Abstract
The aim of this study was to assess the effect of nucleotides administration on growth performance and immune response in post-weaning piglets. Twenty-eight male weaned piglets, homogeneous for age and weight were randomly allocated to two experimental treatments. Treated group (T) was daily orally administered 0.8 g/head of a mixture of nucleotides suspended in 2.1 mL water solution; while control group (C) received 2.1 mL saline solution. Body weight (BW) and average daily gain (ADG) were individually recorded weekly, while feed intake (FI), and gain:feed (G:F) were recorded and calculated on pen basis. Faecal score was evaluated every seven days. On day 0, 9, 18 and 27 blood samples were collected to determine IgA, IgG and haptoglobin concentration. At day 28 all piglets were sacrificed, and tissue samples of ileal Peyer’s patches were collected for the evaluation of IL1α, IL1β, IL6, IL10, TNFα, TLR2, TLR4 and PPARγ gene expression. Nucleotides supplementation significantly increased BW (17.37 vs. 19.00 kg/pig; p = <.01), ADG (.351 vs. .400 kg/d; p < .01), and FI (3.96 vs. 4.39 kg/d; p < .01), but not G:F (.61 vs. .64; p = .29). Faecal consistency was not different between the experimental groups and no occurrence of diarrhoea was reported. IgA and IgG content in blood was not influenced by the treatment, as well as gene expression of inflammatory cytokines in Peyer’s patches. The present trial shows that nucleotide administration is able to improve growth performance of post-weaning piglets, with no effects on inflammatory response and the expression of immune-related genes.
Nucleotides administration increased BW, ADG and FI.
Nucleotides did not affect inflammatory and immune response.
Highlights
Introduction
Over the last years, efficiency and quality of commercial swine production have been significantly improved thanks to breeding and nutritional programmes, as well as management practices. With the aim to further accelerate the production cycle, weaning age has been progressively reduced, and piglets are weaned at 3–4 weeks of age. Weaning is a stressful moment in pigs’ life, accompanied by physiological changes in the gastrointestinal tract (GIT), which become more pronounced when early-weaning practices are adopted (Smith et al. Citation2010). A fast recovery of the intestine is essential for proper growth of weaned piglets, and to this end antimicrobials have been widely used in the past to counteract the adverse effects of weaning (Cromwell Citation2002). However, the general concern about the risk of antibiotic-resistance development led to the ban of their use as growth promoter by the European Union in 2006. In this light, the interest towards alternative substances has strongly increased, including the investigation of feed additives that stimulate growth and cell differentiation of intestinal tract and immune system of piglets (Pluske Citation2013).
Although nucleotides have been extensively investigated during past years, the mechanism of action and the efficacy of these compounds in pig nutrition is still debated (Jang and Kim Citation2019; Patterson et al. Citation2019). Nucleotides are a group of bioactive compounds, composed by a nitrogenous base, a pentose sugar and one or more phosphates. Nucleotides play several roles in biochemical processes; they serve as nucleic acid precursors, physiological mediators, components of coenzymes and sources of cellular energy (Carver and Walker Citation1995; Grimble and Westwood Citation2001; Sauer et al. Citation2011). Nucleotides have also a key role for the maturation of enterocytes and lymphoid cells. Despite intestinal epithelial cells can provide endogenous nucleotides, either via de novo synthesis or via salvage pathway (Carver and Walker Citation1995), dietary supply can become ‘conditionally essential’ in stressful moments, such as weaning. At this stage, the requirement of nucleotides strongly increases to promote the growth of intestinal epithelium and lymphoid cells (Sato et al. Citation1999). Until the moment of weaning, the huge demand of nucleotides for piglets’ GIT development is supplied by sow’s milk, whose nucleotides concentration accounts for up to 20% of the non-protein fraction of milk (Uauy Citation1989).
The main nucleotides in sow milk are represented by uridine 5’monophosphate (UMP), guanosine 5′monophosphate (GMP), adenine 5′monophosphate (AMP), cytidine 5′monophosphate (CMP) and inosine 5′monophosphate (IMP). The average concentration of these nucleotides in sow milk from 7 to 28 days of lactation is 187.9 µmol/mL (UMP), 10.4 µmol/mL (GMP), 7.6 µmol/mL (AMP), 4.2 µmol/mL (CMP) and 1.4 µmol/mL (IMP), as reported by Mateo (Citation2005). However, nucleotides concentration is not consistent over time, but it declines starting from the first week of lactation. At the moment of weaning, the nucleotide contribution of milk fails, and post-weaning diet may not be suitable for proper intestinal maturation because of its low nucleotides content (Martinez-Puig et al. Citation2007). Thus, dietary supplementation of nucleotides may positively contribute to the post-weaning phase (Domeneghini et al. Citation2004).
The aim of this study was to investigate the effect of nucleotides administration to post-weaning piglets on growth performances and immune response.
Materials and methods
This study was performed at the facility of Animal Production Research and Teaching Centre of the Polo Veterinario, Università degli Studi di Milano (Lodi, Italy). All experimental procedures were reviewed and approved by the Ethics Committee of the University of Milano (approval number 34/12). The experimental and notification procedures were carried out in compliance with Directive 86/609/EEC.
Animals, housing, diet and experimental treatment
A total of 28 male weaned pigs (Topig40 x Topigs Fomeva), homogeneous for age (28 ± 1.6 days) and initial body weight (BW) (7.68 ± 0.31 kg), were enrolled in the trial. The animals were included in a completely randomised design based on BW and allocated to two treatments (control – C, and treated – T) of seven replicates each (two animals/replicate).
Animals were housed in one room, with computer-controlled heating and mechanical ventilation systems. Room temperature was maintained at 28 °C at the beginning of the experimental period, and decreased by 1 °C every 3 days, to a final temperature of 24° C at the end of the trial. Each pen was provided with plastic slatted floor, a feeding trough and two drinking nipples. Two stainless steel chains per pen were provided as environmental enrichment. At day 11 and 19 post-partum, piglets were vaccinated against Mycoplasma hyopneumoniae (Ingelvac Mycoflex, Boehringer Ingelheim) and Porcine circovirus type 2 (Ingelvac Circoflex, Boehringer Ingelheim), respectively.
All piglets were fed a standard commercial diet (meal form) formulated to meet or exceed nutrient requirements for post-weaning piglets (NRC 2012) (Table ). Water and feed were provided ad libitum.
Table 1. Ingredients and composition of the diet administered to the animals (as fed basis).
For 28 consecutive days, every morning at 08.00, C group received 2.1 mL of saline solution, while T animals received 0.8 g/head/day of mixture of nucleotides (Prosol spa, Madone, Italy) in a 2.1 mL water solution. The treatments were orally administered to each pig in order to guarantee that animals received the full established dosage.
The orally administered experimental nucleotides mixture contained UMP, GMP, AMP, CMP and IMP. The mixture was formulated and the dosage chosen to provide the respective amount of each nucleotide resembling its average content in sow milk from 7 to 28 days of lactation (Mateo Citation2005).
The chemical composition of the basal diet was analysed at the beginning of the trial. Crude protein, crude fat and crude fibre were determined following the Association of Official Analytical Chemists methods of analysis (AOAC, 2005). Amino-acid content was calculated by AMINODat® 4.0 (Evonik Nutrition & Care GmbH, Germany), while calcium, phosphorus, and ME contents in the diet were calculated by INRA-CIRAD-AFZ Feed tables.
Samples and measurements
Individual BW and pen feed intake (FI) were recorded on weekly basis (at days 0, 7, 14, 21 and 28) by electronic scale (Ohaus ES100L, Pine Brook, New Jersey; sensitivity ± 0.02 kg). Average daily gain (ADG) and gain:feed ratio (G:F) were subsequently determined.
Faecal score was recorded weekly on pen basis by subjective four-point scale, where 1 = firm and 4 = watery, according to Wellock et al. (Citation2007).
Blood samples were collected in the morning, after an overnight fasting, from the same one piglet per pen at days 0, 9, 18 and 27. Samples were collected from cranial vena cava into a 10-mL vacuum tube with ethylenediaminetetraacetic acid (VT100STK, 0.1 mL EDTA) as anticoagulant. Samples were subsequently analysed for haptoglobin (Hp), IgA, and IgG content. The concentration of Hp was determined by colorimetric assay (Tridelta Phaserange serum haptoglobin assay, cat. no. TP-801) and expressed on the basis of a standard curve (Cooke and Arthington Citation2013). Intra-assay CV and inter-assay CV were 7.41% and 6.18%, respectively. IgA and IgG were measured by porcine-specific ELISA kit according to the recommendations of the manufacturer (Bethyl Laboratories, Montgomery, TX, USA). All samples were assayed in duplicate.
Piglets were sacrificed at day 28, and 10 mg samples of ileal Peyer’s patches were obtained from each animal. Ileal segments containing Peyer’s patches were collected approximately 5 cm before the ileocecal valve. The pieces of tissue were cut longitudinally along the side of the intestine opposite the Peyer’s patches, gently rinsed with saline solution and stripped of the underlying smooth muscle layer. Peyer’s patches were then excised from the tissue samples with a lancet and immediately stored in 1.5 mL cryovials with 0.9 mL RNAlater solution (Invitrogen, Life Technologies Ltd, Paisley, UK), and frozen at −80 °C for further analyses.
Immune-related genes quantification by RT-qPCR
Total RNA was extracted with TRIzol Reagent (Invitrogen, Life Technologies Ltd, Paisley, UK) and purified with a commercial kit (Macherey-Nagel, Oensingen, Switzerland), according to the manufacturer’s recommendations. The RNA concentration was quantified by use of the NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The purity of RNA (A260/A280) was ∼2. Specific mRNAs were amplified and quantified using the iScriptTM One Step RT-PCR for Probes reagent (Bio-Rad, CA, USA), according to the manufacturer’s instructions. RT-q-PCR was performed with CFX384 Real-Time System (Bio-Rad, CA, USA). Thermal protocol was: 50 °C for 10 minutes for reverse transcription and then 95 °C for 10 seconds/60 °C 30 seconds for 40 cycles. For assessment of melting curves, PCR products were incubated at 55 °C for 60 s then the temperature was increased to 95 °C at 0.5 °C increments for 10 s.
Samples from ileal Peyer’s patches were analysed for the expression of interleukin 1α (IL1α), interleukin 1β (IL1β), interleukin 6 (IL6), interleukin 10 (IL10), peroxisome proliferator-activated receptor γ (PPARγ), tumour necrosis factor α (TNFα), toll-like receptor 2 (TLR2), toll-like receptor 4 (TLR4), and β-actin as reference gene.
Primers and probes for real-time qPCR were purchased from Applied Biosystems (Carlsbad, CA, USA) except the set for β -actin quantification (forward primer 5′-ACTCGATCATGAAGTGCGAC-3′, reverse primer 5′-GTGATCTCCTTCTGCATCCTG-3′, Taqman probe 5′-CGTGTTGGCGTAGAGGTCCTTCC-3′), which were designed with IDT software available online, optimised to work in a one-step protocol and were synthesised by Eurofin MWG Operon (Huntsville, AL, USA). The relative expression levels were determined by normalising the Ct of the indicated target with the Ct of Sus scrofa β-actin, as the reference gene for normalisation, using the ΔΔCt method.
Statistical analysis
All the data were analysed as a randomised complete block design and an analysis of variance (ANOVA) was conducted by a GLM procedure (SAS version 9.4, SAS Institute Inc., Cary, NC, USA), including the effect of the treatment in the statistical model. The piglet represented the experimental unit for BW, ADG, immunoglobulins, Hp and gene expression. The pen was considered the replicate for faecal score, FI and gain:feed. Significance level was declared at p ≤ .05, while trends were considered at .05 < p < .10; all values in the text and tables are reported as means ± SEM.
Results and discussion
Growth performance and faecal score
Growth performances are shown in Table . The treatment with nucleotides during post-weaning period increased BW, ADG, and FI (p < .01), but did not affect G:F (p = .29). Specifically, no significant differences were detected on BW at the beginning of the trial (p > .05), while the administration of nucleotides increased BW in the last fourteen days (p < .01), with positive trends in the first two weeks of treatment (p = .06 and p = .07, respectively). Treated piglets showed higher ADG and FI in the first (p <. 01) and third (p = .05; p < .01) week of the experiment and overall the trial period (p <.01). Gain:feed was not improved by the administration of nucleotides (p > .05), with the exception of a positive trend during the first week of the trial (p = .010). Nucleotides supplementation did not influence the consistency of the faeces (3.14 vs. 3.43 ± .168, respectively, for C and T, p > .05) and no incidence of diarrhoea was observed during the experimental period.
Table 2. Effect of nucleotides supplementation on growth and slaughtering performances, and on faecal score.
Over the years, several studies investigated the effect of nucleotides on animals’ performances, but results are still contrasting or, at least, variable (Lee et al. Citation2007; Martinez-Puig et al. Citation2007; de Andrade et al. Citation2016). The results of our trial on growth performance confirm the positive effect of nucleotides administration in the first week post-weaning, as reported by Weaver and Kim (Citation2014), but also a further improvement of growth rates in the subsequent weeks of the experiment was observed. Conversely, the effectiveness of nucleotides administration during the first month post-weaning evidenced in our trial is in contrast with Superchi et al. (Citation2012) who reported improved BW and ADG only starting from day 35 of administration.
The discrepancy in the findings may possibly be related to the source and dosages of nucleotides used, the biological differences between the animals used in the different trials, and the experimental conditions applied.
Inflammatory and immune response
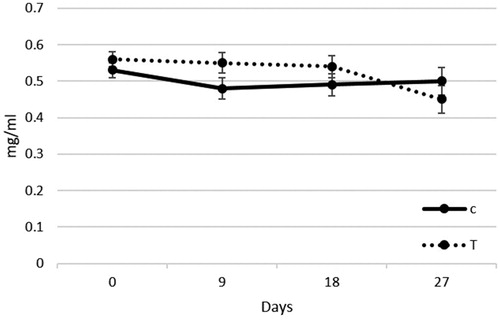
Haptoglobin is positive acute-phase protein, and as such it is a useful marker of inflammation. In the present trial nucleotide administration did not influence Hp concentration in T piglets with respect to C group (.53 vs. .50 ± .060 mg/mL; p = .47), highlighting no differences between the two groups (Figure ).
Figure 1. Haptoglobin plasma content in post-weaning piglets receiving nucleotides supplementation. Values are expressed as mg/mL. C: control; T: treated.
Ileum is highly susceptible to gut pathological events which may concern weaning piglets, and therefore its possible structural and functional changes linked to nutraceutical administration are of a predictive value in the view of judging intestinal defensive responsiveness (Domeneghini et al. Citation2004).
Some published papers targeted the ileum to evaluate the effect of feed additives with presumed beneficial effect on performance and inflammatory- and immune-related parameters (Chen et al. Citation2018; Li et al. Citation2018; Cao et al. Citation2019) In addition, in our study we were expecting the major effects in gene expression occurring in this tract of the intestine according to the results reported by Schokker et al. (Citation2015), who outlined as in 55-day-old piglets only the ileum displayed differences in immune-related processes when compared to the jejunum. The authors attributed this result to the fact that, in differentiated and matured ileum, immunological structures like Peyer’s patches are much more abundant as compared to jejunum.
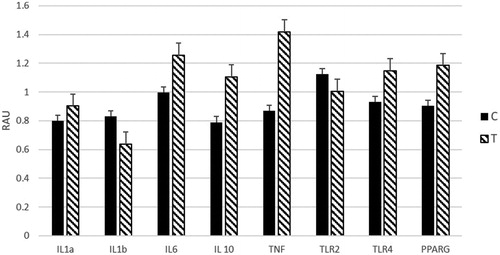
In our trial, we did not observe differences in gene expression levels of inflammatory cytokines at ileal Peyer’s patches level between the two groups (Figure ) in contrast with Waititu et al. (Citation2017). This discrepancy could be attributed to the sanitary challenge performed by these authors, differently from our trial, that could have enhanced the effectiveness of nucleotides administration.
Figure 2. Effect of administration of nucleotides on gene expression at ileal Peyer’s patches level. Values are expressed as relative arbitrary unit (RAU). C: control; T: treated.
However, for a complete comparative study, accounting also for JPP could lead to better elucidate the mechanisms of action of nucleotides.
In the present trial the plasma concentrations of IgA (.36 vs. .38 ± .040 mg/mL, respectively, for C and T) and IgG (4.95 vs. 4.91 ± .180 mg/mL, respectively, for C and T) were not affected by nucleotides supplementation (p = .23 and p = .68, respectively) in accordance with Moore et al. (Citation2011), but in contrast with Lee et al (Citation2007) and Sauer et al (Citation2012) as regard to IgA. The lack of the expected improvement of the immune response could be related to the absence of an experimentally induced impairment of immune response (Mateo Citation2005). This hypothesis is supported by the aforementioned results reported by Waititu et al. (Citation2017), emphasising the requirement of immune stimuli to evaluate the immunomodulatory properties of the product.
Conclusions
In conclusion, our results showed a positive effect of nucleotides supplementation on growth performances, with no significant variations on the immune response. To better evaluate the efficacy of nucleotides supplementation on immune response, an experimentally induced challenge could be required.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Cao G, Tao F, Hu Y, Li Z, Zhang Y, Deng B, Zhan X. 2019. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 10:2926–2934.
- Carver JD, Walker WA. 1995. The role of nucleotides in human nutrition. J Nutr Biochem. 6:58–72.
- Chen L, Li S, Zheng J, Li W, Jiang X, Zhao X, Li J, Che L, Lin Y, Xu S, et al. 2018. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechnol. 9:62.
- Cooke RF, Arthington JD. 2013. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J Anim Physiol Anim Nutr (Berl). 97:531–536.
- Cromwell GL. 2002. Why and how antibiotics are used in swine production. Anim Biotechnol. 13:7–27.
- de Andrade C, de Almeida VV, Sbardella M, do Prado Perina D, de Lima Silva F, Berenchtein B, Costa LB, Miyada VS. 2016. Performance and intestinal health of weanling pigs fed with dietary nucleotides. Semina: Ciências Agrárias. 37:2181–2191.
- Domeneghini C, Di Giancamillo A, Savoini G, Paratte R, Bontempo V, Dell’Orto V. 2004. Structural patterns of swine ileal mucosa following L-glutamine and nucleotide administration during the weaning period. An histochemical and histometrical study. Histol Histopathol. 19:49–58.
- Grimble GK, Westwood OM. 2001. Nucleotides as immunomodulators in clinical nutrition. Curr Opin Clin Nutr Metab Care. 4:57–64.
- Jang KB, Kim SW. 2019. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J Anim Sci. 97:4875–4882.
- Lee DN, Liu SR, Chen YT, Wang RC, Lin SY, Weng CF. 2007. Effects of diets supplemented with organic acids and nucleotides on growth, immune responses and digestive tract development in weaned pigs. J Anim Physiol Anim Nutr (Nutr). 91:508–518.
- Li Q, Gabler NK, Loving CL, Gould SA, Patience JF. 2018. A dietary carbohydrase blend improved intestinal barrier function and growth rate in weaned pigs fed higher fiber diets. J Anim Sci. 96:5233–5243.
- Martinez-Puig D, Manzanilla E, Morales J, Borda E, Pérez J, Piñeiro C, Chetrit C. 2007. Dietary nucleotide supplementation reduces occurrence of diarrhoea in early weaned pigs. Livestock Science. 108:276–279.
- Mateo CD. 2005. Aspects of nucleotide nutrition in pigs [dissertation]. Brookings (SD): South Dakota State University.
- Moore KL, Mullan BP, Pluske JR, Kim JC, D'Souza DN. 2011. The use of nucleotides, vitamins and functional amino acids to enhance the structure of the small intestine and circulating measures of immune function in the post-weaned piglet. Anim Feed Sci Technol. 165:184–190.
- Patterson R, Heo JM, Wickramasuriya SS, Yi YJ, Nyachoti CM. 2019. Dietary nucleotide rich yeast extract mitigated symptoms of colibacillosis in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. Anim Feed Sci Technol. 254:114204.
- Pluske JR. 2013. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J Anim Sci Biotechnol. 4:1.
- Sato N, Nakano T, Kawakami H, Idota T. 1999. In vitro and in vivo effects of exogenous nucleotides on the proliferation and maturation of intestinal epithelial cells. J Nutr Sci Vitaminol. 45:107–118.
- Sauer N, Eklund M, Bauer E, Ganzle MG, Field CJ, Zijlstra RT, Mosenthin R. 2012. The effects of pure nucleotides on performance, humoral immunity, gut structure and numbers of intestinal bacteria of newly weaned pigs. J Anim Sci. 90:3126–3134.
- Sauer N, Mosenthin R, Bauer E. 2011. The role of dietary nucleotides in single-stomached animals. Nutr Res Rev. 24:46–59.
- Schokker D, Zhang J, Vastenhouw SA, Heilig HG, Smidt H, Rebel JM, Smits MA. 2015. Long-lasting effects of early-life antibiotic treatment and routine animal handling on gut microbiota composition and immune system in pigs. PLoS One. 10:e0116523.
- Smith F, Clark JE, Overman BL, Tozel CC, Huang JH, Rivier JEF, Blisklager AT, Moeser AJ. 2010. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am J Physiol Gastrointest Liver Physiol. 298:G352–363.
- Superchi P, Saleri R, Borghetti P, De Angelis E, Ferrari L, Cavalli V, Amicucci P, Ossiprandi MC, Sabbioni A. 2012. Effects of dietary nucleotide supplementation on growth performance and hormonal and immune responses of piglets. Animal. 6:902–908.
- Uauy R. 1989. Dietary nucleotides and requirements in early life. In: Lebenthal E, editor. Textbook of gastroenterology and nutrition in infancy. New York: Raven Press; p. 265–280.
- Waititu SM, Yin F, Patterson R, Yitbarek A, Rodriguez-Lecompte JC, Nyachoti CM. 2017. Dietary supplementation with a nucleotide-rich yeast extract modulates gut immune response and microflora in weaned pigs in response to a sanitary challenge. Animal. 11:2156–2164.
- Weaver AC, Kim SW. 2014. Supplemental nucleotides high in inosine 5’-monophosphate to improve the growth and health of nursery pigs. J Anim Sci. 92:645–651.
- Wellock I, Fortomaris P, Houdijk J, Kyriazakis I. 2007. Effect of weaning age, protein nutrition and enterotoxigenic Escherichia coli challenge on the health of newly weaned piglets. Livestock Sci. 108:102–105.
