Abstract
The aim of this research is to propose equations to estimate body weight (BW) from body measures (BMs) in the Cornigliese sheep, an endangered breed reared in Italy for meat purpose. BW and BMs were submitted to correlation and multiple regression analyses. Two datasets were used: (i) 303 animals (178 females, 125 males, BW 4.00–117.80 kg), on which BW, height at withers (HW), chest circumference (ChC) and body length (BL) were measured; (ii) 156 animals (109 females, 47 males, BW 5.15–117.80 kg) out of 303 on which croup height (HCr), chest width (ChW), chest depth (ChD) and croup width (CrW) were also considered. On each dataset, two regression models were applied, one containing all variables (models 1 and 3, respectively, for datasets 1 and 2) and the other one comprising groups of variables, selected by means of the stepwise procedure (models 2 and 4, respectively, for datasets 1 and 2). BW resulted correlated with all BMs (from 0.852 for ChW to 0.950 for ChC; p < .001). Models 1 and 3 fitted the data better than models 2 and 4, both for all animals and for females and males separately. We concluded that BW could be predicted from BMs also in Cornigliese sheep breed. The best fits were obtained when the highest number of measures was included in the model (models 1 and 3). Nevertheless, models 2 and 4 could be used more easily in extensive sheep breeding than models 1 and 3, since they require less parameters.
The estimation of body weight is important in a meat type sheep breed for choosing the optimal slaughter time.
A reduction of work for farmer is positive, mainly in field conditions of extensive rearing, where scales are not easily available.
Body weight could be predicted from body measures in Cornigliese sheep breed with good precision and accuracy.
Highlights
Introduction
In sheep meat breeds, it is very important to accurately estimate the weight of the animals for several reasons: firstly, for choosing the most convenient time for slaughtering, secondly, for checking the health status of animals (Mahmud et al. Citation2014) and, lastly, for breeding purposes (Sarti et al. Citation2003). Benefits are also expected on animal management, in terms of adherence to the food plan or in case of sudden weight losses linked to parasitic or chronic diseases. While in intensive sheep farms the measurement of weight does not normally represent an issue, due to the advanced management level and the availability of basic equipment, on extensive farms it might be problematic. This is because the weighting scales are normally not available in field conditions, or either they are difficult to use outside; moreover, some modern technologies (automated weighting procedures, image processing) are not usually widespread in such conditions (Wilson Citation2014). Nevertheless, a delay in the weight of slaughter, compared to what is defined as optimal by studies on the development of the tissues of animals in relation to sex (Sabbioni et al. Citation2018, Citation2019), causes a negative economic impact on the profitability of the farm. For this reasons, several studies have addressed the problems related to the correct registration of live weight in extensive sheep breeding, with reference both to local breeds in rural areas of developing countries (Topai and Macit Citation2004; Sowande and Sobola Citation2008; Kunene et al. Citation2009; Yilmaz et al. Citation2013) and to more selected breeds in Western countries (Sarti et al. Citation2003; Petrović et al. Citation2012). It is therefore useful in practice to estimate the body weight (BW) from equations obtained starting from body measures (BMs) which are easily accessible. In literature, many equations are available, but they differ in relation to breed, sex, age, attitude, geographic context and type of BM of the animals under study. For example, age could be considered as an important factor affecting BW estimation (Kunene et al. Citation2009), but its use in prediction equations is not always applicable at practical level with an acceptable precision, especially in those conditions in which there is a lack of recordings and the only way to determine the age is by a dentition check. The same authors reported different equations in relation to age-gender interaction: in this way, the usefulness of the equation is reduced for in-field applications.
In Italy, the use of chest girth to estimate live weight in sheep was proposed by Sarti et al. (Citation2003) for Appenninica and Merinizzata Italiana breeds, aimed to performance recording of individuals under selection. Cornigliese sheep is an Italian local breed raised in Emilia-Romagna region, used mainly for meat purpose, with a limited number of animals (approximately 1500), a late development (Sabbioni et al. Citation2016a, Citation2016b) and a strong difference between sexes at slaughter (Sabbioni et al. Citation2018), with reference to fat deposition. Due to its late development and gender-related differences in fatness, the choice of the optimal moment for slaughtering is more related to the weight than to the age. Moreover, due to the wool growth, a visual estimate of BW is not easy to implement. The Cornigliese sheep breed is not actually under selection, being considered endangered, following the FAO (Citation2015) risk-status classification. Nevertheless, it is important for increasing the value of the individuals used for meat production and for gaining carcases with a good profitability for farmers, and a meat with a good acceptability for consumers, to have an accurate estimate of live weight. Sabbioni et al. (Citation2018) showed that carcases from females of Cornigliese sheep breed slaughtered at high BWs, contain 1.9 times fat more than males. In a more recent study (Sabbioni et al. Citation2019), they found that females from the same BW-class showed intramuscular fat 1.6 times higher than males. Since the importance of accurately estimate BW in the Cornigliese sheep breed, in this study we aimed to propose equations to determine the BW from BMs which can in turn be used in practice by farmers.
Materials and methods
The trial was carried out at farm level, under the control of the public veterinary service and complied with the Italian laws on animal experimentation and ethics (LD 04/03/2014, n.26).
The trial involved 303 animals (178 females and 125 males) of the Cornigliese sheep, from one flock located in the area of origin of the breed (mountain area of the province of Parma, Italy), at about 800 m of altitude (latitude 44°24′50″04 N; longitude 10°7′30″72 E).
The animals, individually ear-tagged, were submitted to the standard rearing conditions suggested for this breed (Sabbioni et al. Citation2016a). The farrowing season was typically from October to February. The wool shearing took place twice a year, in spring and in autumn.
During a period of two years, all animals in the flock, ageing at the beginning of the trial 0–5 years, were weighted and measured at regular intervals: 1-month interval from birth to 6-month, 2-month intervals until 12-month and 4-month intervals later.
BW was recorded with the use of a dynamometer (model CCS-300K, UWE, Taiwan), while BMs were taken by means of a flexible metre, a Lydtin’s rod and a measuring compass. The body measurements recorded on all 303 animals (dataset 1) were
HW: height at withers, from the top of withers to the ground;
ChC: chest circumference, behind the posterior edge of the shoulders at the point of least perimeter;
BL: body length, from the anterior edge of shoulder to the posterior edge of ischium.
On a subsample of 156 animals (109 females and 47 males) out of 303, in addition to the previously mentioned measures, also the following ones were recorded (dataset 2):
HCr: height at croup, from the top of the croup to the ground;
ChW: chest width, behind the shoulder;
ChD: chest depth, from the withers to the sternum;
CrW: croup width, between the trochanters.
BW and BMs were primarily submitted to descriptive statistics, then to Pearson correlation analysis and, subsequently, to multiple regression analysis to assess prediction models for BW estimation.
On each of the two datasets, two different regression models were applied in SAS (Citation2014), one defined as complete, containing all measured variables (models 1 and 3, respectively, for datasets 1 and 2) and one containing fewer independent variables, selected by means of the stepwise procedure (models 2 and 4, respectively, for datasets 1 and 2). The goodness of fit of the four models was assessed by means of R2 and RMSE, as the criterion for choosing the best model during the stepwise procedure was the Akaike Information Criterion (AIC) (Judge et al. Citation1985).
Results and discussion
Descriptive statistics () revealed that BW showed a coefficient of variation at least 2–3 times greater than BMs; this was expected and already described by Sabbioni et al. (Citation2016a). The same relationship between BW and BMs was noted likewise by Walstra (Citation1980) with reference to allometric coefficients and explained because of the three-dimensions of the BW measure compared to BMs, which are mono dimensional ones. Females revealed a variability of BW and BMs lower than males, except for ChC. BW resulted highly correlated with all BMs (from 0.852 with ChW to 0.950 with ChC; p < .001) (). Moreover, BMs were highly correlated among themselves (from 0.747 between BL and ChW to 0.985 between HW and HCr; p < .001). No gender effect was showed in correlation coefficients between BW and BMs or among BMs. Younas et al. (Citation2013) reported similar coefficients of correlation between BW and BMs in Hissardale sheep breed; they also noted that correlations declined with age. Contrarily to our results, Shirzeyli et al. (Citation2013) found that BL was the most correlated linear measure with BW. Petrović et al. (Citation2012) found lower phenotypic correlations than those here reported between BW and BMs in Merinolandschaf breed of sheep (from 0.183 between CrW and BW at birth to 0.421 between ChC and BW at weaning). Moreover, they noted that phenotypic correlations were lower compared to the genetic correlations, due to more complex genetic and residual factors. The difference between Cornigliese and a Merinos-derived breed, such as Merinolandschaf, was unexpected, because of the genetic history of Cornigliese sheep breed (related to ancient crosses with Spanish Merinos) and recent results from Ceccobelli et al. (Citation2015). They compared 5 sheep breeds (Cornigliese, Spanish Merino, Bergamasca, Appenninica and Palmera) and found some level of admixture between Cornigliese and Spanish Merinos at low (2–3) number of clusters, as at higher number of clusters (5) the two breeds were correctly separated. Additionally, Yakubu (Citation2012) found lower correlations (between 0.43 and 0.76) than ours between BW and BMs. The high correlations reported in the present study between BW and BMs strengthened the hypothesis of estimating the former by means of the latter and were justified by the wide range of BW of animals, covering all categories from neonatal lambs to adults. The prediction equations of BW from BMs are reported in . The complete model applied to all individuals with the highest number of independent variables (model 3) showed a greater adaptation than the complete model with fewer independent variables (model 1), as shown by the higher value of R2 (0.936 vs. 0.910) and lower values of RMSE (7.190 vs. 8.211 kg). The same finding was shown in males (R2 0.965 vs. 0.871; RMSE 4.985 vs. 7.053), as in females the RMSE was lower (7.870 vs. 8.427) but the R2 was quite similar (0.916 vs. 0.919). The complete models (1 and 3) fitted the data better than the reduced models (2 and 4), respectively, both for all animals and for females and males, given the higher R2 and, in most cases, the lower RMSE. Finally, model 4 gave best fits compared to model 2, showing lower AIC values both for all animals (778 vs. 1586) and for females (563 vs. 942) and males (140 vs. 622) separately. Tadesse and Gebremariam (Citation2010) reported increased accuracy as more BMs were included in the prediction equations; nevertheless, they consider that at farm level a simpler equation, with only ChC as independent variable for BW estimation, could be still effectively used. In the case of an in-field application of the BW estimation equations, a smaller degree of precision, together with a greater ease of use, would be certainly preferable.
Table 1. Descriptive statistics of body weight and body measures in Cornigliese sheep breed.
Table 2. Phenotypic correlation coefficients among body weight and body measures in Cornigliese sheep breed.
Table 3. Multiple regression coefficients for body weight (kg) estimation from body measures (cm) in Cornigliese sheep breed.
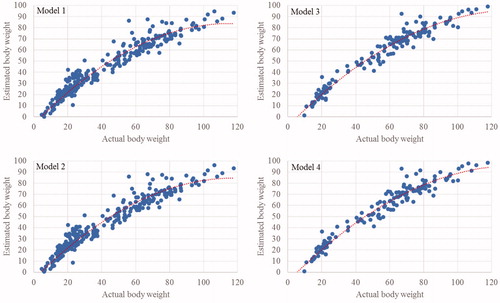
Regression equations of real vs. estimated BW obtained for the models 1–4 are reported in . High values of R2 were obtained (0.940–0.961), confirming that in Cornigliese sheep the live weight can be predicted starting from body measurements, as in other sheep breeds. Therefore, the choice of the model to use is highly dependent on several factors, where the most important is the convenience of use, which is reflected into the number of measurable variables.
Figure 1. Actual body weight vs. estimated body weight. Model 1: the underlying regression equation is: y = –0.0068x2 + 1.5643x – 6.7174; R2 = 0.944. Model 2: the underlying regression equation is: y = –0.0065x2 + 1.5373x – 6.3193; R2 = 0.940. Model 3: the underlying regression equation is: y = –0.0058x2 + 1.5527x – 8.5986; R2 = 0.961. Model 4: the underlying regression equation is: y = –0.0057x2 + 1.5380x – 8.2466; R2 = 0.958.
Conclusion
We concluded that BW could be predicted by means of linear BMs in Cornigliese sheep breed. The best fits were obtained when the highest number of BMs was included in the model. Nevertheless, reduced models could be used more easily in practice in the extensive sheep breeding, since they require less parameters to be measured in the population. Further studies are ongoing to verify the presence of latent factors which might impact the weight gain and size of animals. To this aim, we are considering the application of the principal component analysis. This ongoing study will potentially contribute to increase the economic interest in the breed, while aiming at its genetic preservation.
Ethical approval
All authors declare that this study follows the principles of the Declaration of Helsinki.
Acknowledgments
Part of the results was presented at the 23rd National Congress of the Animal Science and Production Association (ASPA 2019, Sorrento, Italy, 11–14 June 2019).
Disclosure statement
None of the authors has a financial or personal relationship with other people or organisations that could inappropriately influence this publication.
Additional information
Funding
References
- Ceccobelli S, Karsli T, Di Lorenzo P, Marozzi G, Landi V, Sarti FM, Sabbioni A, Lasagna E. 2015. Genetic diversity of Cornigliese sheep breed using STR markers. Small Rumin Res. 123:62–69.
- FAO. 2015. The second report on the state of the world’s animal genetic resources for food and agriculture. Rome (Italy): FAO Commission on Genetic Resources for Food and Agriculture.
- Judge GG, Griffiths WE, Hill RC, Lütkepohl H, Lee TC. 1985. The theory and practice of econometrics. 2nd ed. New York (NY): John Wiley.
- Kunene NW, Nesamvuni AE, Nsahlai IV. 2009. Determination of prediction equations for estimating body weight of Zulu (Nguni) sheep. Small Rumin Res. 84:41–46.
- Mahmud MA, Shaba P, Zubairu UY. 2014. Live body weight estimation in small ruminants – a review. Glob J Anim Sci Res. 2:102–108.
- Petrović MP, Caro Petrović V, Ružić Muslić D, Ilić Z, Spasić Z, Stojković J, Milenković M. 2012. Genetic and phenotypic aspects of the body measured traits in Merinolandschaf breed of sheep. Biotech Anim Husb. 28:733–741.
- Sabbioni A, Beretti V, Ablondi M, Righi F, Superchi P. 2018. Allometric coefficients for carcass and non-carcass components in a local meat-type sheep breed. Small Rumin Res. 159:60–74.
- Sabbioni A, Beretti V, Righi F, Superchi P. 2016a. Allometric coefficients for body measures and morphometric indexes in a meat-type sheep breed. Small Rumin Res. 144:248–254.
- Sabbioni A, Beretti V, Zambini EM, Superchi P. 2016b. Carcass and meat parameters in Cornigliese sheep breed as affected by sex and age-class. Ital J Anim Sci. 15:2–9.
- Sabbioni A, Beretti V, Zambini EM, Superchi P, Ablondi M. 2019. Allometric coefficients for physical-chemical parameters of meat in a local sheep breed. Small Rumin Res. 174:141–147.
- Sarti F, Castelli L, Bogani D, Panella F. 2003. The measurement of chest girth as an alternative to weight determination in the performance recording of meat sheep. Ital J Anim Sci. 2:123–129.
- SAS. 2014. SAS/STAT 13.2 user’s guide. Cary (NC): SAS Institute Inc.
- Shirzeyli FH, Lavvaf A, Asadi A. 2013. Estimation of body weight from body measurements in four breeds of Iranian sheep. Songklanakarin J Sci Technol. 35:507–511.
- Sowande OS, Sobola OS. 2008. Body measurements of West African dwarf sheep as parameters for estimation of live weight. Trop Anim Health Prod. 40:433–439.
- Tadesse A, Gebremariam T. 2010. Application of linear body measurements for live body weight estimation of highland sheep in Tigray region, North-Ethiopia. J Drylands. 3:203–207.
- Topai M, Macit M. 2004. Prediction of body weight from body measurements in Morkaraman Sheep. J Appl Anim Res. 25:97–100.
- Walstra P. 1980. Growth and carcass composition from birth to maturity in relation to feeding level and sex in Dutch Landrace pigs. Wageningen (The Netherlands): H. Veenman and Zonen B.V.
- Wilson RF. 2014. Optimising a weighing protocol for sheep [master’s thesis]. Lincoln (New Zealand): Lincoln University.
- Yakubu A. 2012. Application of regression tree methodology in predicting the body weight of Uda sheep. Anim Sci Biotech. 45:484–490.
- Yilmaz O, Cemal I, Karaca O. 2013. Estimation of mature live weight using some body measurements in Karya sheep. Trop Anim Health Prod. 45:397–403.
- Younas UM, Bhatti AJA, Pasha TN, Ahmad N, Nasir M, Hussain A. 2013. Inter-relationship of body weight with linear body measurements in Hissardale sheep at different stages of life. J Anim Plant Sci. 23:40–44.
