 1 mT variant and the 5 mT variant was statistically significant ().
Figure 4. Melanophore index in sea trout larvae (Salmo trutta trutta) under geomagnetic field (control) and under static magnetic field of 1, 3 and 5 mT. A – head region – ANOVA F = 30.588, p = .0000; B –trunk region – ANOVA F = 17.541, p = .00000.
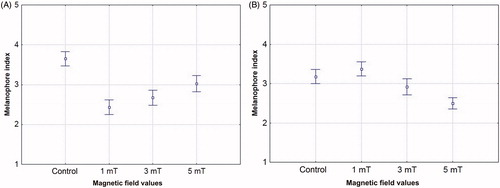
In the trunk region, the greatest expansion of melanin was observed in the 1 mT variant, and the largest aggregation in the 5 mT variant. The difference between the control series and the 5 mT variant was statistically significant ().
All the test for responses of the embryonic and larval melanophores to magnetic field were replicated (2 study seasons); results obtained in both seasons were similar and showed no significant differences (p > .05).
Recently, in view of the rapidly intensifying application of power technologies, more and more attention has been paid to effects of man-generated magnetic fields on living organisms. In the aquatic environments, the attention is focussed on effects of magnetic fields generated by submarine cables deployed on the seafloor and by power transmission lines placed at and near the shores (Fey, Greszkiewicz et al. Citation2019; Fey, Jakubowska et al. Citation2019; Formicki et al. Citation2019). As already mentioned, magnetic fields in aquatic environments affect the organisms inhabiting those systems. Magnetic field is known to retard embryonic development of fish, for example embryos of zebra fish (Danio rerio), sea trout (Salmo trutta), rainbow trout (Oncorhynchus mykiss) and roach (Rutilus rutilus) (Skauli et al. Citation2000; Chebotareva et al. Citation2009). We observed a delay in development in the embryos kept under magnetic field. Their development was delayed by several dozen degree-days, and the delay was directly proportional to the field intensity.
Only few studies deal with the effect of artificially generated magnetic field on fish melanophores. The research carried out on skin fragments (in vitro) obtained from trout shows that exposure to magnetic field of several hundred militesla (160 mT, 250 mT, 310 mT, 380 mT and 600 mT) induces pigment expansion in melanophores compared to the control group (Wannitikul et al. Citation1993). On the other hand, in adult black tetra (Gymnocorymbus ternetzi) magnetic field of several tesla causes movement of melanin to the centre of the cell (Testorf et al. Citation2002). This may be due to the high values of the applied field, or to the characteristics of colour change in different fish individuals and species.
Some factors, for example colchicine, high hydrostatic tension as well as vinblastine, do not disturb the movement of melanosomes in pigment cells (Chen and Wang Citation1993). On the other hand, a number of factors, such as light, chemical compounds, toxins or bacteria induce melanophore reactions visible as aggregation or expansion of melanin (Dwivedi Citation1978; Chaplen et al. Citation2002).
Microtubules are responsible for the movement of melanosomes in melanophores, which is associated with changes in the cell appearance (Bruno et al. Citation2008). These long tubular structures may be elongated, and melanin particles, while dispersing and coming together, slip inside them (Fujii Citation2000). It can also be assumed that magnetic field affects the reactions of melanin molecules in the pigment cells.
The response of pigment cells to magnetic field may also be caused by crystals of magnetite (Fe3O4) which have been detected in cells of many fish species, in particular the migratory fish (Sakaki et al. Citation1990; Ogura et al. Citation1992). Up to now it has not been proven that magnetic particles were connected directly to the nervous system which significantly affects skin colour changes in animals, however the existing connection – microtubules, between magnetite crystals and ion channels – could open or close ion channels in membranes and in this way induce changes in cell potential, which in turn translates into melanophore reactions (Kirschvink et al. Citation2001).
Our research on melanophores in the sea trout embryos and newly hatched larvae shows that relatively small-intensity static magnetic fields can cause reactions in the form of changes in the number of cells in embryos, and movement of melanin into the central areas of melanophores in embryos and larvae.
Studies on magnetic field effects on early developmental stages of salmonids should be carried out on account of a potential application of the results in production of those commercially important and valuable ichthyotaxa. In salmonid aquaculture, the timing of “eyeing”, i.e. the time when melanin appears in the embryonic eyeballs is very important for handling of the eggs and embryos. The term “eyed eggs” is even used in the culture of stocking material. In our study, application of 1 to 5 mT magnetic fields delayed somewhat the appearance of melanophores in the embryonic eyeballs and on larval bodies relative to the control, which seems advantageous as all the organs in both the embryos and larvae had been already developed. For this reason, results of studies on magnetic field effects on embryonic and post-embryonic development of fish may be, in the future, used in intensive production of stocking material for aquaculture. Salmonids are highly valued for the quality of their meat, skin and meat colouration having a bearing on the price. This study, involving melanophores of trout embryos and larvae, demonstrated their responses to static magnetic fields. It would be interesting to follow responses of other pigment cells such as erythrophores to static magnetic fields. This is a new research question, not addressed so far in fish studies, and seems worthy of tackling.
Conclusions
The 1 to 5 mT magnetic fields resulted in a delayed appearance of melanophores in developing trout embryos and larvae as well as in reduced numbers of the melanophores. This may be advantageous because eventually, on termination of the experiment, all the larvae were uniformly coloured, evidencing all the handling to have been safe for the fish. The behaviour of melanophores on the head and trunk of the trout larvae kept in the magnetic field was changed, compared to the control. The largest aggregation of melanin was observed in the larvae exposed to the 5 mT magnetic field. Results of this study may be applied to aquaculture practice to modulate the timing of pigment cell appearance in salmonids; in a wider sense, the results may prove useful in the production of various colour forms in fish. In addition, results of this study are important for assessing effects of static magnetic fields on animals, which is essential in environments experiencing man-generated magnetic fields. However, the current state of knowledge on the magnetic sense is still far from adequate and requires further detailed studies.
Ethical approval
All procedures involving animals were evaluated and approved by the guidelines of the Animal care and Use Committee of the West Pomeranian University of Technology in Szczecin (Poland).
Acknowledgements
This work was partly supported by the Ministry of Agriculture and Rural Development in Poland (research task no 14/84/2019) and the project no 00001-6521.1-OR1600002/17/18 financed by the Sectoral Operational Programme “Fisheries and See 2014–2020”.
Disclosure statement
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of this article.
References
- Bruno L, Echarte MM, Levi V. 2008. Exchange of microtubule molecular motors during melanosome transport in Xenopus laevis melanophores is triggered by collisions with intracellular obstacles. Cell Biochem Biophys. 52:191–201.
- Brysiewicz A, Formicki K, Tański A, Wesołowski P. 2017. Magnetic field effect on melanophores of the European whitefish Coregonus lavaretus (Linnaeus, 1758) and vendace Coregonus albula (Linnaeus, 1758) (Salmonidae) during early embryogenesis. Eur Zool J. 84:49–90.
- Chaplen FWR, Upson R, McFadden P, Kolodziej W. 2002. Fish chromatophores as cytosensors in a microscale device: detection of environmental toxins and bacterial pathogens. Pigm Cell Res. 15:19–26.
- Chebotareva YV, Izyumov YG, Krylov VV. 2009. The effect of an alternating electromagnetic field upon early development in roach (Rutilus rutilus: Cyprinidae, cypriniformes). J Ichthyol. 49:409–415.
- Chen JS, Wang SM. 1993. The role of microtubules in pigment translocation in goldfish xanthophores. Arch Histol Cytol. 56:451–458.
- Daiwile AP, Naoghare PK, Giripunje MD, Prasada Rao PD, Ghosh TK, Krishnamurthi K, Alimba CG, Sivanesan S. 2015. Correlation of melanophore index with a battery of functional genomic stress indicators for measurement of environmental stress in aquatic ecosystem. Environ Toxicol Pharmacol. 39:489–495.
- Diebel CE, Proksch R, Green CR, Neilson P, Walker MM. 2000. Magnetite defines a magnetoreceptor. Nature. 406:299–302.
- Dwivedi DK. 1978. The effects of drugs on the melanophores of teleost Rasbora daniconius. Aust J Pharm Sci. 7:29–31.
- Fey DP, Greszkiewicz M, Otremba Z, Andrulewicz E. 2019. Effect of static magnetic field on the hatching success, growth, mortality and yolk-sac absorption of larval northern pike Esox lucius. Sci Total Environ. 647:1239–1244.
- Fey DP, Jakubowska M, Greszkiewicz M, Andrulewicz E, Otremba Z, Urban-Malinga B. 2019. Are magnetic and electromagnetic fields of anthropogenic origin potential threats to early life stages of fish? Aquat Toxico. 209:150–158.
- Formicki K, Korzelecka-Orkisz A, Tański A. 2019. Magnetoreception in fish. J Fish Biol. 95:73–91.
- Formicki K, Szulc J, Korzelecka‐Orkisz A, Tański A, Kurzydłowski JK, Grzonka J, Kwiatkowski P. 2015. The effect of a magnetic field on trout (Salmo trutta Linnaeus, 1758) sperm motility parameters and fertilisation rate. J Appl Ichthyol. 31:36–146.
- Formicki K, Tanski A, Sadowski M, Winnicki A. 2004. Effects of magnetic field on fyke net performance. J Appl Ichthyol. 20:402–406.
- Formicki K. 2008. Magnetoreception: Chapter 14. In: Finn RN, Kapoor BG, editors. Fish larval physiology. USA: Science Publisher. 461–491.
- Fujii R. 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 13:300–319.
- Kirschvink JL, Walker MM, Diebel CE. 2001. Magnetite-based magnetoreception. Curr Opin Neurobiol. 11:462–467.
- Nicoloso L, Negrini R, Milanesi E, Crepaldi P. 2008. Identification of polymorphism in the SCL24A5 gene of cattle. Ital J Anim Sci. 7:505–512.
- Ogura M, Kato M, Arai N, Sasada T, Sakaki Y. 1992. Magnetic particles in chum salmon (Oncorhynchus keta). Extraction and transmission electron microscopy. Can J Zool. 70:874–877.
- Patil S, Jain A. 1993. Role of calcium in melanosome aggregation within Labeo melanophores. J Biosci. 18:83–91.
- Sadowski M, Winnicki A, Formicki K, Sobociński A, Tański A. 2007. The effect of magnetic field on permeability of egg shells of salmonid fishes. Acta Icth et Piscat. 37:129–135.
- Sakaki Y, Motomiya T, Kato M, Ogura M. 1990. Possible mechanism of biomagnetic sense organ extracted from sockeye salmon. IEEE Trans Magn. 26:1554–1556.
- Sawaguchi Y, Kimura K, Misawa K, Arisawa J. 2003. Influence of the 50 Hz magnetic field on the body color and the amount of urine of goldfish. Proceedings from the 2003 IEEE International Symposium on Electromagnetic Compatibility. 11–16 May, Istanbul, Turkey, pp. 828–831.
- Skauli KS, Reitan JB, Walther BT. 2000. Hatching in zebrafish (Danio rerio) embryos exposed to a 50 Hz magnetic field. Bioelectromagnetics. 21:407–410.
- Tesch FW, Wendt T, Karlsson L. 1992. Influence of geomagnetism and salinity on orientation of the eel Anguilla anguilla L. as evident from laboratory experiments. Ecol Freshw Fish. 1:52–60.
- Testorf MF, Öberg PÅ, Iwasaka M, Ueno S. 2002. Melanophore aggregation in strong static magnetic fields. Bioelectomagnetics. 23:444–449.
- Walker MM, Dennis TE, Kirschvink JL. 2002. The magnetic sense and its use in long-distance navigation by animals. Curr Opin Neurobiol. 12:735–744.
- Wannitikul P, Winnicki A, Formicki K. 1993. Effect of constant magnetic field on fish melanophores in vivo and in vitro. In: Blank M, editor. Proceedings of the electricity and magnetism in biology and medicine; San Francisco; San Francisco Press. p. 849–850.