 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The effects of two stunning methods (carbon monoxide asphyxia, CO, and electroshock, E) on blood plasma parameters, rigor index, fillet pH and shape changes, ATP breakdown and Adenylate Energy Charge (AEC) in muscle immediately post mortem were investigated in rainbow trout (Oncorhynchus mykiss) kept in tanks containing water set at 8 °C or 12 °C. Both the methods here adopted induced a stress-response which, however, was not able to affect the rigor mortis development and fillet pH. The fillets from the E group showed the strongest length contraction and width increase at 48 h post mortem. The CO stunning method exhibited the highest levels of both ATP and AEC in the muscle immediately after death, equal to 2.28 µmol/g and 0.83, respectively, while 1.12 µmol/g and 0.64 values were found in the E group. In addition, we found that the water temperature might interact with the stunning method and minimise the stress response. In the present trial, the most suitable use of the CO stunning method would be coupled with 12 °C of rearing water temperature to better preserve ATP and AEC in muscles.
CO stunning preserves ATP and AEC in trout muscle better than electrical stunning
Water temperature might interact with the stunning and minimise the stress response
CO stunning is suggested to be coupled with 12 °C of water temperature
Electricity should be avoided at 8 °C of water temperature
Highlights
Keywords:
Introduction
Conditions of anxiety, pain, suffering or fear above all have ethical implications, since the human being is considered to be responsible for the effective respect of the rights and welfare of other living animals, as stated in the declaration of UNESCO (Citation1978). Operations concerning stunning and slaughtering processes can cause different degrees of stress and disturbances that may affect meat quality (Poli Citation2009).
The European Food Safety Authority (EFSA Citation2004) has classified the methods used to slaughter fish in two main groups: stunning/slaughtering methods and slaughtering without stunning methods. In the first group, percussive, spiking and electrical (E) stunning/slaughtering methods are listed; whereas, in the second, carbon dioxide (CO2) narcosis, carbon monoxide (CO), asphyxiation in the air, asphyxia in ice/ice slurry, dry salt/ammonia bath and exsanguinations are included. Carbon monoxide is not widely applied in fish, but it has been used in animal euthanasia for long time (Smith Citation2001). Studies in Atlantic salmon (Bjørlykke et al. Citation2011, Citation2013), tilapia (Mantilla et al. Citation2008), and the early study of Concollato et al. (Citation2019) on rainbow trout (Oncorhynchus mykiss) suggest that CO can be an excellent fish sedative that does not elicit any visible stress response. Indeed, as previously reviewed by Concollato et al. (Citation2015), CO binds with strong affinity to the haem group of haemoglobin (Hb) and myoglobin (Mb) displacing oxygen and producing carboxy-haemoglobin (COHb) and carboxy-myoglobin (COMb), which cannot transport oxygen molecules. This strong binding (Kalin Citation1996) is the major cause of CO stunning effectiveness, thanks to the exclusion of Hb in the O2’s transport. The CO also influences cellular respiration through the inhibition of many enzymes, such as cytochromes, which possess haem groups similar to those of Hb and Mb, causing the suppression of oxidative phosphorylation (Prescott et al. Citation1996). Considering Atlantic salmon, Bjørlykke et al. (Citation2011) and Concollato et al. (Citation2014) highlighted that fish exposed to CO did not express aversive reactions and were easily slaughtered by percussion. Therefore, studying the CO effects on stress parameters (Concollato et al. Citation2014) of Atlantic salmon and addressing their quality changes (Secci et al. Citation2016), it was evident that CO treatment resulted in an increased level of catecholamines, an earlier onset of rigor mortis because of the rapid pH decrease in response to the lactate production, and higher drip losses compared to percussive stunned/killed fish. In addition, the CO treatment ensured the lowest yellowness index (b*) to rainbow trout fillets during 7 days of refrigerated storage and the absence of textural attribute impairments even if CO treated trout fillets had a perceivable lowest odour intensity and a highest juiciness (Concollato et al. Citation2019).
Despite the encouraging results obtained, CO utilisation as stunning and killing method deserves more attention. Indeed, it is well known that the stress response is species-specific, thus the same stunning/killing method cannot be considered equally suitable among different species. In this sense, Concollato et al. (Citation2016) have previously shown that CO and E treatments were able in preserving muscle energy of farmed rainbow trout immediately after death even if E was suggested as the most appropriate stunning/slaughtering method. Hence, the aim of the present study was to compare CO and E as stunning procedures and find out if the temperature of water where rainbow trout are maintained might interact with them. For this reason, two different water temperatures (8 °C and 12 °C) for maintaining fish were selected to compare welfare and physical changes until rigor mortis resolution of CO-stunned trout and electrical treated ones, using electricity as control group since it is one of the most common stunning methods.
Material and methods
Experimental set-up
The experimental protocol was designed according to the guidelines of the current European Directive 2010/63/EU put into law in Italy with D. Lgs. 26/2014 on the care and use of experimental animals. The trial was conducted by certified personnel at the Edmund Mach Foundation’s experimental farm, located in S. Michele all’Adige, Trento (Italy). During the experiment, the safety of each operator was guarantee by the presence of the firemen of Trento province (Italy) who monitored CO concentration in the air using portable gas detectors (GasBadge Pro, Oakdale, PA, USA).
dFour hundred rainbow trout (Oncorhynchus mykiss W.; mean weight: 729 ± 105 g) were randomly distributed into 4 tanks containing 3600 L of freshwater each (20.3 ± 2.90 kg/m3) set at two different temperatures. Indeed, the water temperature was kept at 12 °C in tanks 1 and 2, whereas the water temperature was 8 °C in tanks 3 and 4. Temperature was monitored by a common thermometer. All the tanks were aerated, the level of dissolved oxygen was monitored by an oxymeter OXI 340i (WTW, Oberbayern, Germany) and the average registered level was 9.0 ± 0.5 mg O2/L. Fish stayed in that tanks for 4 days during which they were fed the same commercial diet; a 24 h fasting period was adopted before killing. Two stunning methods were applied: electroshock (E, tank 1: E_12 °C; tank 4: E_8 °C) performed by the electronic teaser GOZLIN TEQ002 (GOZLIN, Modena, Italy) for 30 s at 180 V once hauled fish out of water, and asphyxia with carbon monoxide (tank 2: CO_12 °C; tank 3: CO_8 °C). Specifically, 100% food grade CO (SIAD, Bergamo, Italy) was flushed into the tanks for about 49 min, time at which water was super-saturated of CO and fish were declared as stunned upon visual inspection (no swimming activity or ventilation) as suggested by Roth et al. (Citation2003). Finally, trained staff percussively killed all the fish, both from E and CO groups, using a wooden knob. Overall, eighteen fish per experimental group, representative of the corresponding experimental group in terms of mean live weight and variability, were sampled. Eight fish/experimental group underwent the analyses reported in the present manuscript, whereas the other ten fish/group were allotted to the quality evaluation, however the results are not here included. Detailing, 5 fish/experimental group were considered for blood plasma analysis, Rigor Index and pH evolution measurements, whereas other 3 fish/group were examined for fillet shape change assessment and adenosine 5′-triphosphate (ATP) and related metabolites content analysis, as detailed below. To note that no broken spines, haemorrhages or other injuries were observed in the fish analysed.
Blood plasma parameters and cortisol level
Immediately after percussion, blood samples were collected from the caudal vein of 5 fish/experimental group. Blood was placed in heparinised tubes, centrifuged at 4000 xg for 10 min; the resultant plasma was transferred into Eppendorf tubes and stored at −80 °C until analyses.
Plasma lactate and glucose were analysed using MaxMat PL (MaxMat S.A., Montpellier, France). Cortisol was determined using ELISA (RE52061, IBL International GmbH, Hamburg, Germany) and K+ was analysed with selective ion electrode (Cobas c 111, Roche Diagnostics Ltd., Rotkreuz, Switzerland).
Rigor index, pH evolution, fillet shape changes during rigor mortis and ATP determination
After slaughtering, the same 5 fish/experimental group used for blood sampling were, as well, considered for Rigor Index and pH evolution measurements. For Rigor Index and pH measurement, fish were individually tagged, weighed and stored in polystyrene boxes with ice, maintained in a cold room at 1 ± 1 °C until rigor mortis resolution, which occurred at about 76 h post mortem.
Rigor mortis (n = 5) evolution was assessed by the Rigor Index (RI), calculated according to Bito et al. (Citation1983), using the following formula:
where L0 (cm) is the vertical distance between the base of the caudal fin and the table surface utilised as a support base for the fish, measured immediately after the death (T0), whereas Lt (cm) is the vertical distance between the base of the caudal fin and the table surface at the selected time intervals (4, 9, 15, 24, 33, 39, 48, 57 and 76 h post mortem).
The pH was measured in triplicate on the cranial part of epaxial fillet portion, using a Mettler Toledo FiveEasy™/FiveGo™ pH metre (Mettler-Toledo Ltd, Leicester, UK).
On the other 3 fish/treatment mentioned above, manual filleting in pre rigor condition was performed. Afterwards, 1 g of muscle was sampled from the cranial epaxial portion of the right fillets (n = 3) to assess ATP and its catabolite contents. Specifically, ATP and its catabolites [i.e. adenosine 5′-diphosphate (ADP), adenosine 5′-monophosphate (AMP), inosine 5′-monophosphate (IMP), inosine (Ino) and hypoxanthine (Hx)] were detected and quantified by the HPLC analysis, based on Burns and Ke (Citation1985) method. The HPLC apparatus comprised a pump system (Beckman mod. 125-S, Brea, CA, USA) equipped with a UV detector (Beckman mod. 166, Brea, CA, USA) with absorbance fixed at 254 nm, analogic interface (Beckman mod. 406, Brea, CA, USA), Ultrasphere ODS Reverse Phase column (Beckman, Brea, CA, USA; length: 250 mm, internal diameter: 4.6 mm; particle size: 5 µm; pore size: 80 Å), Ultrasphere ODS pre-column (ID: 4.6 mm, length: 45 mm), and 20-µl fixed loop. The mobile phase was KH2PO4, 0.5 M, pH 7.0. Standards were purchased from Sigma-Aldrich (St. Louis, MO, USA).
From ATP and related catabolites, the Adenylate Energy Charge (AEC) = (0.5 ADP + ATP)/(AMP + ADP + ATP) (Atkinson Citation1968) was also calculated. Our main interest was to detect the amount of those parameters involved in the first period of freshness evolution such as ATP and AEC in the muscle immediately post mortem (T0), in relation with the stunning methods applied and the rearing water temperature.
Moreover, the left fillets (n = 3), maintained in a cold room at 1 ± 1 °C, were used to assess the shape changes during rigor mortis evolution, by taking pictures at 0, 4, 9, 15, 24 and 48 h post mortem with a NIKON D3000 camera with lens Nikkor 18-55. Picture of the fillets was then analysed by the Software Adobe Photoshop CS4 for the following parameters: perimeter, area, maximum length and maximum width.
Statistical analysis
Data were analysed using the General Linear Model procedures of the statistical analysis software SAS 9.1 (SAS Citation2004) for Windows. A two-way ANOVA tested the stunning methods (S, two levels: CO and E) and the water temperatures (T, two levels: 8 °C and 12 °C) as fixed effects. The stunning method × water temperature interaction (S×T) was also tested. Tukey’s test was utilised to determine the differences when the p value was under .05. Fish weight was considered as covariate for all the analysed variables.
Results
Blood plasma parameters and cortisol level
Fish subjected to CO stunning method showed significantly higher (p < .001) glucose levels (Table ) than the E ones, while water temperature did not exert any influence on this parameter. The highest lactate amount was exhibited by CO group with respect to E one, whereas no significant differences in cortisol were detected between the two experimental groups.
Table 1. Blood plasma parameters and cortisol levels in rainbow trout reared at 8 °C or 12 °C and stunned by asphyxia with CO (CO) or electricity (E) (n = 5 fish/experimental group).
The temperature at which fish were maintained significantly affected lactate and K+ concentrations in blood plasma. As for lactate levels, a significantly (p < .05) higher value was observed at 12 °C, whereas for K+ levels a significantly (p < .001) higher value was found at 8 °C. Significant (p < .01) interaction between rearing temperature (T) and stunning method (S) emerged for K+ levels (Table ). Fish reared at 8 °C showed the highest values of K+ levels, with CO stunning method showing significantly higher K+ values than E stunning method (p < .01), whereas the fish reared at 12 °C showed the lowest values among all the other groups.
Table 2. Results of the significant interaction (stunning × temperature, S × T) emerged for K+ (mM) plasma content.
ATP content, rigor index, pH evolution, and fillet shape changes during rigor mortis
The results on the effect of water rearing temperatures and stunning methods on the contents of ATP, AEC and the fillet shape parameters measured immediately post mortem (T0) in rainbow trout muscle are summarised in Table . The fillets from CO experimental group resulted in significantly higher (p < .05) amount of ATP and AEC level (p < .001) than E group. ATP and AEC in fish fillets were significantly affected by the water temperature: at 12 °C both parameters showed higher values (p < .05 and p < .001, respectively) than those found on fillets of the 8 °C experimental group (Table ). However, a significant (p < .01) interaction S × T was found for AEC value (Table ), indicating that at 8 °C the CO stunning method significantly increased the AEC value than E stunning method (p < .01), whereas at 12 °C the stunning method did not alter this parameter.
Table 3. ATP contents, AEC values, and fillets shape changes immediately post mortem in muscle of rainbow trout reared at 8 °C or 12 °C and stunned/slaughtered by asphyxia with CO (CO) or electricity (E) (n = 3 fish/experimental group).
Table 4. Results of the significant interaction (stunning × temperature, S × T) emerged for AEC values determined immediately post mortem (T0) in muscle of rainbow trout reared at 8 °C or 12 °C and stunned by asphyxia with CO (CO) or electricity (E).
Both temperature and stunning method experimental factors did not affect pH evolution or rigor mortis, at any of the considered time points (Figures and ).
Figure 1. Post mortem evolution of rainbow trout pH as affected by rearing water temperature [8 °C, (A); 12 °C, (B)], and stunning methods (asphyxia with CO, CO; electricity, E). Values are presented as means (n = 5 fish/experimental group). Vertical bars indicate the standard deviation.
![Figure 1. Post mortem evolution of rainbow trout pH as affected by rearing water temperature [8 °C, (A); 12 °C, (B)], and stunning methods (asphyxia with CO, CO; electricity, E). Values are presented as means (n = 5 fish/experimental group). Vertical bars indicate the standard deviation.](/cms/asset/87f1071e-6a34-4080-994d-f83d186a5c72/tjas_a_1759465_f0001_b.jpg)
Figure 2. Post mortem evolution of rainbow trout Rigor Index (RI, %) as affected by rearing water temperature [8 °C, (A); 12 °C, (B)], and stunning methods (asphyxia with CO, CO; electricity, E). Values are presented as means (n = 5 fish/experimental group). Vertical bars indicate the standard deviation.
![Figure 2. Post mortem evolution of rainbow trout Rigor Index (RI, %) as affected by rearing water temperature [8 °C, (A); 12 °C, (B)], and stunning methods (asphyxia with CO, CO; electricity, E). Values are presented as means (n = 5 fish/experimental group). Vertical bars indicate the standard deviation.](/cms/asset/bc2b33a0-9f1a-4b14-b812-4c17b7a60423/tjas_a_1759465_f0002_b.jpg)
Fillet shape changes occurred during the rigor mortis development, expressed as the percentage of the corresponding initial value, are shown in Figures and . Perimeter and area were not affected (p < .05) by the experimental stunning procedures (Figure ). Only the fillet length contraction significantly differed between the stunning groups at 9, 15 and 48 h post mortem, with the CO group showing the lower length reduction in comparison to the E group fillets. The maximum contraction value of the fillets was reached only at the end of the monitoring time (i.e. 48 h): 86% and 87% of the initial length in the E stunning group and CO stunning group, respectively, being the difference statistically significant (p < .05).
Figure 3. Effect of asphyxia with CO (CO) or electricity (E) stunning methods on perimeter, area, maximum length and maximum width in fillets of rainbow trout, measured at different times after death, expressed as percentage of the value measured immediately after death (n = 3 fish/experimental group).
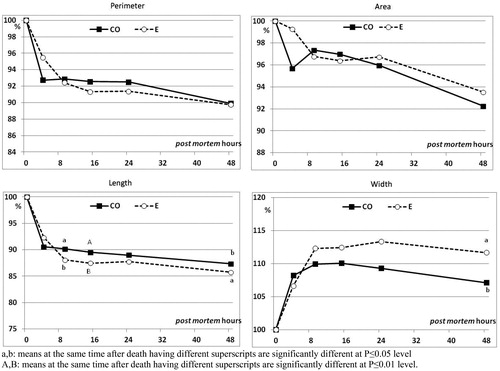
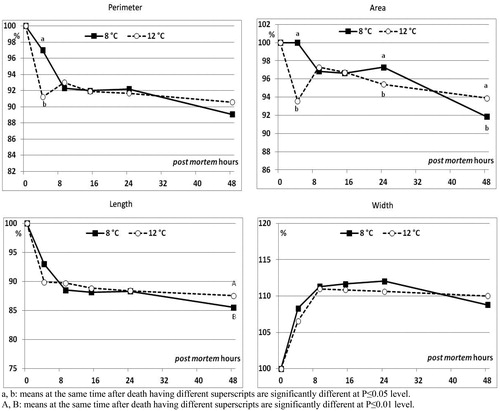
Figure 4. Effect of rearing temperature (water set at 8 or 12 °C) on perimeter, area, maximum length and maximum width in fillets of rainbow trout, measured at different times after death, expressed as percentage of the value measured immediately after death (n = 3 fish/experimental group).
The most rapid fillet width increase was observed for the E stunning group, with values being significantly different between the two stunning methods only at 48 h post mortem (111.7% for E group and 107.1% for CO group; p ≤ .05).
The rearing water temperature also had an effect on the fillet shape: area and length were significantly affected at the end of the period monitored (Figure ), whereas perimeter reduction was found to differ due to the water temperature only at 4 h post mortem, with the group reared at 8 °C showing the lowest reduction compared to that reared at 12 °C (p < .05).
The area shrinking differed due to rearing water temperature at 3 time points: the 12 °C group exhibited significantly higher (p ≤ .05) shrinking values than the 8 °C one both at 4 and 24 h (corresponding to 93.6% and 95.4% of shrinking, respectively). Contrariwise, the trend was reversed (p ≤ .05) at 48 h post mortem, with 8 °C and 12 °C groups showing the 92% and the 94% area shrinking, respectively.
Discussion
Blood plasma parameters and cortisol level
The stunning methods and the rearing water temperatures had significant effects on some blood parameters. It deserves to mention that fish stunned by E method were hauled out of the tank and stunned almost immediately, whereas rainbow trout stunned by CO were, on overall, treated for a prolonged time. It would indicate that the CO fish would have had more time to mobilise glycogen stores leading to a higher rise in the plasma glucose levels, compared to the E-stunned fish. Barton and Iwama (Citation1991) stated that the modifications in glucose metabolism are a general response to stress in captured fish, therefore providing extra-energy resources to enable the animal to maintain the homeostasis, which was evident in the present study.
The glucose levels of the CO group was similar to those found in rainbow trout subjected to an acute stressor (around 7.2 mM as a consequence of 2 min of handling/confinement) (Magnoni et al. Citation2019), while the control group used in the same study had a glucose level equal to 5.3 mM, which was similar to that found for the E group in the present study, corresponding to the limit of the physiological level.
Anaerobic condition caused by a severe stress is well known to generate breakdown of muscle glycogen to lactate, and the latter is then released in the blood stream (Wood Citation1991; Thomas et al. Citation1999). The highest lactate level was detected for the CO group, whereas the E group, which presented the lowest glucose level, showed also the lowest lactate amount, however being within the physiological threshold. Milligan and Girard (Citation1993) in rainbow trout 8 h after being chasing for 5 min (5.30 ± 2.25 mM) reported similar values to that of the CO group of the present study. The general increase in lactate production with temperature clearly shows the positive relationship between fish metabolism and temperature.
In general, resting levels of cortisol in fish can vary considerably due to stress response. For instance, Merkin et al. (Citation2010) observed that the cortisol levels of sea-farmed rainbow trout significantly increased from 9 ng/mL at farm to 57 ng/mL in response to transportation, registering values of 187 ng/mL due to short-term crowding and 145 ng/mL due to long term crowding. Besides, other pre-slaughter procedures (air exposure, chasing and increased water temperature) affected the cortisol responses in goldfish (Carassius auratus) individuals, with concentrations ranging from 9.1 to 516.0 ng/mL (Cockrem et al. Citation2019). However, we have not to ignore that the cortisol levels detected here for the CO and E groups were not statistically different and fell within a low-stress range (Barton and Iwama Citation1991).
As far as plasma K+ level, its increase reported in fish could derive from strenuous exercise, intracellular acidification (Ultsch et al. Citation1981), acute stress and haemoconcentration (Mc Donald and Milligan Citation1997). The significant S × T interaction observed here revealed a not negligible effect of the water temperature in which the trout were kept in relation to the different stunning methods. The K+ concentration in plasma of the CO_8 °C rainbow trout was the highest among all the other groups. This fact supports the findings from Ultsch et al. (Citation1981) and Mc Donald and Milligan (Citation1997) and lies in the CO action in displacing O2 and favouring the cellular acidification.
ATP content, Rigor Index and pH evolution, fillet shape changes during rigor mortis
The results obtained from the ATP analysis revealed a higher preservation of energy in the CO muscle than in the E one, in line with literature. As an example, Mishima et al. (Citation2005) in horse mackerel (Trachurus japonicus) slaughtered by temperature shock, at 8 h post mortem found values (∼ 2.10 µmol/g) similar to those of the CO group of this trial, whereas at 12 h post mortem, when slaughtered by cutting the brain, they found values similar to those observed for the E group (∼ 1.20 µmol/g).
The different stress degree sustained by all the groups and the variation in the actual time of death affected the post mortem AEC (Adenylic Energetic Charge) values, that were significantly higher for the CO group. Berg et al. (Citation1997) reported for stressed (stunning with CO2) Atlantic salmon AEC values similar to those registered for the E group only 3 h after slaughter (0.66 ± 0.07 vs. 0.64 ± 0.08, respectively), which were comparable to values of unstressed group from the same trial (netted individually and killed within 25 seconds by a blow to the head) at about 20 h post mortem. Similar AEC values to those of the CO group (0.83 ± 0.08) were found by Erikson et al. (Citation1999) in Atlantic salmon being chased for 1 h before slaughtering (0.88 ± 0.04) and by Schulte et al. (Citation1992) in rainbow trout being exercised to exhaustion for 30 min before slaughter (0.84 ± 0.011).
The positive interaction found showed that the water rearing temperature exerted an important effect. Reduction of fish muscle temperature substantially removes thermal energy accessible for the muscle degradation that starts within hours after slaughter (Skjervold et al. Citation2001). Electroshock seemed to be the treatment which deplete more energy at 8 °C, instead no differences emerged between the two stunning methods at 12 °C. This seems to show an evident effect of the temperature since the treatments applied were not different.
Despite the stunning procedures affected the lactate content, the Rigor Index and pH evolution in fish stunned by CO or E at the two rearing temperatures seem to follow the same pattern during the 76 h of monitoring, in the two groups of trout.
The maximum shrinking in length (∼14%) found in the E group fillets 48 h post mortem was similar to that observed, at 11 and 48 h after death, by Misimi et al. (Citation2008) on both stressed (chased to exhaustion for 30 min) and unstressed (anesthetised with AQUI-S™) Atlantic salmon, respectively. It is interesting to note that CO seemed to reduce the fillet length shrinking through all the monitoring period (48 h), with respect to electroshock stunning/slaughtering method (E group). During rigor, the E fillet width increased as a consequence of the major length decrease: width increased up to +11.7% at about 9 h after death, time corresponding with the maximum rigor of the whole fish. Misimi et al. (Citation2008) recorded a width increase (∼ +6%) in unstressed Atlantic salmon 45 h post mortem similar to the one raised by the CO group 48 h after death. Rearing water temperatures had a slight greater impact on the fillet shape changes, independently from the stunning methods applied. Despite the differences observed on fillet shape change, a comparison with the literature on the effect of rearing water temperature was not possible, because similar studies have not been conducted to the authors knowledge.
The behaviour recorded seems to suggest that fillets obtained from fish of the CO group begin to contract and change their shape earlier than fillets obtained from fish of the E group, although significant differences are quite poor. This result, quite in line with the values registered for blood parameters, must be related with the greater depletion of muscle energy in the case of fish that have suffered greater stress condition at the time of death.
Conclusions
In conclusion, this study confirms once more that the stunning procedure is a stressful event for fish. Both the methods here adopted induced a stress-response which, however, was not able to affect the rigor mortis development, that is considered as a promising indicator for the quality maintenance during the storage. In addition, the rearing water temperature should be carefully chosen because of its possible role in minimising the stress response at slaughter. For instance, in the present case, the most suitable use of the CO stunning method would be coupled with 12 °C of rearing water temperature.
Acknowledgments
The authors express their gratitude to the Fondazione Edmund Mach and ASTRO (Associazione Troticoltori Trentini) for providing the facilities to realise this study. Thanks are also due to SIAD Company (Bergamo, Italy) for providing CO.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Atkinson DE. 1968. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 7:4030–4034.
- Barton B, Iwama G. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis. 1:3–26.
- Berg T, Erikson U, Nordtvedt TS. 1997. Rigor mortis assessment of Atlantic salmon (Salmo salar) and effects of stress. J Food Sci. 62:439–446.
- Bito M, Yamada K, Mikumo Y, Amano K. 1983. Studies on rigor mortis of fish: 1. Differences in the mode of rigor mortis among some varieties of fish by modified Cutting’s method. Bull Tokai Reg Fish Res Lab. 109:89–93.
- Bjørlykke GA, Kvamme BO, Raae AJ, Roth B, Slinde E. 2013. Slaughter of Atlantic salmon (Salmo salar L.) in the presence of carbon monoxide. Fish Physiol Biochem. 39:871–879.
- Bjørlykke GA, Roth B, Sørheim O, Kvamme BO, Slinde E. 2011. The effects of carbon monoxide on Atlantic salmon (Salmo salar L.). Food Chem. 127:1706–1711.
- Burns GB, Ke PJ. 1985. Liquid chromatographic determination of hypoxanthine content in fish tissue. AOAC. 68:444–447.
- Cockrem JF, Bahry MA, Chowdhury VS. 2019. Cortisol responses of goldfish (Carassius auratus) to air exposure, chasing, and increased water temperature. Gen Comp Endocr. 270:18–25.
- Concollato A, Bjørlikke GA, Kvamme BO, Sørheim O, Slinde E, Olsen RE. 2015. The effect of carbon monoxide on slaughter and processing of fish. In: Preedy VR, editor. Processing and impact on active components in food. London: Elsevier; p. 427–431.
- Concollato A, Dalle Zotte A, Vargas SC, Cullere M, Secci G, Parisi G. 2019. Effects of three different stunning/slaughtering methods on physical, chemical, and sensory changes in rainbow trout (Oncorhynchus mykiss). J Sci Food Agric. 99:613–619.
- Concollato A, Olsen RE, Vargas SC, Bonelli A, Cullere M, Parisi G. 2016. Effects of stunning/slaughtering methods in rainbow trout (Oncorhynchus mykiss) from death until rigor mortis resolution. Aquaculture. 464:74–79.
- Concollato A, Parisi G, Olsen RE, Kvamme BO, Slinde E, Dalle Zotte A. 2014. Effect of carbon monoxide for Atlantic salmon (Salmo salar L.) slaughtering on stress response and fillet shelf life. Aquaculture. 433:13–18.
- EFSA. 2004. Opinion of the Scientific Panel on Animal Health and Welfare on a request from the Commission related to welfare aspects of the main systems of stunning and killing the main commercial species of animals. Efsa J. 45:1–29.
- Erikson U, Sigholt T, Rustad T, Einarsdottir IE, Jørgensen L. 1999. Contribution of bleeding to total handling stress during slaughter of Atlantic salmon. Aquaculture. 7:101–115.
- Kalin JR. 1996. Diagnosis of carbon monoxide poisoning by current approaches in forensic toxicology. In: Habben K, editor. Current approaches in forensic toxicology. Columbia (NC): The Forensic Toxicologist Certification Board.
- Magnoni LJ, Novais S, Eding E, Leugen I, Lemos MF, Ozorio RO, Geurden I, Prunet P, Schrama JW. 2019. Acute stress and an electrolyte-imbalanced diet, but not chronic hypoxia, increase oxidative stress and hamper innate immune status in a rainbow trout (Oncorhynchus mykiss) isogenic line. Front Physiol. 10:453.
- Mantilla D, Kristinsson HG, Balaban MO, Otwell WS, Chapman FA, Raghavan S. 2008. Carbon monoxide treatments to impart and retain muscle color in tilapia fillets. J Food Science. 73:C390–C399.
- Mc Donald DG, Milligan CL. 1997. Ionic, osmotic and acid-base regulation in stress. In Iwama GK, Pickering AD, Sumpter JP, Schreck CB, editors. Fish stress and health in aquaculture. Cambridge (UK): Society for Experimental Biology.
- Merkin GV, Roth B, Gjerstad C, Dahl-Paulsen E, Nortvedt R. 2010. Effect of pre-slaughter procedures on stress responses and some quality parameters in sea-farmed rainbow trout (Oncorhynchus mykiss). Aquaculture. 309:231–235.
- Milligan CL, Girard SS. 1993. Lactate metabolism in rainbow trout. J Exp Biol. 180:175–193.
- Mishima T, Nonaka T, Okamoto A, Tsuchimoto M, Ishiya T, Tachibana K, Tsuchimoto M. 2005. Influence of storage temperatures and killing procedures on post-mortem changes in the muscle of horse mackerel caught near Nagasaki Prefecture. Fisheries Sci. 71:187–194.
- Misimi E, Erikson U, Digre H, Skavhaug A, Mathiassen JR. 2008. Computer vision-based evaluation of pre- and postrigor changes in size and shape of Atlantic cod (Gadus morhua) and Atlantic salmon (Salmo salar) fillets during rigor mortis and ice storage: effects of perimortem handling stress. J Food Science. 73:E57–E68.
- Poli BM. 2009. Farmed fish welfare-suffering assessment and impact on product quality. Ital J Anim Sci. 8:139–160.
- Prescott LM, Harley JP, Klein DA. 1996. Microbiology. London (UK): William. C. Brown.
- Roth B, Imsland A, Moeller D, Slinde E. 2003. Effect of electric field strength and current duration on stunning and injuries in market-sized Atlantic salmon held in seawater. N Am J Aquacult. 65:8–13.
- SAS. 2004. SAS/STAT User’s Guide (Release 9.1). Cary (NC): SAS Institute.
- Schulte PM, Moyes CD, Hochachka PW. 1992. Integrating metabolic pathways in post-exercise recovery of white muscle. J Exp Biol. 166:181–195.
- Secci G, Serra A, Concollato A, Conte G, Mele M, Olsen RE, Parisi G. 2016. Carbon monoxide as stunning/killing method on farmed Atlantic salmon (Salmo salar): effects on lipid and cholesterol oxidation. J Sci Food Agric. 96:2426–2432.
- Skjervold PO, Fjaera SO, Østby PB, Einen O. 2001. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture. 192:265–280.
- Smith AS. 2001. Laboratory animal science. Oslo (Norway): The Norwegian Reference Center for Laboratory Animal Science and Alternatives.
- Thomas PM, Pankhurst NW, Bremner HA. 1999. The effect of stress and exercise on post-mortem biochemistry of Atlantic salmon and rainbow trout. J Fish Biol. 54:1177–1196.
- Ultsch GR, Ott ME, Heisler N. 1981. Acid-base and electrolyte status in carp (Cyprinus carpio) exposed to low environmental pH. J. Exp. Biol. 93:65–80.
- UNESCO. 1978. Universal Declaration of the Right of Animals [WWW Document]. [accessed 2020 Oct 1]. Available from: http://jose.kersten.free.fr/aap/pages/uk/UDAR_uk.html
- Wood CM. 1991. Acid-base and ion balance, metabolism, and their interactions, after exhaustive exercise. J Exp Biol. 160:285–308.
