Abstract
The objective was to study the effect of the partial substitution of soybean meal and oil with full-fat silkworm (Bombyx mori L.) meal (SWM) in the diet of growing chickens, on their growth and meat quality traits. A total of 195 1-day-old chicks of both sexes were allocated to 15 littered floor pens and assigned to three dietary treatments (5 replicates/treatments) until 8 weeks of age: a commercial diet (Control) and other two diets with an inclusion of either 25% (SWM25) or 50% (SWM50) SWM. At 8 weeks of age, two males/replicates were slaughtered and carcases dissected to compute yields. Pectoralis major muscle was subjected to pH and L*a*b* colour values, proximate composition and fatty acid profile analysis. All chickens showed satisfactory growth performance throughout the trial, with the best growth being observed in the SWM25 group. Carcase traits remained unaffected by the dietary treatment, but SWM25 chickens had a higher breast yield (p < .05) than the Control group. The pH of SWM50 breasts was higher than Control (p < .01). Dietary treatments affected meat protein content, differing between SWM25 and SWM50 (22.2 versus 23.3%, respectively; p < .05). SWM dietary inclusion increased n-3 PUFA and lowered the n-6 PUFA proportions in a level-dependent manner. Consequently, n-6/n-3 ratio diminished, thus improved, with the dietary SWM inclusion. Results showed that it is possible to partly substitute soybean meal/oil with SWM in the diet of chickens, ensuring satisfactory performance and carcase traits, and providing meat with a healthier n-6/n-3 ratio.
Silkworm (Bombyx mori) chrysalis is a possible sustainable feed ingredient for growing chickens, alternative to conventional soybean.
The dietary inclusion of silkworm full-fat meal in chicken diets provided satisfactory growth performance, carcase and meat quality traits.
The dietary inclusion of full-fat silkworm meal in chicken diets enriched meat lipids with omega-3 fatty acids.
Highlights
Introduction
The supply of an adequate protein amount together with a balanced amino acid profile is essential for poultry species to provide satisfactory growth performance: to this purpose, soybean and fish meal are currently the main feed ingredients (Agazzi et al. Citation2016). In recent years, however, the continuously increasing price of these feedstuffs is making the economic sustainability of the poultry meat industry problematic, particularly in some developing countries (Gale and Arnade Citation2015). Together with the economic issue, soybean and fish meal are also not seen as sustainable, as they have a substantial environmental impact: soy needs a relevantly high amounts of water and land to grow, whereas fishmeal is mainly composed of blue fish, whose natural global stocks are rapidly dwindling due to overexploitation (van Huis and Oonincx Citation2017). Furthermore, they are nutrient sources which can also be consumed by humans, thus threatening food security, a concept which will be increasingly stressed in the near future due to the increasing World population (Horlings and Marsden Citation2011). In this scenario, the search for new and sustainable feed ingredients for farmed animals, has identified in insects one of the promising alternatives to replace the conventional feedstuffs (Veldkamp and Bosch Citation2015). Insects are potentially highly sustainable as they are poikilothermic and characterised by a high feed conversion efficiency, they require low amounts of water and land to be farmed, and many of them can be successfully grown on organic side streams, converting low-value organic by-products into high-value protein (van Zanten et al. Citation2014) In parallel, insects, which can either be used as a whole or being separated into protein meal and fat, are excellent sources of nutrients: in general, they have a high protein content with a nutritive value similar to soybean meal, they are good sources of fatty acids, vitamins and microminerals (Rumpold and Schlüter Citation2013). In recent years, most research efforts have been directed towards insect species such as the black soldier fly (Hermetia illucens), the housefly (Musca domestica) and the yellow mealworm (Tenebrio molitor): they generally provided positive outcomes when tested as part of diets intended for different poultry species (Pieterse et al. Citation2014; Biasato et al. Citation2016; Cullere et al. Citation2016, Citation2018; Dalle Zotte et al. Citation2019).
Another insect species of potential interest for poultry diets is the silkworm (Bombyx mori), more precisely the chrysalis (or spent silkworm pupae), which is a by-product derived from the silk production. They are obtained in such large quantities (8 kg of wet pupae for 1 kg of raw silk) that despite being consumed in South East Asia as traditional food and as agricultural fertiliser, most of them are discarded as waste (Yu et al. Citation2018), thus representing a nutrient loss and also an environmental treat.
The spent silkworm pupae are rich in nutrients, so as to be often categorised as functional food (Kwon et al. Citation2012). They have a high protein content (roughly higher than 50%) with an amino acid profile which was reported to be similar to that of fish meal (Ullah et al. Citation2017a). They are also rich in lipids (about 30%) whose 30% is represented by the healthy polyunsaturated fatty acids (PUFA). Among them, nearly the 40% is represented by the α-linolenic acid which is an essential fatty acid (FA), precursor of n-3 FA (Guil-Guerrero et al. Citation2018). Furthermore, spent silkworm pupae contain vitamins (mainly B group and vitamins A and E) and minerals (mainly potassium, phosphorus, zinc). For these reasons, the spent silkworm pupae could potentially be used as a top-class unconventional feed source for poultry diets. Existing research on this topic has been exclusively conducted in developing countries and mainly focussed on performance traits, while aspects related to the nutritional quality of the meat have not been investigated yet. As it was recently reviewed by Sheikh et al. (Citation2018), available scientific data are extremely variable as different studies testing the same soybean/fishmeal substitution levels on chickens provided controversial results. Therefore, the new scientific insights that the present research study wanted to explore are: (1) try to contribute to establish a sort of reference threshold for the dietary incorporation of the spent silkworm pupae into chicken diets, and (2) to provide knowledge about its effect on the nutritional quality of the animal product which is consumed. Therefore, the present research studied the effect of a partial substitution of soybean meal and a total substitution of soybean oil with full-fat silkworm meal (SWM) in the diet for growing chickens on their growth performance, carcase traits and meat quality.
Material and methods
Experimental design and bird management
The study was carried out in the experimental poultry farm of the Sylhet government (Bangladesh) and it was approved by the Animal Experimentation Ethics Committee, Sylhet Agricultural University, Sylhet (approval number AUP2018001).
One hundred ninety-five 1-day-old Rhode Island Red × Fayoumi crossbred (Sonali) chicks of both sexes (n = 99 males and n = 96 females) were used for the experiment. Chicks were obtained from a local hatchery: they were vaccinated against Newcastle disease, infectious bronchitis, infectious bursal disease and fowl poxvirus. The experimental design consisted of three different dietary treatments, having 5 replicates each (13 chicks/replicate). Chicks were randomly assigned to 15 floor pens (275 × 91 cm) located in an open-sided shed. Pen bedding consisted of 5 cm-depth dried rice husk. From the first day until the end of the experiment (8 weeks of age), chicks received the following experimental corn–soybean meal-based mashed diets: a commercial diet (Control) which was formulated according to the nutritional requirements of the Sonali crossbred, a diet in which the 25% of soybean meal was replaced by silkworm (B. mori) chrysalis meal (SWM25), and a diet in which the 50% of soybean meal was replaced by SWM meal (SWM50): in both SWM25 and SWM50 diets, the inclusion replaced also the 100% soybean oil.
Ingredients of the experimental diets are listed in Table . Diets and water were offered ad libitum throughout the experiment and birds were subjected to 18 L:6D photoperiod (30 lux). Temperature and relative humidity were monitored but not controlled. Individual body weight (BW) and pen feed intake (FI) were recorded weekly to calculate individual daily weight gain (DWG) and pen feed conversion ratio (FCR). Chicken health status and mortality were monitored daily.
Table 1. Ingredients of the experimental diets (g/kg as fed).
Slaughter, carcase dissection, sampling procedure and breast muscle physical analysis
At 8 weeks of age, two male chickens/replicate/treatment were randomly selected, weighed and slaughtered at a commercial slaughterhouse located near the poultry farm: chickens were electrically stunned (120 V, 200 Hz), bled, soft-scalded (53 °C for 2 min), plucked and eviscerated to obtain commercial carcases, which included head, neck, wings, shanks and giblets (heart, liver and gizzard). Carcases were subsequently air-chilled (precooling at 5 °C for 60 min, followed by chilling at 0 °C for 90 min), stored at +4 °C and transported to the Department of Poultry Science, Sylhet Agricultural University (Bangladesh). For each treatment, carcases were weighed; yields on the slaughter weight were subsequently calculated using the carcase, gizzard, liver, heart, leg, thigh, drumstick and breast weights. Twenty-four hours post mortem the Pectoralis major muscle was subjected to pH (pHu; portable pH metre FG2-Five GoTM; Mettler Toledo, Greifensee, Switzerland) and instrumental colour (RM200QC colorimeter; X-Rite Co., Neu-Isenburg, Germany) measurements (L*a*b*; CIE Citation1976). All pHu and colour readings were taken in duplicate. Five breast muscles were randomly selected (one per replicate)/treatment and individually ground with a Retsch Grindomix GM 200 (7000 g for 10 s), frozen at −40 °C, freeze-dried and ground again (7000 g for 5 s) to obtain a fine powder. Samples were then shipped to the Department of Animal Medicine, Production and Health of the Padova University (Italy) for proximate composition and fatty acid (FA) profile determinations.
Chemical analysis of the silkworm meal and the experimental diets
Dried silkworms and experimental diets were sampled and finely ground; their chemical composition was analysed in duplicate following the Association of Official Analytical Chemists (Association of Official Analytical Chemists Citation2000) methods to determine dry matter (method no. 934.01), crude protein (method no. 2001.11), crude fibre (method no. 978.10) and ash (method no. 967.05) content. Ether extract (EE) was determined after acid hydrolysis (EC 1998). Calcium and P analyses were performed by ICP-OES (Spectro Ciros Vision EOP) after microwave digestion (AOAC Citation2000, method no. 999.10). For the SWM, only dry matter, crude protein, ether extract, and ash content were analysed. The analysed chemical composition of the SWM and of the experimental diets is depicted in Table . The FA profile of the SWM and of the experimental diets is listed in Table .
Table 2. Chemical composition (g/kg, as is basis) of the silkworm chrysalis meal (SWM) and of the experimental diets.
Table 3. Fatty acid profile (% of total FAME) of the of the Bombyx mori pupae meal (SWM) and of the experimental diets.
Meat proximate composition and FA profile determination
The proximate composition of the freeze-dried breast meat samples were analysed in accordance with the AOAC (Citation2000) methods. The lipid extraction was performed by Accelerated Solvent Extraction (M-ASE) using the binary mixture of solvents chloroform: methanol 1:2, combining the traditional Folch method (Folch et al. Citation1957) with that provided by Lee et al. (Citation1996). The total lipid content was determined gravimetrically after the removal of the solvent by evaporation under nitrogen stream at 50 °C. The FA profile was determined as follows: samples were trans methylated using a methanolic solution of H2SO4 (4%) in order to determine fatty acid methyl esters (FAME). A biphasic separation was obtained by adding 0.5 ml of distilled water and 1.5 ml of N-heptane to each sample. FAME were quantified by gas chromatography (Shimadzu GC17A), equipped with an Omegawax 250 column (30 m × 0.25 µm × 0.25 µm) and FID detector. Helium was used as the carrier gas at a constant flow of 0.8 mL/min. The injector and detector temperature was 260 °C. Peaks were identified based on commercially available FAME mixtures (37-Component FAME Mix, Supelco Inc., Bellefonte, PA). The results are expressed as % of total detected FAME.
Statistical analysis
Live weight and DWG data were subjected to a two-way ANOVA with experimental diet (Control, SWM25 and SWM50) and sex as fixed effects, and their interaction, following the GLM procedure of the SAS 9.1.3 statistical analysis software for Windows (SAS Institute Citation2008). Repeated data (growth traits) were analysed by a PROC MIXED and pen was considered as random effect (experimental unit: the single chicken). For growth traits, the initial weight was used as covariate. For FI, FCR (experimental unit: the pen), carcase and meat physicochemical traits (experimental unit: the single carcase) a one-way ANOVA tested the effect of the experimental diets. Post-hoc pairwise contrasts were evaluated by Bonferroni adjustments: p < .05, p < .01 and p < .001 were assigned as significance levels.
Results
Table shows the effect of dietary inclusion of 25 and 50% SWM to chicken diets on daily weight gain (DWG), which was measured at weekly intervals. The diets significantly affected (p < .0001) DWG at weeks 2, 7 and 8 and, more importantly, the overall DWG: SWM25 provided the best results both for males (13.5 versus 13.0 versus 12.5 g for SWM25, Control and SWM50 chickens, respectively) and females (12.7 versus 12.1 versus 11.7 g for SWM25, Control and SWM50 chickens, respectively). As for sex, it significantly affected the DWG throughout the experiment (1–8 weeks), with males showing always higher (p < .0001) DWG than females. A significant (p < .05) interaction was also observed between dietary level of SWM and bird sex for DWG at weeks 4, 5 and 7, showing a reduced sex effect in SWM-fed chickens. The effect of the dietary inclusion of SWM on body weight of birds separated by sex is depicted in Figure ; in both sexes the diet effect started after 4 weeks of experimental diet feeding, with the highest body weight for SWM25 birds at week 7, but lower than SWM50 at week 8. The feed intake (FI) differed (p < .05) between SWM25 and SWM50 groups (40.8 versus 39.2 g/day/bird, respectively), with the Control group being intermediate (39.7 g/day/bird; Table ). Despite this, the FCR did not differ significantly among the experimental groups. All birds were in good health throughout the study and no mortalities occurred. The dietary inclusion of SWM did not overall affect the chicken carcase traits (Table ), with the exception of the heart percentage and breast yield: the heart percentage was higher in the SWM25 group compared to the Control, with SWM50 being intermediate (p < .05). The same pattern was also observed for the breast yield, found higher in the SWM25 group compared to the Control (p < .05). The pHu of the Pectoralis major muscle was significantly higher in SWM50 than in Control birds (6.13 versus 5.93, p < .01), however, the L*a*b* colour values did not show any statistically significant change.
Figure 1. (a) Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on the weekly body weight (g) of male chickens. a–cDifferent superscript letters differ for p < .05. A,BDifferent superscript letters differ for p < .01. (b) Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on the weekly body weight (g) of female chickens. a,bDifferent superscript letters differ for p < .05. A,BDifferent superscript letters differ for p < .01.
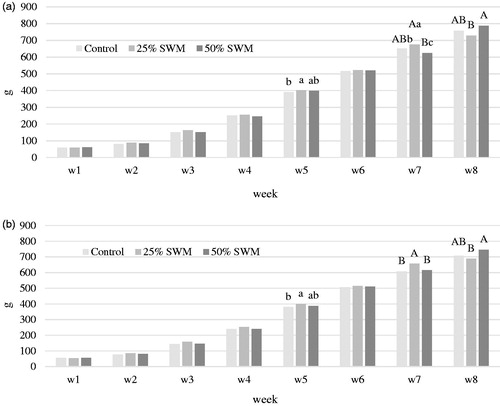
Table 4. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on chickens’ daily weight gain (g) for every week of the experiment.
Table 5. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on feed intake (FI) and feed convertion rate (FCR) of chickens.
Table 6. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on carcase traits, internal organs and breast meat traits of male chickens.
The proximate composition of the chicken breast was modified by the dietary treatment (Table ). Specifically, the protein content was lower in SWM25 than in SWM50 and Control breasts (22.2 versus 23.3 g/100 g meat; p < .05). Instead, SWM25 breasts showed lower ash content than Control breasts (1.05 versus 1.11 g/100 g meat; p < .05) and SWM50 breasts presented intermediate mean value 1.09 g/100 g meat.
Table 7. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on the breast meat proximate composition (g/100 g meat) of male chickens.
The inclusion of the SWM in the diets for growing Sonali chickens had a moderate effect on the meat FA profile (Table ). Overall, saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) FA proportions remained similar in the three dietary groups. Despite this, some significant effects on single FAs were observed: the C18:1 n-11 decreased with increased dietary SWM inclusion level (2.05 versus 1.81 versus 1.65% total FAME, for Control, SWM25 and SWM50 breasts, respectively; p < .05). The silkworm meal increased the proportion of linolenic acid (C18:3 n-3) in the meat in an inclusion level-dependent manner: 0.88 versus 1.88 versus 3.20% for Control, SWM25 and SWM50 breasts, respectively (p < .001). This effect was also quantitatively (mg/100 g meat) appreciable, as shown in Figure . This resulted in a higher n-3 PUFA proportion for the highest SWM inclusion level (p < .01) and had a lowering effect on the n-6/n-3 ratio on both SWM groups (7.5 and 4.9, for SWM25 and SWM50, respectively), compared to the Control group (14.0; p < .001). To the latter result, the n-6 proportion also contributed as it linearly decreased from the Control to the SWM50 breast meats (24.1 versus 21.3 versus 20.9% for Control, SWM25 and SWM50 breasts, respectively; p < .05 between Control and SWM50).
Figure 2. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on the breast muscle C18:3 n-3 (mg/100 g of meat) of male chickens. a–cDifferent superscript letters differ for p < .05. *C18:3 n-3: 6.75 versus 15.0 versus 28.4 mg/100 g meat for Control, SWM25 and SWM50 treatments, respectively.
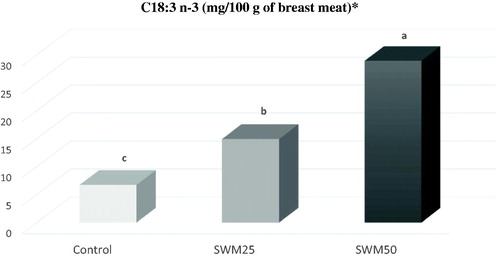
Table 8. Effect of the dietary inclusion of 0% (Control), 25% (SWM25) and 50% (SWM50) silkworm meal on the breast meat fatty acid profile (% of total FAME) of male chickens.
Discussion
Observed protein and ether extract content in SWM are coherent with data reported in literature (Makkar et al. Citation2014). However, in this sense, appreciable variations can be observed (crude protein: 50–72%; ether extract: 6–37%), mainly depending on the silkworm chrysalis diets (i.e., different mulberry leaves nutrients composition), stage of harvesting, management conditions, methods of processing and storage (Ojewola et al. Citation2005; Ijaiya and Eko Citation2009; Makkar et al. Citation2014). The extremely variable composition of nutrients could be problematic in the formulation of livestock diets, therefore it would be advisable to standardise the silkworm production procedures if the secondary purpose (primary purpose is the silk yarn) is to obtain raw material for feed formulation. This, together with the inclusion level and the type of product tested, has contributed to the heterogeneous productive performance obtained in poultry until now (i.e., full-fat or defatted SWM) (Makkar et al. Citation2014).
In this study, the feed intake and the daily weight gain were observed to be the lowest at the highest SWM inclusion level, likely due to both lower palatability and the increased dietary chitin content, which is a component of the exoskeleton of silkworm chrysalis, which has been reported to reduce the digestibility of nutrients in chickens (Makkar et al. Citation2014; Ullah et al. Citation2017b). However, chitin was also found to act as prebiotic by improving the immune response of birds (Bovera et al. Citation2015) and by increasing the caecal production of butyric acid, which is considered the prime energy source for enterocytes (Khempaka et al. Citation2011).
Moreover, silkworm meal contains growth-promoting factors (ecdysteroid), which are reported to stimulate protein synthesis and tissue formation (Fagoonee Citation1983). Benefits in growth performance were found in broiler chickens (Fagoonee Citation1983; Konwar et al. Citation2008) and in Japanese quails (Koudela et al. Citation1995).
The chitin effect on chicken health status could not be evaluated in the present study as all birds under study were healthy and no cases of morbidity/mortality were observed throughout the trial. However, if considering chicken live performance, the lower inclusion level (SWM25) provided the best benefits, likely attributable to the lower dietary chitin content.
The lack of differences for carcase traits and internal organ proportion among dietary treatments confirms that the two SWM inclusion levels did not negatively affect bird relative growth and health. Our results do not support the findings by Khatun et al. (Citation2003) and Ijaiya and Eko (Citation2009) who observed the highest slaughter yield for the dietary groups containing higher level of fish meal replacement with silkworm meal, and authors explained this result as due to the better amino acid profile of SWM compared to fish meal. In our study, however, a higher breast meat incidence was observed for the SWM25 group compared to the Control and SWM50 groups, thus partly agreeing with the assumption by the above-mentioned authors, as the amino acid composition of SWM proteins generally better meets animal requirements than soybean meal (Makkar et al. Citation2014). Nonetheless, the higher breast yield observed for SWM25 chickens could also just be as a result of their higher feed intake.
Data show a linear increase of the breast pH from the Control to the highest SWM inclusion level. Unfortunately, to our knowledge, no studies have explored the relationship between dietary SWM inclusion and meat pH, and, when testing other insects in poultry feeding, i.e., defatted black soldier fly (H. illucens) meal, no differences were found for breast pH of broiler chickens (Schiavone et al. Citation2019). Only in a recent study (Zeng et al. Citation2019), which tested the effect of the dietary inclusion of mulberry leaves (the only dietary source of B. mori) on finishing pigs, loins from mulberry leaves-fed pigs showed higher ultimate pH compared to control diet-fed pigs (p < .08), attributing the higher muscle pH to the quercetin present in mulberry leaves, which can inhibit muscle glycolysis by the down-regulation of glycolysis-related enzymes, reduce lactic acid accumulation in muscle after slaughter. It is likely that bioactive compounds with hypoglycaemic activity present in silkworms (Rattana et al. Citation2019) could act similarly, thus explaining the higher muscle pH found in chickens in the present study. The lowest amount for protein content, which was found in the meat of male chickens fed SWM25 likely depended on their lower growth rate recorded the week before slaughter, counterbalancing the opposing trend observed the previous week. Therefore, this result does not suggest a direct relationship with the dietary SWM inclusion. Further research with a larger number of samples is needed to better understand and corroborate this result.
The breast meat lipids from chickens fed the Control, SWM25 and SWM50 diets showed similar proportions of SFA, MUFA and PUFA. This has been expected as the FA classes of the experimental diets did not differ substantially.
The higher proportion of C18:3 n-3 (α-linolenic acid) in meat lipids which was found in SWM-fed chickens was expected. Unlike other feed ingredients from terrestrial animals, silkworm αlipids contain high amounts α-linolenic acid, with reported values ranging from 11 to 45% of the total fatty acids (Rao Citation1994; Usub et al. Citation2008). In our study, the SWM dietary supplementation positively and linearly increased the n-3 FA amount in the chicken breasts, with the α-linolenic acid showing an increase by a factor of 2.2 in SWM25 and by a factor of 4.2 in SWM50. EPA + DHA contents (data not shown, as the difference among treatments was not significant) also increased with SWM dietary inclusion level (40.9, 48.6 and 55.5 mg/100 g meat for Control, SWM25 and SWM50), improving the food nutritional claim ‘source of omega-3 FA’, that may only be declared if the product contains at least 40 mg of the sum of EPA + DHA/100 g of product (European Commission).
Due to the reduction of the n-6 FA with the SWM dietary supplementation, the n-6/n-3 ratio of the breast meat in SWM groups sharply decreased, falling within the recommended ratio (4:1 to 10:1) by nutrition experts worldwide to decrease the risk of cardiovascular diseases (Simopoulos Citation1989; Fernandes Citation2002). These low n-6/n-3 ratio are difficult to reach in meat food products from mammal livestock, with conventional feedstuffs, thus SWM dietary supplementations may play an important role in achieving this.
Furthermore, considering that cardiovascular diseases contribute significantly to global disability and of mortality in humans, and that recent studies (Zhang et al. Citation2020) have positively associated n-6/n-3 ratios with the risk of depressive symptoms, any effort to reduce the n-6/n-3 PUFAs ratio in the diets of the population of Western countries would concur to partly alleviate suffering and social discomfort.
Conclusions
The present study showed that silkworm (B. mori) is a rich source of crude protein and provides lipids with a high amount of omega-3 fatty acids. Silkworm meal may be used to replace 25% soybean meal (7% dietary inclusion) in the RIR × Fayoumi crossbred chicken diets, promoting good growth performance, carcase traits and proximate composition. Increasing the SWM inclusion level to 50% replacement (14% dietary inclusion) favourably modifies the fatty acid profile of the chicken breast meat, significantly reducing the n-6/n-3 PUFAs ratio.
Acknowledgements
The authors thank Zahurul Islam, Bangladesh Institute of Nuclear Agricultural, for his technical support. The linguistic support of Elizabeth Gleeson is also acknowledged.
Disclosure statement
The authors state no conflict of interest.
Additional information
Funding
References
- Agazzi A, Invernizzi G, Savoini G. 2016. New perspectives for a sustainable nutrition of poultry and pigs. J Dairy Vet Anim Res. 3:00079.
- Association of Official Analytical Chemists. 2000. Official methods of analysis. 17th ed. Arlington, VA: AOAC.
- Biasato I, De Marco M, Rotolo L, Renna M, Lussiana C, Dabbou S, Capucchio MT, Biasibetti E, Costa B, Gai F, et al. 2016. Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J Anim Physiol Anim Nutr. 100(6):1104–1112.
- Bovera F, Piccolo G, Gasco L, Marono S, Loponte R, Vassalotti G, Mastellone V, Lombardi P, Attia YA, Nizza A. 2015. Yellow mealworm larvae (Tenebrio molitor, L.) as a possible alternative to soybean meal in broiler diets. Br Poult Sci. 56(5):569–575.
- CIE 1976. Recommendations on uniform colour spaces, colour differences, equations. Psychometric colour terms. Suppl. 2, CIE Publications n.15. Paris, France: Commission Internationale de l’Eclairage, Colorimetry.
- Cullere M, Tasoniero G, Giaccone V, Acuti G, Marangon A, Dalle Zotte A. 2018. Black soldier fly as dietary protein source for broiler quails: meat proximate composition, fatty acid and amino acid profile, oxidative status and sensory traits. Animal. 12(3):640–647.
- Cullere M, Tasoniero G, Giaccone V, Miotti-Scapin R, Claeys E, De Smet S, Dalle Zotte A. 2016. Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal. 10(12):1923–1930.
- Dalle Zotte A, Singh Y, Michiels J, Cullere M. 2019. Black soldier fly (Hermetia Illucens) as dietary source for laying quails: live performance, and egg physico-chemical quality, sensory profile and storage stability. Animals. 9(3):115.
- European Commission. 1998. Commission Directive 98/64/EC of 3 September 1998 establishing community methods of analysis for the determination of amino acids, crude oils and fats, and Olaquindox in feeding stuffs and amending Directive 71/393/EEC. https://op.europa.eu/en/publication-detail/-/publication/856db9b7-6f1d-4d6e-a778-c766c9fa1776. [Accessed 11 2020 April].
- Fagoonee I. 1983. Possible growth factors for chickens in silkworm pupae meal. Br Poultry Sci. 24(3):295–300.
- Fernandes J. 2002. Nutrition and health Recommendations of the health council of the Netherlands regarding energy, proteins, fats and carbohydrates. Ned Tijdschr Geneeskd. 146(47):2226–2229.
- Folch J, Lees M, Stanley G. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 226(1):497–509.
- Gale F, Arnade C. 2015. Effects of rising feed and labour costs on China’s chicken price. Int Food Agribus Man. 18:137–150.
- Guil-Guerrero JL, Ramos-Bueno RP, González-Fernández MJ, Fabrikov D, Sánchez-Muros MJ, Barroso FG. 2018. Insects as food: fatty acid profiles, lipid classes, and sn-2 fatty acid distribution of Lepidoptera larvae. Eur J Lipid Sci Technol. 120(6):1700391.
- Horlings LG, Marsden TK. 2011. Towards the real green revolution? Exploring the conceptual dimensions of a new ecological modernisation of agriculture that could ‘feed the world. Glob Environ Change. 21(2):441–452.
- Ijaiya AT, Eko EO. 2009. Effect of replacing dietary fish meal with silkworm (Anaphe infracta) caterpillar meal on performance, carcass characteristics and haematological parameters of finishing broiler chicken. Pak J Nutr. 8(6):850–855.
- Khatun R, Howlider MAR, Rahman MM, Hasanuzzama M. 2003. Replacement of fish meal by silkworm pupae in broiler diets. Pak J Biol Sci. 6(11):955–958.
- Khempaka S, Chitsatchapong C, Molee W. 2011. Effect of chitin and protein constituents in shrimp head meal on growth performance, nutrient digestibility, intestinal microbial populations, volatile fatty acids, and ammonia production in broilers. J Appl Poult Res. 20(1):1–11.
- Konwar P, Konwar BK, Ahmed HF, Nath NC, Ghosh MK. 2008. Effect of feeding silkworm pupae meal with enzyme supplementation on growth performance of broilers. Ind Vet J. 85:47–49.
- Koudela K, Tenora J, Bajer J, Mathova A, Sláma K. 1995. Stimulation of growth and development in Japanese quails after oral administration of ecdysteroid-containing diet. Eur J Entomol. 92:349–349.
- Kwon M-G, Kim D-S, Lee J-H, Park S-W, Choo Y-K, Han Y-S, Kim J-S, Hwang K-A, Ko K, Ko K. 2012. Isolation and analysis of natural compounds from silkworm pupae and effect of its extracts on alcohol detoxification. Entomol Res. 42(1):55–62.
- Lee CM, Trevino B, Chaiyawat M. 1996. A simple and rapid solvent extraction method for determining total lipids in fish tissue. J AOAC Int. 79(2):487–492.
- Makkar HPS, Tran G, Heuzé V, Ankers P. 2014. State of the art on use of insects as animal feed. Anim Feed Sci Technol. 197:1–33.
- NRC-National Research Council, subcommittee on Poultry Nutrition. 1994. Nutrient requirements of poultry (9th revised edition, National Academy of Sciences). Washington, DC: National Academy of Sciences.
- Ojewola GS, Okoye FC, Ukoha OA. 2005. Comparative utilization of three animal protein sources by broiler chickens. Int J Poultry Sci. 4(7):462–467.
- Pieterse E, Pretorius Q, Hoffman LC, Drew DW. 2014. The carcass quality, meat quality and sensory characteristics of broilers raised on diets containing either Musca domestica larvae meal, fish meal or soya bean meal as the main protein source. Anim Prod Sci. 54(5):622–628.
- Rao PU. 1994. Chemical composition and nutritional evaluation of spent silk worm pupae. J Agric Food Chem. 42(10):2201–2203.
- Rattana S, Katisart T, Butiman C, Sungthong B. 2019. Total flavonoids, total phenolics, 1-deoxynojirimycin content and alpha-glucosidase inhibitory activity of Thai silkworm races (Bombyx mori Linn.). Pak J Pharm Sci. 32:2539–2544.
- Rumpold BA, Schlüter OK. 2013. Nutritional composition and safety aspects of edible insects. Mol Nutr Food Res. 57(5):802–823.
- Schiavone A, Dabbou S, Petracci M, Zampiga M, Sirri F, Biasato I, Gai F, Gasco L. 2019. Black soldier fly defatted meal as a dietary protein source for broiler chickens: effects on carcass traits, breast meat quality and safety. Animal. 13(10):2397–2405.
- Sheikh IU, Banday MT, Baba IA, Adil S, Nissa SS, Zaffer B, Bulbul KH. 2018. Utilization of silkworm pupae meal as an alternative source of protein in the diet of livestock and poultry: a review. J Entomol Zool Stud. 6:1010–1016.
- Simopoulos AP. 1989. Summary of the NATO advanced research workshop acids: biological effects and nutritional essentiality. J Nutr. 119(4):521–528.
- Statistical Analysis Software for Windows. 2008. Statistics version 9.1.3 ed. Cary, NC: SAS Institute.
- Ullah R, Khan S, Hafeez A, Asad S, Nazir AK, Naila C, Naseer A. 2017a. Silkworm (Bombyx mori) meal as alternate protein ingredient in broiler finisher ration. PJZ. 49(4):1463–1470.
- Ullah R, Khan S, Khan N, Mobashar M, Sultan A, Ahmad N, Lohakare J. 2017b. Replacement of soybean meal with silkworm meal in the diets of white leghorn layers and effects on performance, apparent total tract digestibility, blood profile and egg quality. IJVHSR. 5:200–207.
- Usub T, Lertsatitthanakorn C, Poomsa-Ad N, Wiset L, Yang L, Siriamornpun S. 2008. Experimental performance of a solar tunnel dryer for drying silkworm pupae. Biosyst Eng. 101(2):209–216.
- Van Huis A, Oonincx D. 2017. The environmental sustainability of insects as food and feed. A review. Agron Sustain Dev. 37(5):43.
- Van Zanten HHE, Oonincx D, Mollenhorst H, Bikker P, Meerburg BG, deBoer I. 2014. Can the environmental impact of livestock feed be reduced by using waste-fed housefly larvae? In Proceedings of the 9th International Conference on Life Cycle Assessment in the Agri-Food Sector; October 8–10; San Francisco, USA. ACLCA, Vashon, WA, p. 1455–1461.
- Veldkamp T, Bosch G. 2015. Insects: a protein-rich feed ingredient in pig and poultry diets. Anim Front. 5:45–50.
- Yu XB, Shen YY, Cui QM, Chen Y, Sun W, Huang XZ, Zhu Y. 2018. Silkworm (Bombyx mori) has the capability to accumulate C20 and C22 polyunsaturated fatty acids. Eur J Lipid Sci Technol. 120(2):1700268.
- Zeng Z, Jiang J-j, Yu J, Mao X-b, Yu B, Chen D-w. 2019. Effect of dietary supplementation with mulberry (Morus alba L.) leaves on the growth performance, meat quality and antioxidative capacity of finishing pigs. J Integr Agric. 18(1):143–151.
- Zhang R, Sun J, Li Y, Zhang D. 2020. Associations of n-3, n-6 fatty acids intakes and n-6:n-3 ratio with the risk of depressive symptoms: NHANES 2009–2016. Nutrients. 12(1):240.
