Abstract
This experiment was conducted to study the effects of γ-amino butyric acid (GABA) on growth performance, blood biochemical indices and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment. One hundred and forty four, 28-day-old male yellow-feathered broilers were separated into 2 groups with 6 replicates and 12 birds per replicate. The two experimental groups included: control group, which fed the basal diet, and GABA supplemented group, which fed the basal diet supplemented with 100 mg/kg GABA. After 7 days of feeding experimental diet at 26 °C, birds were fed the experimental diet and exposed to a high temperature environment at 35 °C and 60% RH for 14 days. Results revealed that average daily gain (ADG, p = .004) and average daily feed intake (ADFI, p = .003) of the GABA group were higher than control group, feed: gain ratio (F/G; p = .023) and mortality (p = .042) were lower than control group. The serum levels of glucose (GLU; p = .016), total cholesterol (T-CHO; p = .001), and low density lipoprotein (LDL; p = .001) as well as the activities of aspartate-aminotransferase (AST; p = .008), lactate dehydrogenase (LDH; p = .042) and creatine kinase (CK; p = .032) of the GABA group were lower than control group, while villus length (p = .016) of jejunum was higher than control group. In conclusion, adding 100 mg/kg GABA to diet can improve growth performance, reduce serum intracellular enzyme activities, protect the organs and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment during 36-49 days of age.
GABA improves growth performance of yellow-feathered broilers exposed to a high temperature environment.
GABA protects the intestine of yellow-feathered broilers exposed to a high temperature environment.
Highlights
Introductions
High environmental temperature during bird rearing is one of the most important and dangerous factors affecting poultry production, as it negatively affects the growth performance, reproduction, and organ development (St-Pierre et al. Citation2003; Silvestre et al. Citation2009; Rhoads et al. Citation2010; Rispoli et al. Citation2011). Additionally, global warming and intensive production may even aggravate this negative impact on poultry production (Sobhan Citation1994). In the past few years, several strategies have been suggested to reduce the impact of high temperature environment on poultry production, but many of these recommendations are neither practicable nor feasible because of higher cost (retrofitting poultry houses or adding refrigeration equipment) and more complex management (changing breeding density or feeding patterns). Meanwhile, nutritional manipulation is a convenient and feasible approach to alleviate these negative impact caused by high temperature environments (Chand et al. Citation2014; Dhama et al. Citation2015). For example, broilers were fed a high-protein diet and high-metabolisable energy (ME) diet could increase growth performance, immune function and meet quality of Ross-308 and Cobb-500 broilers exposed to heat stress (Attia et al. Citation2018). They also found that increasing protein with energy concentration may be a useful tool in improving the productive performance, meat quality, blood haematological and biochemical traits, antioxidants and immunity of broiler chickens exposed to heat stress (Attia and Hassan Citation2017). However, compared with the method of adjusting nutrient levels by changing diet composition, the method of directly using thermal stress relief additives has the advantages of simple operation, convenience and quickness.
Earlier studies added some additives to diets of poultry to find the way to alleviate the negative effects of thermal environment, and achieved some achievements. For example, Şenay et al. (Citation2019) found that the extracts of pomegranate and apple peel could be used as alternative natural antioxidant sources for vitamin E in the diets to relieve the heat stress of Japanese quail. Liu et al. (Citation2014) reported that resveratrol could relieve heat stress in black-boned chickens. Al-Sagan et al. (Citation2020) found that 3.2% of fennel seed powder could be used as an agent for enhancing the Ross-308 broiler’s tolerance during chronic heat stress condition from 19 to 41 days of age. There had also some additives that are used to alleviate heat stress, such as trace elements, vitamins, probiotics, etc. (Rehman et al. Citation2017; Zhu et al. Citation2017).
In our previous studies, we found that γ-amino butyric acid (GABA) may be one such nutritional element to increase performance of poultry exposed to a high temperature environment. GABA was an important neurotransmitter present in the nervous system and other tissues (Chen et al. Citation2002), 100 mg/kg GABA appeared to play an important regulatory role during heat stress by improving growth rate, feed conversion rate and reducing the level of triglyceride (TG), total cholesterol (T-CHO) as well as the activities of creatine kinase (CK), lactate dehydrogenase (LDH) while increasing some digestive enzymes in Arbour Acres (AA) broilers exposed to a high temperature environment (Hu et al. Citation2016). Study also found that adding 50 mg/kg GABA to diet could promote the development and tissue structure of thymus and bursal density, increased plasma IL-2, IgG, IgM levels and newcastle disease (ND) antibody titres, thereby improving the body’s immune function of Wenchang broilers exposed to a high temperature environment (Tang and Chen Citation2016). It was also reported that 0.5% GABA solution orally improves the growth performance of Ross 308 broilers exposed to a high temperature environment by increasing villus height in the jejunum (Al Wakeel et al. Citation2017). Other studies also found the effect of GABA on heat stress. For example, Dai et al. (Citation2012) adding 100 mg/kg GABA to diet of AA broilers, they found it might offer a potential nutritional strategy to prevent cyclic heat stress-related depression in AA broiler meat chemical composition and quality.
Yellow-feathered broilers are widely distributed in the Southeast Asia, which were reported to have fresher meat and a more delicious taste compared to other strains of birds (Wang et al. Citation2017). However, these broilers are typically free range allowing them to be easily affected by the thermal environment. Besides, with the increasing need for yellow-feathered broilers, the intensive production also has potential heat stress problems. Moreover, unlike heat stress researches in more popular commercial broiler strains such as AA and Ross broilers, there have fewer studies on how to alleviate this negative impact of yellow-feathered broilers exposed to a high temperature environment. Thus, our study selected GABA, which has a stress-relieving effect in white-feather broilers, and verified its heat-alleviation effect on yellow-feathered broilers, and this results could provide some scientific support for resisting heat stress in large-scale production of yellow-feathered broilers.
Materials and methods
Experimental bird, feeding and management
This experiment was approved by the Animal Welfare Committee of the Institute of Animal Science, Chinese Academy of Agricultural Sciences. A total of one hundred and forty four, 28-day-old male yellow-feathered broilers (Xueshan chicken, Yangzhou Lihua Animal and Poultry Co., Ltd., Yangzhou, Jiangsu Province, P. R. China) were separated into 2 groups with 6 replicates and 12 birds per replicate. The two experimental groups included: control group, which fed the basal diet, and GABA supplemented group, which fed the basal diet supplemented with 100 mg/kg GABA (ZAU Feed Science Institute, Hangzhou, Zhejiang Province, P. R. China), which dosage is based on the results of previous researches and the recommendation of the commercial company. On 29 d, chickens were fed with experimental diet and in normal temperature conditions (26 °C, 60% RH). After 7 days of dietary treatment (feeding 7 days was to eliminate the insufficient of GABA intake due to insufficient feed intake of broilers in thermal environment), the birds were weighed (36d) and transferred into an environmental animal chamber (Kooland Technology Co., Ltd., Peking, P. R. China) and exposed to a high temperature environment at 35 °C (24 h/d) and 60% RH for 14 days. Basal diet was formulated and pelleted to meet or exceed NY/T33-2004 nutrient requirements (breeding standards for yellow-feathered broiler chickens in P. R. China) (Table ). Birds had ad libitum access to before diet and drinking water, indoor ventilation and 24 h of light per day as well as regular cleaning and disinfection of their environment. At 28 days of age, birds are vaccinated against infectious bursal vaccine and no further vaccination is required to avoid errors due to vaccination. Other measures were the same as the breeding standards of yellow-feathered broilers. The size of the cage was 1.2 m * 0.9 m, which ensured that feeding density was 0.09 m2/bird.
Table 1. Composition and nutrient levels of the basal diet (air dry basis).
Sample collection
At the end of the experiment (49 d), 2 birds per cage were selected for blood collection from the brachial vein before being slaughtered by severing the jugular vein. To ensure the 2 birds were representative of the entire pen, sample birds were selected based on the average body weight of each replicate. Intestinal samples were collected from the middle of the jejunum and ileum, and then stored in 10% formalin for histopathological examination.
Growth performance
Body weight of yellow-feathered broilers was measured at the beginning of the temperature increasing (36d) and the end of the high temperature environment trial (49 d). Feed intake was recorded. Average daily gain (ADG), average daily feed intake (ADFI) and feed: gain ratio (F/G) were also calculated.
Blood biochemical indices
Blood samples were centrifuged at 4 °C, and 2,800 xg for 10 min (DL-5M type, Xiangyi Power Testing Instrument Co., Ltd., Changsha, Hunan Province, P. R. China). The serum was then transferred into micro tubes and stored at −20 °C. Glucose (GLU), triglyceride (TG), total cholesterol (T-CHO), high density lipoprotein (HDL), and low density lipoprotein (LDL) in serum were determined by an automatic biochemical analyser (UniCel DxC 800 Synchron, Beckman Coulter, USA). The test kits were purchased from BGH-biochemical Co., Ltd., Zhongshan, Guangdong Province, P. R. China.
The activities of alanine-amino transferase (ALT), aspartate-aminotransferase (AST), γ-glutamyl transferase (GGT), lactate dehydrogenase (LDH) and creatine kinase (CK) in serum were measured by test kits, which were purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu Province, P. R. China.
Intestinal morphology
Tissue specimens of jejunum and ileum were rapidly fixed in 10% formalin solution for 24 h, and then serial concentrations of ethyl alcohol were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 °C in a hot air oven for 24 h. Paraffin tissue blocks were prepared for transverse sectioning by a microtome (Leica manual rotary slicer, RM 2235, Leica Inc., Germany). The tissue sections were collected on glass slides, deparaffinized, stained with haematoxylin and eosin (H&E), and then examined for histological features under a light microscope (50× magnification, DM-1000, Leica Microsystems Inc., Germany), respectively. Villus length and crypt depth were measured with Leica Application Suite X software in 3 fields per slide and 3 villi per field, using the methods of Caruso et al. (Citation2012). Villus length was measured from the crypt-villus junction to the villus top, whereas crypt depth was measured from the muscular mucosa to the crypt-villus junction, so that villus length: crypt depth ratio (V/C) could be calculated.
Statistical analysis
All parametric data were firstly processed to 6 data per group in Excel 2013, and then analysed using independent-sample T test in SPSS 17.0 (SPSS Inc., Chicago, IL) according to the method of Argyrous (Citation2000). Data was expressed as Mean and SEM. Probability values less than 0.05 were considered statistically significant differences (p < .05).
Results
Growth performance
Growth performance of yellow-feathered broilers from 36-49 d was presented in Table . BW (p = .015), ADG (p = .004) and ADFI (p = .003) of the GABA group were much higher than control group, F/G (p = .023) and mortality (p = .042) were less than control group.
Table 2. Effects of dietary supplemented of GABA on the growth performance of yellow-feathered broilers exposed to a high temperature environment from 36–49 d of age.
Blood biochemical indices
Blood biochemical indices of yellow-feathered broilers at 49 d of age were presented in Table . GLU (p = .016), LDL (p = .001) and T-CHO (p = .001) in serum of the GABA group were lower than control group.
Table 3. Effects of dietary supplemented of GABA on blood biochemical indices of yellow-feathered broilers exposed to a high temperature environment from 36–49 d of age.
The activities of some enzymes in serum of yellow-feathered broilers at 49 days of age were presented in Table . AST (p = .008), LDH (p = .042) and CK (p = .032) of the GABA group were lower than control group.
Table 4. Effects of dietary supplemented of GABA on the activities of serum enzymes of yellow-feathered broilers exposed to a high temperature environment from 36–49 d of age.
Intestinal morphology
Intestinal morphology of yellow-feathered broilers at 49 d of age was presented in Table and Figures and . Villus height in the jejunum of the GABA group was longer than control group (p = .016). Trends towards longer villus height (p = .057) and a greater V/C ratio (p = .073) in the ileum were also detected in the GABA group. The longer villi in the jejunum (Figure ) and ileum (Figure ) of GABA group as compared to the control group. .
Figure 1. Jejunum mucosal morphology of yellow-feathered broilers exposed to a high temperature environment. C: control group; GABA: GABA group.
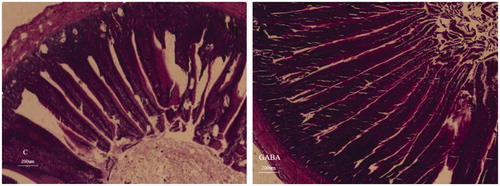
Figure 2. Ileum mucosal morphology of yellow-feathered broilers exposed to a high temperature environment. C: control group; GABA: GABA group. The longer villi in the jejunum (Figure ) and ileum (Figure ) of GABA group as compared to the control group. Besides, it was obvious that the villi in the GABA group was arranged more tightly than that in the control group, which might increase the amount of villi or the area of absorption.
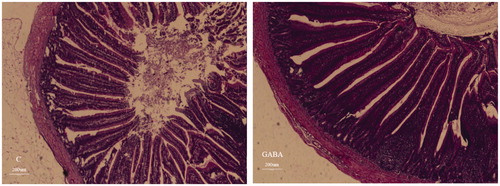
Table 5. Effects of dietary supplemented of GABA on the intestinal morphology of yellow-feathered broilers exposed to a high temperature environment from 36–49 d of age.
Discussion
High temperature is a major problem in tropical and subtropical regions, often resulting in heavy economic losses in poultry production (Nardone et al. Citation2010; Attia and Hassan Citation2017; Attia et al. Citation2018). Perhaps because it is widely distributed in the central nervous system, GABA appears to be a new treatment for improving performance during heat stress. Studies reported that when birds were in a high temperature environment, GABA could inhibit the sympathetic nervous system and increase feed intake (Jonaidi and Noori, Citation2012) and weight gain (Chand et al. Citation2014). It was also confirmed that GABA played an effective role in growth performance through increasing body weight and feed utilisation of broilers exposed to a high temperature environment whether added 100 mg/kg to the diet or 0.5% solution orally (Hu et al. Citation2016; Al Wakeel et al. Citation2017). Scholars gave Roman hens experimental diet supplemented with 25, 50, 75, 100 mg/kg GABA, it suggested that 50 mg/kg GABA might improve laying performance (Zhang et al. Citation2012), which might through inhibiting the secretion of cholecystokinin and suppressing the stomach satiety centre (Kato et al. Citation2001). However, Zhu et al. (Citation2015) found that laying performance increased linearly with the addition 100 mg/kg of GABA in HY-line variety brown chickens exposed to heat stress environment.
Similar to these aforementioned studies with other poultry breeds, our results were the first to demonstrate that 100 mg/kg GABA improves growth performance of yellow-feathered broilers exposed to a high temperature environment, and we found that GABA could increase ADG and ADFI while decrease F/G of yellow-feathered broilers, and at the same time, it decreased the mortality, which further improved production efficiency. Our results suggested that GABA could alleviate the negative effects of a high temperature environment on yellow-feathered broilers, and compared with previous study, we also found that the optimal dose of GABA varies between broilers and laying hens, and even in the research of different breeds of laying hens, different results were obtained.
Serum concentration of GLU and TG might be an indicator of the negative impact caused by a high temperature (Attia and Hassan Citation2017; Attia et al. Citation2018), which indicated changes in carbohydrate and fat metabolism during heat stress (Luo et al. Citation2018). Attia et al. (Citation2016) found that heat stress significantly decreased the level of GLU in serum of laying hens. However, Xie et al. (Citation2015) reported that plasma TC and GLU content were significantly elevated during acute thermal heat stress, while plasma TG, GLU were not significantly different from control birds during prolonged thermal heat stress. These differences may be due to the breed and age of experimental animals, and the condition of heat stress. Study also found that levels of GLU and TG in serum were decreased when Roman laying hens exposed to a high temperature environment (Zhang et al. Citation2012), and adding 50 mg/kg GABA to diet significantly increased GLU levels. Our experiment also showed that the levels of LDL and T-CHO were significantly decreased in 100 mg/kg GABA supplemented of yellow-feathered broilers. This research clearly confirms that GABA can reduce the negative impact on blood biochemical indices caused by a high temperature. The possible mechanism might be that additive suppressed the expression of HSPs, which improves insulin sensitivity, leading to glycolysis and fat synthesis, and regulation of serum GLU, T-CHO, and TG contents under heat stress (He et al. Citation2019). However, no significant differences appeared in the levels of TG, which was not the same as previous research (Zhang et al. Citation2012). One possible reason might be that yellow-feathered broilers are a local chicken breed with slow growing rate in P. R. China, which are less absolute surface area and thus less exposure to heat stress (Attia et al. Citation2011).
High environmental temperature alters activities of some enzymes in the serum of chickens (Melesse et al. Citation2011), as ALT, GGT, LDH and CK, intracellular enzymes that were released into the blood only when tissue and organs are damaged (Liu Citation2008). So their level in serum is important indices to evaluation the health of tissue and organs (Hu et al. Citation2016). Studies reported that serum ALT and AST activities were increased under heat stress (Hosseini-Vashan et al. Citation2016; Wan et al. Citation2017), while adding100mg/kg GABA to diet decreased the activities of LDH and CK in Arbour Acres broilers exposed to a hot environment (Hu et al. Citation2016), which might be related to its antioxidant function (Dai et al. Citation2011; Martínez-Tomé et al. Citation2001). The same trend was obtained in our study as the activities of AST, LDH and CK in serum were significantly decreased when 100 mg/kg GABA was supplemented in the diet. Therefore, it seemed to reflect that GABA might protect the organ from heat stress in yellow-feathered broilers exposed to a high temperature environment.
Small intestine in poultry is composed of duodenum, jejunum, and ileum, and each component contributes to various aspects of nutrient absorption and digestion. Generally speaking, duodenum is the principal place of food breakdown (Klasing, Citation1999), jejunum primarily absorbs and assimilates nutrients, while the ileum plays an essential role in fermentation (Ewing and Cole, Citation1994). Mucosal morphology is the key to digestion and absorption of nutrients, which reflects the gut health overall (Ducatelle et al. Citation2018). However, the structure of the small intestine was easy to break, including villi fracture, shortening of the villus height, deeper crypts, and mucosal epithelial cell exfoliation when heat stress occurred (Yu et al. Citation2010), and the reason might that chickens would produce excess oxygen free radicals under high environmental temperature, which induced severe damage to intestinal mucosa (Tan et al. Citation2010). It had been confirmed that 0.5% GABA solution orally played a protective role in improving absorption of nutrients by relieving this negative impact of heat stress on intestinal mucosa of Wenchang broilers (Mujahid et al. Citation2005; Chen et al. Citation2015). GABA might alleviate the damage to intestine of broilers exposed to a high temperature environment through protecting villus height, crypt depth and mucous membrane thickness of Ross 308 broilers (Al Wakeel et al. Citation2017). Another view was that GABA was translated into glutamine, which stimulates protein synthesis and improves the intestinal barrier after severe trauma, such as high temperature (Li et al. Citation2002). Besides, potential protective mechanism of GABA during heat stress is that it decreases intestinal permeability and promotes the proliferation of crypt cells and intraluminal secretions to protect the intestinal barrier (Sun et al. Citation2004). Moreover, these effects of GABA might be related to the increased activity of GSH-Px, which ultimately decreases the formation of oxygen free radicals in chickens exposed to a high temperature (Chen et al. Citation2013).
Our study found that villus height of jejunum in GABA group was higher than the control group. Results also showed that trends towards longer villi and a greater V/C ratio of ileum, and the villi was arranged more tightly in GABA group, which might increase the amount of villi or the area of absorption. This indicated that GABA could promote the development of intestinal villi in both the length and the number, by protecting the intestine of yellow-feathered broilers exposed to a high temperature environment.
Conclusions
In conclusion, adding 100 mg/kg GABA to diet can improve growth performance, reduce serum intracellular enzyme activities, protect the organs and intestinal morphology of yellow-feathered broilers exposed to a high temperature environment during 36–49 days of age.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Al-Sagan AA, Khalil S, Hussein EO, Attia YA. 2020. Effects of fennel seed powder supplementation on growth performance, carcass characteristics, meat quality, and economic efficiency of broilers under thermoneutral and chronic heat stress conditions. Animals. 10(2):206.
- Al Wakeel RA, Shukry M, Abdel Azeez A, Mahmoud S, Saad MF. 2017. Alleviation by gamma amino butyric acid supplementation of chronic heat stress-induced degenerative changes in jejunum in commercial broiler chickens. Stress. 20(6):562–572.
- Argyrous G. 2000. Statistics for social and health research: with a guide to SPSS. Sage.
- Attia YA, El AEHEA, Abedalla AA, Berika MA, Al-Harthi MA, Kucuk O, Sahin K, Abou-Shehema BM. 2016. Laying performance, digestibility and plasma hormones in laying hens exposed to chronic heat stress as affected by betaine, vitamin C, and/or vitamin E supplementation. SpringerPlus. 5(1):1619.
- Attia YA, Hassan RA, Tag El-Din AE, Abou- Shehema BM. 2011. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Animal Nutr. 95(6):744–755.
- Attia YA, Hassan SS. 2017. Broiler tolerance to heat stress at various dietary protein/energy levels. European Poultry Sci. 81:1612–9199.
- Attia YA, Al-Harthi MA, Sh. Elnaggar A. 2018. Productive, physiological and immunological responses of two broiler strains fed different dietary regimens and exposed to heat stress. Ital J Anim Sci. 17(3):686–697.
- Caruso M, Demonte A, Neves VA. 2012. Histomorphometric study of role of lactoferrin in atrophy of the intestinal mucosa of rats. Health. 04(12):1362–1370.
- Chand N, Naz S, Khan A, Khan S, Khan RU. 2014. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int J Biometeorol. 58(10):2153–2157.
- Chen Z, Wang T, Huang L, Fang D. 2002. Effects of GABA on the heat stress broilers. Zool Res. 23(4):341–344.
- Chen Z, Tang J, Sun YQ, Xie J. 2013. Protective effect of γ-amino butyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J Anim Plant Sci. 23(6):1634–1641.
- Chen Z, Xie J, Hu MY, Shao Z. F, Li MH. 2015. Protective effects of γ-aminobutyric acid (GABA) on the small intestinal mucosa in heat-stressed Wenchang chicken. J Anim Plant Sci. 25(1):78–87.
- Dai SF, Gao F, Zhang WH, Song SX, Xu XL, Zhou GH. 2011. Effects of dietary glutamine and gamma-amino butyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Anim Feed Sci Tech. 168(1-2):51–60.
- Dai SF, Gao F, Xu XL, Zhang WH, Song SX, Zhou GH. 2012. Effects of dietary glutamine and gamma-aminobutyric acid on meat colour, pH, composition, and water-holding characteristic in broilers under cyclic heat stress. Br Poultry Sci. 53(4):471–481.
- Dhama K, Latheef SK, Mani S, Samad HA, Karthik K, Tiwari R, Khan RU, Alagawany M, Farag MR, Alam GM, et al. 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production-A review. International J of Pharmacology. 11(3):152–176.
- Ducatelle R, Goossens E, De Meyer F, Eeckhaut V, Antonissen G, Haesebrouck F, Van Immerseel F. 2018. Biomarkers for monitoring intestinal health in poultry: present status and future perspectives. Vet Res. 49(1):43.
- Ewing W, Cole DJA. 1994. The living gut: introduction to micro-organisms in nutrition. Co Tyrone N, Ireland: Context Publications Dungannon.
- He S, Li S, Arowolo MA, Yu Q, Chen F, Hu R, He J. 2019. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow‐feather broilers under heat stress. Anim Sci J. 90(3):401–411.
- Hosseini-Vashan S. J, Golian A, Yaghobfar A. 2016. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets with tomato pomace. Int J Biometeorol. 60(8):1183–1192.
- Hu H, Bai X, Shah AA, Wen AY, Hua JL, Che CY, He SJ, Jiang JP, Cai ZH, Dai SF. 2016. Dietary supplementation with glutamine and gamma-amino butyric acid improves growth performance and serum parameters in 22- to 35-day-old broilers exposed to hot environment. J Anim Physiol Anim Nutr. 100(2):361–370.
- Jonaidi H, Noori Z. 2012. Neuropeptide Y-induced feeding is dependent on GABAA receptors in neonatal chicks. J Comp Physiol A. 198(11):827–832.
- Kato S, Araki H, Kawauchi S, Takeuchi K. 2001. Body temperature dependency in baclofen-induced gastric acid secretion in rats relation to capsaicin-sensitive afferent neurons. Life Sci. 68(17):1951–1963.
- Klasing K. C. 1999. A vian gastrointestinal anatomy and physiology. Semin A Vian Exotic Pet Med. 8(2):42–50.
- Li JY, Lu Y, Hu S, Sun D, Yao YM. 2002. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. WJG. 8(1):168–171.
- Liu LL, He JH, Xie HB, Yang YS, Li J. C, Zou Y. 2014. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poultr Sci. 93(1):54–62.
- Liu XX. 2008. Identification of serum m-AST in alcoholic liver disease and non-alcoholic liver disease. Chin J Lab Diagn. 12(2):254–254.
- Luo J, Song J, Liu L, Xue B, Tian G, Yang Y. 2018. Effect of epigallocatechin gallate on growth performance and serum biochemical metabolites in heat-stressed broilers. Poultr Sci. 97(2):599–606.
- Martínez-Tomé M, García-Carmona F, Murcia MA. 2001. Comparison of the antioxidant and pro-oxidant activities of broccoli amino acid with those of common food additives. J Sci Food Agric. 81(10):1019–1026.
- Melesse A, Maak S, Schmidt R, Von Lengerken G. 2011. Effect of long-term heat stress on some performance traits and plasma enzyme activities in Naked-neck chickens and their F1 crosses with commercial layer breeds. Livest Sci. 141(2-3):227–231.
- Mujahid A, Yoshiki Y, Akiba Y, Toyomizu M. 2005. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult Sci. 84(2):307–314.
- Nardone A, Ronchi B, Lacetera N, Ranieri MS, Bernabucci U. 2010. Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci. 130(1-3):57–69.
- Rehman Z, Chand N, Khan R. U. 2017. The effect of vitamin E, L-carnitine, and ginger on production traits, immune response, and antioxidant status in two broiler strains exposed to chronic heat stress. Environ Sci Pollut Res. 24(34):26851–26857.
- Rhoads ML, Kim J.W, Collier RJ, Crooker BA, Boisclair YR, Baumgard LH, Rhoads RP. 2010. Effects of heat stress and nutrition on lactating Holstein cows: II. Aspects of hepatic growth hormone responsiveness. J. Dairy. Sci. 93(1):170–179.
- Rispoli LA, Lawrence JL, Payton RR, Saxton AM, Schrock GE, Schrick FN, Middlebrooks BW, Dunlap JR, Parrish JJ, Edwards JL. 2011. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction. 142(6):831–843.
- Şenay S, Islim P, Tugay A. 2019. Supplementation of natural antioxidants to reduced crude protein diets for Japanese quails exposed to heat stress. Revista Brasileira de Ciência Avícola. Braz J Poult Sci. 21(1):1–14.
- Silvestre FT, Kamimura S, Arteche AC, Bartolome J, Pancarci SM, Thatcher WW. 2009. Reproductive responses following postpartum suppression of ovarian follicular development with a deslorelin implant during summer heat stress in lactating dairy cows. Anim Reprod Sci. 111(2-4):320–337.
- Sobhan MA. 1994. Climate change–its impacts on Bangladesh (No. CONF-940426-). Pittsburgh, PA: Air and Waste Management Association.
- St-Pierre NR, Cobanov B, Schnitkey G. 2003. Economic losses from heat stress by US livestock industries1. J Dairy Sci. 86:E52–E77.
- Sun DW, Zhan Y, Xu ZR. 2004. Study on dietary nutrients regulation animal intestinal mucosal immune. CHN J Anim Sci. 40(5):36–38.
- Tan GY, Yang L, Fu YQ, Feng JH, Zhang MH. 2010. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult Sci. 89(1):115–122.
- Tang J, Chen Z. 2016. The protective effect of γ‐aminobutyric acid on the development of immune function in chickens under heat stress. J Anim Physiol Anim Nutr. 100(4):768–777.
- Wan X, Jiang L, Zhong H, Lu Y, Zhang L, Wang T. 2017. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim Sci J. 88(8):1239–1246.
- Wang H, Zhang X, Wang G, Jia K, Xu X, Zhou G. 2017. Bacterial community and spoilage profiles shift in response to packaging in yellow-feather broiler, a highly popular meat in Asia. Front Microbiol. 8:2588.
- Xie J, Tang L, Lu L, Zhang L, Lin X, Liu H. C, Odle J, Luo X. 2015. Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poultr Sci. 94(7):1635–1644.
- Yu J, Yin P, Liu F, Cheng G, Guo K, Lu A, Zhu X, Luan W, Xu J. 2010. Effect of heat stress on the porcine small intestine: a morphological and gene expression study. Comp Biochem Physiol A: Mol Integr Physiol. 156(1):119–128.
- Zhang M, Zou X. T, Li H, Dong XY, Zhao W. 2012. Effect of dietary gamma-aminobutyric acid on laying performance, egg quality, immune activity and endocrine hormone in heat-stressed Roman hens. J Anim Sci. 83(2):141–147.
- Zhu YW, Li WX, Lu L, Zhang L. Y, Ji C, Lin X, Liu HC, Odle J, Luo XG. 2017. Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers. Poultr Sci. 96(7):2351–2359.
- Zhu YZ, Cheng JL, Ren M, Yin L, Piao XS. 2015. Effect of γ-aminobutyric acid-producing Lactobacillus strain on laying performance, egg quality and serum enzyme activity in Hy-Line brown hens under heat stress. Asian Australas J Anim Sci. 28(7):1006–1013.
