Abstract
The aim of this study was to evaluate the antioxidant capacity, the antimicrobial proprieties of algae Ascophyllum nodosum and Schizochytrium spp. against one of major swine enteric pathogen Escherichia coli O138 by broth macro-dilution method in Luria–Bertani (LB) medium. The antimicrobial effect of the algal extracts at supplementation of 0.12%, 0.06% and 0.03% (v/v) on E. coli O138, genetically characterised by PCR, was evaluated by following the bacterial growth. The antioxidant activity was determined by the ABTS Radical Cation Decolorisation Assay. In particular, the log10 E. coli used as control resulted significantly higher than 0.12% at 3 hours (8.82 ± 0.07 and 8.18 ± 0.07 log10 cells/mL, respectively; p<.01) suggesting an inhibitory activity related to the dose. No effect activity was observed with Schizochytrium spp. against E. coli growth. A. nodosum and Schizochytrium spp. exhibited antioxidant capacity (p<.05). The combination of them (1:1) exhibited antioxidant activity suggesting a synergistic effect (p<.05). The different proprieties of algal species that can modulate the O138 E. coli growth, one of the major pathogen of swine species, together with the antioxidant capacity, make them a promising functional feed additive to improve the gut health, therefore further studies are needed to confirm these activities in vivo.
The aim of the study was to evaluate the antimicrobial and antioxidant proprieties of two species of algae: Ascophyllum nodosum and Schizochytrium spp.
Ascophyllum nodosum revealed antimicrobial effect against Escherichia coli O138. Both algae exhibited antioxidant capacity also with a synergistic effect.
Highlights
Introduction
One of today's challenges, in line with the One-Health principles, is to reduce the use of drugs and antibiotics in humans and livestock because of the rise of antibiotic resistance (EFSA and ECDC Citation2013; Dhama et al. Citation2013). In this context, pig farming is one of the most profitable agricultural practices; however, antibiotics have often been used in order to deal with critical phases of a pig's life, such as weaning. Post weaning diarrhoea (PWD), a gastrointestinal disease mainly associated with certain Escherichia coli strains, represents the most common indication for the antimicrobial prescription (Amezcua et al. Citation2002). In particular, the pathogroup of porcine verocytotoxin-producing Escherichia coli, belonging to serogroups O138, O139 and O141, is characterised by a virulence profile responsible for acute and severe enterotoxemia and for important economic losses (Verdonck et al. Citation2002; Rossi et al. Citation2014). Many factors, infectious and non-infectious, are involved in the outbreak of the PWD that is considered a multifactorial disease where nutrition plays a pivotal role (Rossi et al. Citation2013). The reduction of the use of antimicrobials in food-producing animals, replacing them where possible and re-thinking the livestock production system, is essential for the future of animal and public health (EFSA Citation2012; Murphy et al. Citation2017). For these reasons, several functional feed additives need to be evaluated in the weaning phase in order to increase the health status and reduce the need for antibiotics (Windisch et al. Citation2008). In this scenario, algae, besides being a valid source of essential nutrients, can be a very interesting natural source of new compounds with biological activity that could be used as functional ingredients of pig rations (Makkar et al. Citation2016; Madeira et al. Citation2017).
Algae are a heterogeneous group of autotrophic, photosynthetic, aquatic organisms that ranges from single-celled microorganisms defined as microalgae, to huge complex seaweeds (Evans and Critchley Citation2014). Schizochytrium spp., a microalga from Thraustochytrids family, is one of the most commonly studied microalgae in animal nutrition because of its high content in n-3 and n-6 poly-unsaturated fatty acids with observed effects on animal’s products quality and animal health status (Jiang et al. Citation2004; AbuGhazaleh et al. Citation2009; Madeira et al. Citation2017).
Macroalgae are macroscopic, multicellular plant-like organisms with a highly variable composition depending on species, habitat, temperature, light intensity, nutrients concentration of water (Makkar et al. Citation2016). Macroalgae can be divided into three groups: brown algae, red algae and green algae. Although brown algae are of lesser nutritional value than red and green algae, they contain important bioactive compounds. Ascophyllum nodosum is a large, common cold water brown alga and is one of the most used seaweed in animal nutrition, it is rich in minerals, particularly potassium and iodine (Combet et al. Citation2014) and contains polyphenols and phlorotannins, enlisted as bioactive compounds (Munir et al. Citation2013; Makkar et al. Citation2016). Algal biomass can also have a positive impact on food security and the environmental impact: in fact, they are cheap to harvest and grow exclusively in water, preventing the use of arable land. They also release oxygen into the atmosphere and, if included in the diet of ruminants, are able to reduce methane emissions (Fievez et al. Citation2007). Algal culture technology is similar to terrestrial plants agriculture; however, algae possess higher productivity. Plants are in a different scale level with an evolved organisation of tissues, but micro and macroalgae have some advantages compared to them: they are not cultivated in agricultural soils, they can grow in non-potable water, in coastal, arid and marginal areas unsuitable for agricultural purposes (Cardon et al. Citation2008; Gouveia and Oliveira Citation2009; Bochenski et al. Citation2019). Moreover, considering the challenges and the agrozootechnical scenario of the coming years, the enlargement of feed resource base through identification of novel feeds or development of new additives that enhance resources use efficiency would play an important role in sustainable development of the animal productions.
Even if the algae bioactivities have been recognised, considering the heterogeneity of the algae-based commercial products for feed and the intra- and interspecific differences among algae, it is necessary to evaluate their functional proprieties, define the suitable species and the possibility to combine them to enhance their effect. For these reasons, the aim of this study was to evaluate the in vitro antioxidant properties and the antibacterial effect against O138 E. coli, major pathogen in swine livestock, of Ascophyllum nodosum and Schizochytrium spp. in order to establish their possible further integration in weaned pigs’ diets as functional feed additive and as possible alternative to antibiotic compounds.
Materials and methods
Evaluation of nutritional value
Algae dried meal samples were obtained from Italfeed S.r.l. (Milan, Italy). The samples were analysed for the main nutrient components (ether extract, crude proteins, fats, crude fibre, ash) according to AOAC (Citation2005) ‘Official methods of analysis’ in double.
Dry matter (DM) was obtained by inserting samples in preweighted aluminium bags and dried in a forced-air oven at 105 °C for 24 h (AOAC method 930.15).
Ashes (Ash) were obtained using a muffle furnace at 550 °C (AOAC method 942.05). Crude protein (CP) was determined by a Kjeldahl method (AOAC method 2001.11). Ether extract (EE) was determined using ether extraction in the Soxtec system (DM 21/12/1998). Crude fibre (CF) was determined by filtering bag technique [AOCS (Citation2009) method Ba 6a-05]. Mineral content was determined after sample mineralisation. In particular, pulverised samples (0.3 g) were digested by a microwave digestor system (Anton Paar MULTIWAVE-ECO) in Teflon tubes filled with 10 mL of 65% HNO3 by applying a one-step temperature ramp (at 120 °C in 10 min and maintained for 10 min). The mineralised samples were cooled for 20 minutes and they were transferred into the polypropylene test tubes. Mineralised samples were diluted 1:100 with 0.3 M HNO3 in MilliQ Water and the concentration of elements was measured by inductively coupled plasma atomic emission spectrophotometer (ICP; model: Optima 3300 XL, PerkinElmer Inc., Massachusetts, USA).
Escherichia coli characterisation
The O138 E. coli strain was obtained from the Lombardy and Emilia Romagna Experimental Zootechnic Institute (IZSLER, Italy).
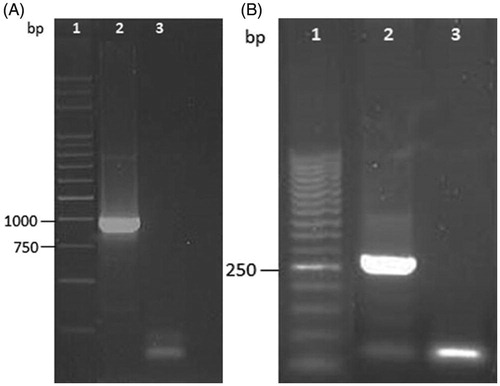
The strain was genetically characterised for the presence of genes codifying for two virulence factors represented by the F18 adhesive fimbriae and the verocytotoxin (VT2e). In particular, specific oligonucleotides were designed for the detection of FedF gene essential for F18 adhesion fimbriae and the B subunit of VT2e, responsible for binding the toxin to the intestinal cell surface before the absorption ().
Table 1. Primers used for polymerase chain reaction (PCR).
Genomic DNA was extracted using phenol/chloroform (1:1) from an overnight culture of E. coli strain and the quality of DNA was evaluated spectrophotometrically (260/280 ratio) and by agarose gel electrophoresis (0.8%, 10 V/cm 2 h) for quantification and to test for the presence of RNA or degraded DNA. The presence of FedF and VT2eB genes was evaluated by polymerase chain reaction (PCR) using specific primer-pairs. PCR was performed using the following experimental conditions: first denaturation 94 °C for 2 minutes, denaturation phase 94 °C for 1 minute, followed by annealing phase 55 °C for 2 minutes and elongation phase 72 °C for 2 minutes (the cycle was repeated 34 times). The volume of reaction mixture was 50 μL, with 5 μL of template (bacterial DNA) added to PCR mixture.
Antimicrobial assay
Antimicrobial extracts from algae Ascophyllum nodosum and Schizochytrium spp. were obtained according to Jiménez et al. (Citation2010) method. Five grams of dried algal meal sample was dissolved in 150 mL of acetone and extracted using a Soxhlet apparatus for 6 hours. After the evaporation of the solvent under vacuum at 50 °C, the residue (120 mg) was resuspended in 20 mL of MilliQ water, filtered with 0.22 µm syringe filter and stored at −20 °C until the analysis.
A liquid culture-based growth inhibition assay with E. coli O138 was performed to evaluate the ability of algae Soxhlet extracts to inhibit bacterial growth. An overnight culture of E. coli O138 in Luria–Bertani (LB) liquid medium was used as the inoculum for the experiments.
The experiment was set up as follows: 10 mL of LB with 120 µL of E. coli culture in liquid LB medium without algal extract was used as a positive control in order to evaluate the bacterial growth without any external influence. Three concentrations of the algal extract were added to a 50-mL tube with 10 mL of LB, to obtain a final concentration of treatment of 0.12%, 0.06% and 0.03% respectively. These concentrations were tested to evaluate if lower concentrations, compared with previous literature (Jiménez et al. Citation2010), were able to exert antimicrobial activity also in order to optimise the possible future inclusion of algae in feed and considering their cost-effective.
120 µL of overnight LB culture of E. coli were then added to each 50-mL tube. The same algal extract concentrations were added to 10 mL of LB without adding 120 µL of E. coli culture for the negative controls. All the samples were then maintained at 37 °C in a shaking incubator for six hours. The growth rate of E. coli was estimated, every hour for six hours, measuring the absorbance with a spectrophotometer (V-630 UV-VIS Spectrophotometer, JASCO, Germany) at an optical density (OD) of 600 nm.
The measured OD was converted in log10 of the number of cells/mL considering 1 OD = 1 × 109 cells/mL (Myers et al. Citation2013).
All assays were performed in technical duplicate and three biological replicates that are meant to verify the replicability of the experiment, using the same procedures repeating the experiment starting from the sample also repeating the test in different days.
Evaluation of antioxidant properties (ABTS assay)
To perform the antioxidant assay, dried meal of Schizochytrium spp. and A. nodosum were extracted using ethanol and water according the method proposed by Machu et al. (Citation2015) with some adaptations. One gram of each algae was dissolved in 10 mL of solvent (pure ethanol or water) and stirred for 24 hours at 23 °C. A mixture of both algae was prepared using 1:1 (w/w) of A. nodosum and Schizochytrium spp. powder extracted with 10 mL of water or ethanol as solvents. The mixture was stirred for 24 hours at 23 °C.
Extracts were centrifugated for 10 min at 3000 rpm, the supernatants were collected and filtered with 0.45-µm syringe filter and stored at −20 °C until the analysis.
The antioxidant activity was tested by adopting an ABTS assay, according to Re et al. (Citation1999).
The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) radical cation was generated by the reaction of 7 mM ABTS with 2.45 mM of K-persulfate. The reaction mixture was left to stand in the dark for 16 hours at room temperature and used within two days. Working solutions of ABTS•+ were obtained by diluting ABTS•+ in ethanol in order to obtain an absorbance of 0.700 ± 0.02 OD at 734 nm at room temperature. First, a calibration curve was obtained using different concentrations (2000 µM, 1500 µM, 1000 µM, 500 µM, 100 µM, 0 µM) of Trolox (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid) as standard. The assay was performed using 10 µL of diluted sample added to 1 mL of working solution (ABTS•+). The absorbance was recorded from 1 to 6 minutes and all determinations were performed in triplicate.
The A. nodosum and Schizochytrium spp. extracts were diluted in their solvents (water and ethanol) and tested in the following concentrations: 10%, 5%, 2%. Secondary, in order to test the synergistic effect, the mixture with an equal concentration of both algae (1:1 w/w) was then tested with the same concentrations: 10%, 5%, 2%. The concentrations tested were prepared diluting the original extract 1:5 (v/v), 1:10 (v/v) and 1:20 (v/v) in line with the range tested by Jiménez et al. (Citation2010) for DPPH assay. The dilutions were also necessary to obtain absorbances comparable with the calibration curve in order to quantify the final results that were expressed as equivalent concentration of Trolox/g after six minutes (TroloxEq/g).
The total antioxidant capacity was expressed as the percentage inhibition (PI), according to the equation: PI = [(AbsABTS•+ - Abs sample)/AbsABTS•+] × 100; here AbsABTS•+ denotes the initial absorbance of diluted ABTS•+, and Abs sample denotes the absorbance of the sample in every 6 min of reaction.
Statistical analysis
Data of antimicrobial and antioxidant assays were analysed using Proc GLIMMIX of SAS 9.4 (SAS Inst. Inc., Cary, NC) (SAS Institute Citation2009). The model included the fixed effect of treatments, time, the interaction between treatment and time. Within significant two-way interactions, slice option was used to separate means within a specific treatment and time and the results are reported as least squares means (LSMEANS) and standard error. Data of µmol TroloxEq/g were compared using Proc GLM. The model included the effect of algae and extraction methods (water and ethanol) and the interaction between algae and extraction methods. Means were considered different when p<.05 and tended to different if .05 < p ≤ .10. Tukey–Kramer studentised adjustments were used to separate the means of extraction methods within the two-way interactions. Results are reported as means and standard deviations.
Results and discussion
In this study, the attention was focalised on the in vitro evaluation of nutraceutical properties of Schizochytrium spp. and Ascophyllum nodosum in order to establish their further use as functional additives.
The obtained results of chemical analysis of algae are in line with literature (Makkar et al. Citation2016) and the commercial feed label (Table ). Both algae were freeze-dried meal with moisture content less than 8% in order to guarantee adequate storage conditions. In general, A. nodosum is characterised by a high content of minerals (more than 20% DM), in particular, it is characterised by a high content of calcium and a low content of phosphorous. This aspect should be considered in the diet‘s formulation in order to maintain a correct balance of these two elements. However, if algae are used as feed additives, they will be included less than 5% in the diet and this percentage should not constitute an important change in the mineral balance. In general, mineral premix additive is always included during the ration formulations, the amount of minerals contained in algae must be considered for their enclosure in the feed in order to respect the admitted levels of European Union regulation (Reg 1081/2003/EC) (EC Citation2003). In particular, some minerals are required as nutrients for the piglets, but they are frequently integrated in excess also to increase animal performances and this aspect could represent a risk for the environment (Hejna et al. Citation2018). Minerals such as cadmium (Cd) are undesirable compounds and Cd could become toxic in higher concentrations. Our results revealed an amount of Cd in line with permitted level of European regulation about feed additives (Directive Citation2002/32/EC). Other minerals like zinc (Zn), copper (Cu) and selenium (Se) are useful to satisfy the requirements of animals, their concentration should be balanced with the mineral content of the ingredients utilised in animal feed, also respecting the safety of the environment (Table ). On the contrary, Schizochytrium spp. contains a lower amount of minerals (5.25% DM), but it represents an important source of lipids. A. nodosum is characterised also by 8.25% (DM) of crude protein content with high biological value (Becker Citation2004). Even if the amount of protein is comparable to the corn meal, it should be considered that the presence of non-protein nitrogen in different algal species can affect the results slightly. In particular, the quantification of the crude protein using the standard conversion value for nitrogen (6.25) could lead to an overestimation of the protein content if non-protein nitrogen is present (Lourenço et al. Citation2004).
Table 2. Chemical composition of dried algae samples of A. nodosum and Schizochytrium spp.
Table 3. Mineral composition of dried algae samples A. nodosum and Schizochytrium spp.
The antimicrobial properties were evaluated against O138 E. coli, one of the major enteric pathogens of weaned piglets responsible for postweaning enteritis and enterotoxaemia that causes significant morbidity and mortality in pigs worldwide. PWD represents an important issue in swine farming that causes important economic losses, at the same time affecting the health of animals and leading to the consumption of antibiotic drugs (Rossi et al. Citation2013). The pathogenicity is usually influenced by the presence of virulence factors, such as VT2e toxin and the F18 adhesive fimbriae (Verdonck et al. Citation2002). The detection of FedF adhesion factor of F18 and of VTe2B gene confirmed the virulence profile of the O138 E. coli strain (Figure ).
Figure 1. (A) Agarose gel (1%) one-dimensional electrophoresis of PCR products for the detection of FedF gene from genomic DNA of O138 Escherichia coli strain. Lane 1: marker 1 kb; lane 2: positive sample; lane 3: negative control sample. (B) Agarose gel (1.5%) one-dimensional electrophoresis of PCR products for the detection of VT2eB gene from genomic DNA of O138 Escherichia coli strain. Lane 1: marker 50 pb; lane 2: positive sample; lane 3: negative control sample.
Significant differences in the bacterial growth were observed among the A. nodosum concentrations, respectively: 0.12%, 0.06%, 0.03% and 0% (E. coli without treatment) (p<.01) (Figure ). In particular, A. nodosum disclosed that the most concentrated treatment (0.12%) exhibited the highest inhibition activity on O138 E. coli growth; on the contrary, the lowest concentrations (0.06% and 0.03%) revealed a bacterial growth comparable to the positive control, indicating that these concentrations did not influence E. coli growth.
Figure 2. Average of E. coli growth (log10 cells/mL) of different concentrations of A. nodosum Soxhlet extract from time 1 to 6 hour. A. nodosum Soxhlet extract concentrations tested were 0.12%, 0.06%, 0.03% and positive control (E. coli). Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.01).
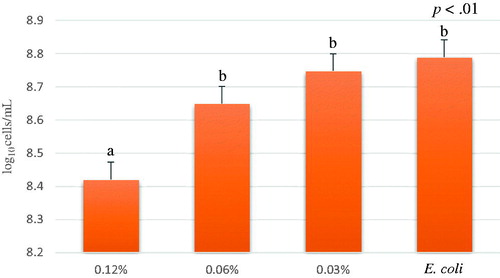
Our results are in line with other findings, demonstrating a significant decrease in bacterial growth in vitro (Dierick et al. Citation2010). Nevertheless, obtained results confirmed the antimicrobial activity also against a wild type strain characterised by a relevant virulence profile.
The inhibitory effect is probably attributed to phlorotannins, which are known to be powerful bacteriostatic and also a bactericidal component of brown algae (Wang et al. Citation2009). Other components of brown seaweed have demonstrated to possess an antibacterial activity, in particular polyphenols, which are a class of secondary metabolites also known for their antimicrobial activity (Daglia Citation2012). It has been demonstrated that polyphenols form different sources are able to produce hydrogen peroxide in aerobic conditions, its production is also directly related to the content of the hydroxyl groups. Tannins possess affinity for bounding proteins and this capacity increases with the number of hydroxyl groups this could explain that phlorotannins exert higher antimicrobial activity compared with terrestrial tannins because they are readily oxidised upon exposure to air and contain a higher number of hydroxyl groups (Wang et al. Citation2009).
Our findings highlight the need to use the highest concentration in order to guarantee the antimicrobial effect. In particular, considering that the extract used in our study was obtained using 5 g of alga in 150 mL of acetone, obtaining 120 mg of solid extract that was then dissolved in 20 mL of water, the final concentration of algal extract resuspended in water was 0.6%. Our study is in line with Gardiner et al. (Citation2008), which used an inclusion percentage of A. nodosum extract in piglet's diet ranging from 0.3% to 0.9% of dry matter.
The effects of the different concentrations of algae tested to evaluate their inhibition capacity over time (Figure ) revealed that after the first hour, the inhibition capacities of the tested concentrations were comparable to the positive control (E. coli). After 2, 3 and 4 hours, there was a significant difference among the most concentrated treatment (0.12%) and the other samples, which were comparable to the positive control (E. coli) (p<.05). The maximum inhibitory effect of algal extract was observed after 3 hours, where the log10 cells/mL of control (E. coli) and 0.12% concentration were significantly different, respectively 8.82 ± 0.07 and 8.18 ± 0.07 (p<.01). No differences were observed at 5 and 6 hours, suggesting an exhaustion of the bioactivity due to algal degradation or to the development of bacterial resistance. According to Zoetendal et al. (Citation2008) the bacterial resistance of E. coli against condensed tannins seems to be related to the activation the BaeSR two-component regulatory system. Phlorotannins contained in A. nodosum possesses similar property to polyphenols, main constituent of tannins, characterised by antimicrobial activity.
Figure 3. Average of E. coli growth (log10 cells/mL) of different concentration of A. nodosum Soxhlet extract through experimental time (from 1 to 6 hour). A. nodosum Soxhlet extract concentrations tested were 0.12%, 0.06%, 0.03% and positive control (E. coli). Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different, means are separated within treatment groups though the experimental time with Tukey (interaction treatment by time p<.05).
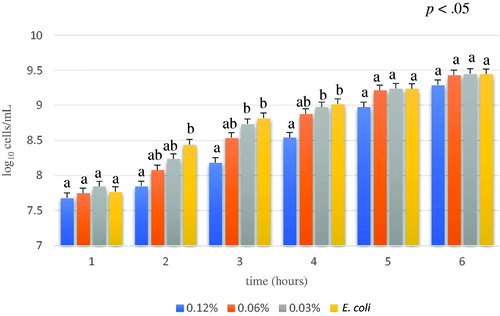
Even if the effect was observed only for a short period, it is important to consider the transit time of the feed in a pig's gastrointestinal tract, which lasts 4 hours, this guaranties the effect throughout the digestion tract.
Results of ABTS assay revealed that A. nodosum ethanol extract antioxidant activity (0.75 ± 0.31 µmol TroloxEq/g) is lower compared to the water extract (p<.05) (Table ) suggesting that this algal species contains a good amount of hydro-soluble antioxidant substances which are easily released in water (Figure ). In line with our results, the study conducted by Machu et al. (Citation2015) disclosed the highest antioxidant capacity adopting the water extraction for brown algae. Machu et al. (Citation2015) tested Eisenia bicyclis that is a brown alga rich in phlorotannins that revealed also antimicrobial capacities against streptomycin resistant Listeria monocitogenes (Kim et al. Citation2018).
Figure 4. Average of percentage of inhibition (PI%) of A. nodosum ethanol extract from 0 to 6 minutes. The ABTS antioxidant assay tested different concentrations of A. nodosum ethanol extract 10%, 5%, 2% and blank. Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.05). ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
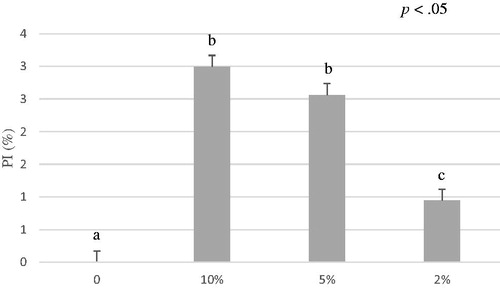
Table 4. Results of ABTS assay of A. nodosum, Schizochytrium spp. and the algal mixture (1:1 w/w) in response to the different extraction methods (water and ethanol).
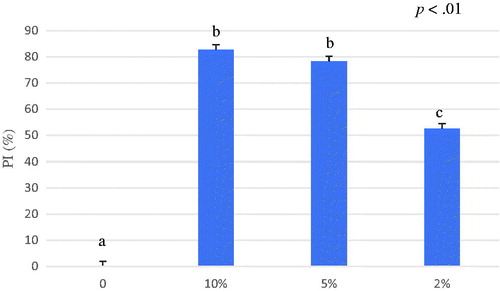
The results have also displayed that the antioxidant activity of A. nodosum water extract exhibited an antioxidant activity of 55.54 ± 16.05 µmol TroloxEq/g after 6 minutes of reaction (Table ) (Figure ). In general, free antioxidant substances react immediately when the sample is added to an ABTS•+ reaction mixture in a similar way to the effect of standard Trolox. Other antioxidants that are not immediately available may require time to be released in order to exert their effect against radicals, for this reason the assay was conducted in six minutes.
Figure 5. Average of percentage of inhibition (PI%) of A. nodosum water extract from 0 to 6 minutes. The ABTS antioxidant assay tested different concentrations of A. nodosum water extract 10%, 5%, 2% and blank. Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.01). ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
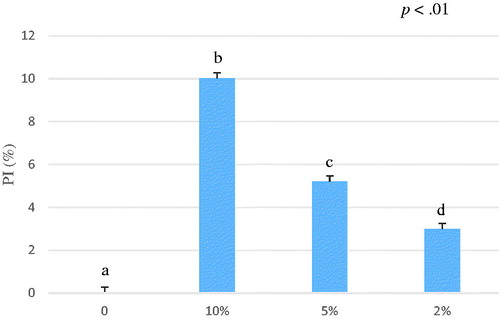
The Schizochytrium spp. ethanol extract exhibited an antioxidant capacity of 2.56 ± 0.53 µmol TroloxEq/g calculated after six minutes of reaction (Table ) (Figure ). The Schizochytrium spp. water extract did not display any activity; in fact, we obtained an opaque extract which was not able to inhibit the ABTS•+ radical. Furthermore, this extract when added to the working solution increased the turbidity of the mixture making the spectrophotometer reading inaccurate. These findings could be due to the high content of lipids of Schizochytrium spp. (Table ) that are known to be not hydro-soluble and water was not able to extract non-polar substances.
Figure 6. Average of percentage of inhibition (PI%) of Schizochytrium spp. ethanol extract from 0 to 6 minutes. The ABTS antioxidant assay tested different concentrations of Schizochytrium spp. ethanol extract 10%, 5%, 2% and blank. Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.01). ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
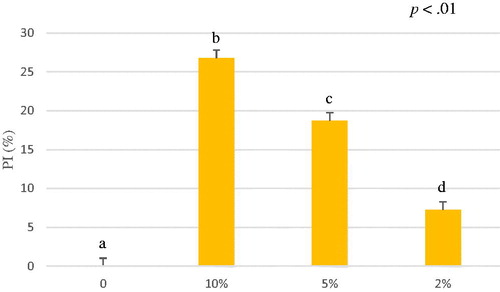
The mixture of A. nodosum and Schizochytrium spp. exhibited an antioxidant capacity of 3.18 ± 0.57 µmol TroloxEq/g for ethanol extract and 10.06 ± 2.73 for water extract (Table ) (Figures and ). The antioxidant capacity (µmol TroloxEq/g) of the mixture was lower than the antioxidant capacity of A. nodosum water extract alone, but the ethanol extracted mixture (3.18 ± 0.57 µmol TroloxEq/g) was comparable to the sum of the antioxidant capacity of both ethanol extracted algae (2.56 ± 0.53 and 0.75 ± 0.31 µmol TroloxEq/g) suggesting that could be possible a synergic effect. Significant differences were observed in a dose-dependent way among the concentration tested expressed as percentage of inhibition (PI%) (p<.01).
Figure 7. Average of percentage of inhibition (PI%) of A. nodosum and Schizochytrium spp. mixture (1:1 w/w) ethanol extract from 0 to 6 minutes. The ABTS antioxidant assay tested different concentrations of A. nodosum and Schizochytrium spp. mixture ethanol extract 10%, 5%, 2% and blank. Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.01). ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
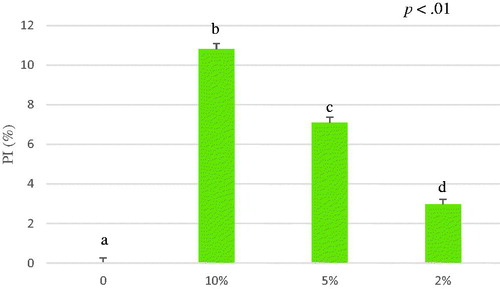
Figure 8. Average of percentage of inhibition (PI%) of A. nodosum and Schizochytrium spp. mixture (1:1 w/w) water extract from 0 to 6 minutes. The ABTS antioxidant assay tested different concentrations of A. nodosum and Schizochytrium spp. mixture water extract 10%, 5%, 2% and blank. Data are shown as least squares means and standard errors. a,bmeans (n = 3) with different superscripts are significantly different (treatment p<.01). ABTS: 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid).
Even if in this study Schizochytrium spp. demonstrated lower antioxidant capacity compared to brown algae, several in vivo studies have been largely demonstrated the capacity of Schizochytrium spp. to improve health status and production efficiency of livestock animals (Mata et al. Citation2010; Lv et al. Citation2015). The content of polyphenols, tocopherols and other minor components acts against oxidation (Pandey and Rizvi Citation2009), in the last decade, interest in the potential health benefits of dietary plant polyphenols as antioxidants has increased rapidly. Schizochytrium spp. contains a large amount of DHA that have many beneficial effects. However, high concentrations of n-3 polyunsaturated fatty acids may increase lipid peroxidation and subsequently induce oxidative stress (Vericel et al. Citation2003). This may explain the lower antioxidant capacity of Schizochytrium spp. compared to Ascophyllum nodosum.
The antioxidant activity of A. nodosum may also be related mainly to phlorotannins, which possess strong antioxidant capacity (Sathya et al. Citation2017). The antioxidant activity of 55.54 ± 16.05 µmol TroloxEq/g observed for A. nodosum is comparable with a strawberry antioxidant capacity (Castrica et al. Citation2019). Other studies also confirm the antioxidant activity of two polysaccharide groups, laminarin and fucoidans which are both present in brown algae (Kadam et al. Citation2015). Phenolic compounds such as flavonoids, phenolic acids, and tannins are considered to be major contributors to the antioxidant capacity of plants. These antioxidants also possess diverse biological activities, such as anti-inflammatory, anti-atherosclerotic and anti-carcinogenic activities. These activities may be related to their antioxidant activity that consists in the electron-transfer capacity from their molecules to the oxidised radical by scavenging the free radicals (Li et al. Citation2007).
Considering that E. coli diseases are commonly multifactorial, all the stressors during the weaning can reduce the immune defences. Antibacterial and antioxidant compounds could help young animals to maintain the health status, improving defences and thus increasing performance. A. nodosum and Schizochytrium spp. have displayed suitable characteristics for animal nutrition also revealing interesting bioactivities. The future trends will be the inclusion of algae as ingredient in feed evaluating the optimal level of inclusion related to their bioactivities in vivo and define their cost opportunity.
Conclusions
Considering that algae represent several advantages form agronomic and environmental point of view, they could become one of the valuable sources of food and feed.
A. nodosum and Schizochytrium spp. revealed interesting bioactivities in vitro and they are promising as future feed additives. Indeed, despite only an antibacterial effect was observed for a limited time, is interesting to notice the synergistic effect supplied from the complementary characteristic of these algal species. A. nodosum can modulate the E. coli growth, Schizochytrium spp. possesses antioxidant capacity and the combination of these two species can enhance the antioxidant power. With the urgent need of innovative functional feed additives as alternative to antibiotics, these algal species should be considered in animal breeding.
Therefore, more studies on the argument are needed to confirm the in vitro obtained results also in livestock conditions. Obtained findings revealed that A. nodosum and Schizochytrium spp. possess antimicrobial and antioxidant activities, these aspects should be considered as promising for innovative feed additives for functional animal nutrition, since each algal species has displayed different effects that could be used in complementary way.
Ethical approval
This study follows the principles of the Declaration of Helsinki.
Acknowledgements
The authors would like to thank Italfeed S.r.l. that provided algae samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- AbuGhazaleh AA, Potu RB, Ibrahim S. 2009. The effect of substituting fish oil in dairy cow diets with docosahexaenoic acid-micro algae on milk composition and fatty acids profile. J Dairy Sci. 92(12):6156–6159.
- Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. 2002. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 66(2):73–78.
- AOAC. 2005. Official Methods of Analysis, 16th ed. Washington, DC: Association of Official Analytical Chemists.
- AOCS. 2009. Approved Procedure Ba 6a-05: Crude fiber analysis in feeds by filter bag technique. In Official Methods and Recommended practices, 4th ed. Champaign, IL: American Oil Chemists’ Society.
- Becker W. 2004. Microalgae in human and animal nutrition. In Richmond A (ed) Handbook of microalgal culture. Blackwell, Oxford. pp. 312–351.
- Bochenski T, Chaturvedi T, Thomsen MH, Schmidt JE. 2019. Evaluation of marine synechococcus for an algal biorefinery in arid regions. Energies. 12(12):2233–2213.
- Cardon ZG, Gray DW, Lewis LA. 2008. The green algal underground: evolutionary secrets of desert cells. Bioscience. 58(2):114–122.
- Castrica M, Rebucci R, Giromini C, Tretola M, Cattaneo D, Baldi A. 2019. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital J Anim Sci. 18(1):336–341.
- Combet E, Ma ZF, Cousins F, Thompson B, Lean ME. 2014. Low-level seaweed supplementation improves iodine status in iodine-insufficient women. Br J Nutr. 112(5):753–761.
- Daglia M. 2012. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 23(2):174–181.
- Dhama K, Chakraborty S, Kapoor S, Tiwari R, Kumar A, Deb R, Rajagunalan S, Singh R, Vora K, Natesan S. 2013. One world, one health-veterinary perspectives. Adv Anim Vet Sci. 1(1):5–13.
- Dierick N, Ovyn A, De Smet S. 2010. In vitro assessment of the effect of intact marine brown macro-algae Ascophyllum nodosum on the gut flora of piglets. Livestock Sci. 133(1–3):154–156.
- Directive. 2002. /32/EC of the European parliament and of the council of 7 May 2002 on undesirable substances in animal feed.
- [EFSA] European Food Safety Authority, [ECDC] European Centre for Disease Prevention and Control. 2013. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2011. EFSA J. 11:3196–3359.
- [EFSA] European Food Safety Authority. 2012. Guidance on risk assessment for animal welfare. EFSA Panel on Animal Health and Welfare (AHAW). EFSA J. 2013;11(5):3196.
- [EC] European Union. 2003. Regulation (EC) No 1831/2003 of the European parliament and of the council of 22 September 2003 on additives for use in animal nutrition (Text with EEA relevance).
- Evans FD, Critchley AT. 2014. Seaweeds for animal production use. J Appl Phycol. 26(2):891–899.
- Fievez V, Boeckaert C, Vlaeminck B, Mestdagh J, Demeyer D. 2007. In vitro examination of DHA-edible micro-algae: 2. Effect on rumen methane production and apparent degradability of hay. Animal Feed Sci Technol. 136(1–2):80–95.
- Gardiner G.E, Campbell A.J, O’Doherty J.V, Pierce E, Lynch P.B, Leonard F.C, Stanton C, Ross R.P, Lawlor P.G. 2008. Effect of Ascophyllum nodosum extract on growth performance, nutrient digestibility, carcass characteristics and selected intestinal microflora populations of grower-finisher pigs. Animal Feed Sci Technol. 141(3-4):259–273.
- Gouveia L, Oliveira AC. 2009. Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol. 36(2):269–274.
- Hejna M, Gottardo D, Baldi A, Dell’Orto V, Cheli F, Zaninelli M, Rossi L. 2018. Nutritional ecology of heavy metals. Animal. 12(10):2156–2170.
- Jiang Y, Fan K-W, Wong R-Y, Chen F. 2004. Fatty acid composition and squalene content of the marine microalga Schizochytrium mangrovei. J Agric Food Chem. 52(5):1196–2000.
- Jiménez JT, O’Connell S, Lyons H, Bradley B, Hall M. 2010. Antioxidant, antimicrobial, and tyrosinase inhibition activities of acetone extract of Ascophyllum nodosum. Chem Papers. 64(4):434–442.
- Kadam SU, Tiwari BK, O'donnell CP. 2015. Extraction, structure and biofunctional activities of laminarin from brown algae. Int J Food Sci Technol. 50(1):24–31.
- Kim HJ, Dasagrandhi C, Kim SH, Kim BG, Eom SH, Kim YM. 2018. In vitro antibacterial activity of phlorotannins from edible brown algae, Eisenia bicyclis against streptomycin-resistant Listeria monocytogenes. Indian J Microbiol. 58(1):105–108.
- Li H, Cheng K, Wong C, Fan K, Chen F, Jiang Y. 2007. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 102(3):771–776.
- Lourenço SO, Barbarino E, Lavín PL, Lanfer Marquez UM, Aidar E. 2004. Distribution of intracellular nitrogen in marine microalgae: calculation of new nitrogen-to-protein conversion factors. Eur J Phycol. 39(1):17–32.
- Lv J, Yang X, Ma H, Hu X, Wei Y, Zhou W, Li L. 2015. The oxidative stability of microalgae oil (Schizochytrium aggregatum) and its antioxidant activity after simulated gastrointestinal digestion: Relationship with constituents. Eur J Lipid Sci Technol. 117(12):1928–1939.
- Machu L, Misurcova L, Vavra Ambrozova J, Orsavova J, Mlcek J, Sochor J, Jurikova T. 2015. Phenolic content and antioxidant capacity in algal food products. Molecules. 20(1):1118–1133.
- Madeira MS, Cardoso C, Lopes PA, Coelho D, Afonso C, Bandarra NM, Prates JA. 2017. Microalgae as feed ingredients for livestock production and meat quality: A review. Livestock Sci. 205:111–121.
- Makkar HP, Tran G, Heuzé V, Giger-Reverdin S, Lessire M, Lebas F, Ankers P. 2016. Seaweeds for livestock diets: a review. Anim Feed Sci Technol. 212:1–17.
- Mata TM, Martins AA, Caetano NS. 2010. Microalgae for biodiesel production and other applications: a review. Renewable Sustainable Energy Rev. 14(1):217–232.
- Munir N, Sharif N, Naz S, Manzoor F. 2013. Algae: a potent antioxidant source. Sky J Microbiol Res. 1(3):22–31.
- Murphy D, Ricci A, Auce Z, Beechinor JG, Bergendahl H, Breathnach R. 2017. EMA and EFSA Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFS2. 15(1):1–245.
- Myers JA, Curtis BS, Curtis WR. 2013. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophysics. 6(1):4–15.
- Pandey KB, Rizvi SI. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longevity. 2(5):270–278.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 26(9–10):1231–1237.
- Rossi L, Di Giancamillo A, Reggi S, Domeneghini C, Baldi A, Sala V, Dell'Orto V, Coddens A, Cox E, Fogher C. 2013. Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model. J Vet Sci. 14(3):263–270.
- Rossi L, Pinotti L, Agazzi A, Dell’Orto V, Baldi A. 2014. Plant bioreactors for the antigenic hook-associated flgK protein expression. Ital J Anim Sci. 13(1):2939–2929.
- SAS Institute. 2009. SAS/STAT® 9.4 User’s Guide. 2nd ed. Cary, NC: SAS Institute Inc.
- Sathya R, Kanaga N, Sankar P, Jeeva S. 2017. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab J Chem. 10:S2608–S2614.
- Verdonck F, Cox E, van Gog K, Van der Stede Y, Duchateau L, Deprez P, Goddeeris BM. 2002. Different kinetic of antibody responses following infection of newly weaned pigs with an F4 enterotoxigenic Escherichia coli strain or an F18 verotoxigenic Escherichia coli strain. Vaccine. 20(23–24):2995–3004.
- Vericel E, Polette A, Bacot S, Calzada C, Lagarde M. 2003. Pro-and antioxidant activities of docosahexaenoic acid on human blood platelets. J Thromb Haemost. 1(3):566–572.
- Wang Y, Xu Z, Bach SJ, McAllister TA. 2009. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian Australas J Anim Sci. 22(2):238–245.
- Windisch W, Schedle K, Plitzner C, Kroismayr A. 2008. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. 86(suppl_14):E140–E148.
- Zoetendal EG, Smith AH, Sundset MA, Mackie RI. 2008. The BaeSR two-component regulatory system mediates resistance to condensed tannins in Escherichia coli. Appl Environ Microbiol. 74(2):535–539.
