Abstract
MicroRNAs (miRNAs) are an abundant class of small non-protein-coding RNAs that regulate gene expression in plants and animals. Skin colour is an important economic consideration in chicken production, and chickens with black skin have high market value. Nevertheless, little research has been conducted on miRNA regulation of melanogenesis in chicken skin. In this study, we sampled the dorsal skins of chickens with black (BS) and white (WS) skin to construct six small RNA libraries. High-throughput sequencing technology was then used to identify which miRNAs were expressed differentially between the BS and WS phenotypes. A total of 645 known and 64 novel miRNAs were identified from the six sequencing libraries. Additionally, the expression of 18 miRNAs was significantly different between the two phenotypes, including 9 miRNAs that were up-regulated, and 9 that were down-regulated. We identified 2 miRNAs, i.e. miR-204 and miR-6631-5p, that may be important for melanogenesis in chicken skin. Our data contribute to the understanding of the molecular mechanism, through miRNA regulation, of melanin formation in chicken skin.
Differentially expressed miRNAs were identified in black skin (BS) and white skin (WS) of black-bone chickens
Eighteen DEMs were detected in BS and WS groups.
miR-204 and miR-6631-5p were potential candidates influencing skin melanogenesis in chicken.
Highlights
Keywords:
Introduction
Skin colour is an important economic consideration in chicken production. The Muchuan black-bone chicken has a deep black skin and is deemed to produce high quality meat (Yu et al. Citation2017). As such, black-bone chickens sell for a higher price than chickens with white skin. However, birds with white or lighter skin can be produced during the breeding process for black-bone chickens, which leads to economic losses. Research on chicken genetics and breeding has largely focussed on identifying genes associated with skin colour with the aim of increasing the amount of melanin in the skin using genetic techniques (Zhang et al. Citation2015a, Citation2015b). To date, more than 150 genes and loci associated with skin colour that influence pigmentation have been discovered (Cieslak et al. Citation2011). Still, the key regulators and regulatory mechanisms that govern skin colour remain unknown.
MicoRNAs (miRNAs) are approximately 19–24 nucleotides (nt) in length and are a type of endogenous non-coding RNA that regulate gene expression by targeting mRNAs for cleavage or translational repression (Bartel Citation2004). Endogenous miRNAs help to regulate various metabolic processes, including cell cycle progression and cell proliferation, differentiation, development, apoptosis, and migration (Brennecke et al. Citation2003; Shenoy and Blelloch Citation2014). Previous research has indicated that miRNAs can target genes or pathways involved in melanocyte biology (Mione and Bosserhoff Citation2015). In other vertebrates, such as ducks (Apopo et al. Citation2015), alpacas (Xue et al. Citation2012), goats (Wu et al. Citation2014), mice (Dynoodt et al. Citation2013), and fish (Yan et al. Citation2013), miRNAs are known to be responsible for pigmentation. However, no studies to date have reported on the role of miRNAs in determining skin colour in chickens. The black-bone chicken has intense pigmentation in its dermal skin layer across its entire body. Because skin colour in the black-bone chicken can vary (including white skin), the black-bone chicken is an ideal model for investigating the molecular mechanism of melanin pigmentation during melanogenesis in chicken skin.
In this study, RNA sequencing (RNA-Seq) was used to investigate miRNA expression profiles in chickens with black and white skin. We were able to identify miRNAs associated with melanogenesis that were differentially expressed during skin colour formation, extending the knowledge of miRNAs involved with melanin formation in chicken skin.
Materials and methods
Ethics statement
All experimental procedures were approved by the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, revised in June 2004) and the Animal Care and Use Committee of the Leshan Normal University.
Animal selection and phenotype measurement
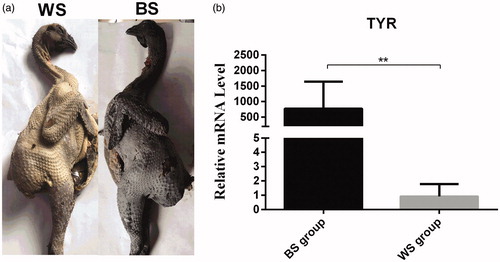
A total of 133 female Muchuan black-bone chickens (40 weeks old) were purchased from the Muchuan County Black Phoenix Black-Bone Chicken Industry Co., Ltd. (Leshan, China) for use in this study. All chickens had free access to feed and water and were exposed to natural light (12 h/day) and temperatures of 17–25 °C during the study. The dorsal skin colour of each chicken was measured using a portable colorimeter (3NH NR10QC; Shenzhen 3NH Technology Co., Ltd., Shenzhen, China). Colours were recorded as L*, a lightness value ranging from 0 (completely black) to 100 (completely white); a*, a colour scale ranging from green (negative) to red (positive); and b*, a colour scale ranging from blue (negative) to yellow (positive) (Škrlep and Čandek-Potokar Citation2007). While measuring, the colorimeter was in full contact with the skin to avoid losing any light from the instrument. Of the 133 chickens, we selected three individuals with black skin (group BS) and three with white skin (group WS) for RNA samples (Supplementary Table S1). Phenotypic differences between the two groups were confirmed by quantifying the expression of the tyrosinase (TYR) gene.
Tissue collection and RNA isolation
The chickens in the BS and WS groups were killed by exsanguination to obtain skin samples. The dorsal skins were immediately frozen in liquid nitrogen and then stored at −80 °C. Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. RNA degradation and contamination were monitored using 1% agarose gels. RNA purity and concentrations were checked using the NanoPhotometer® spectrophotometer (Implen, Westlake Village, CA) and the Qubit® RNA Assay Kit of the Qubit® 2.0 Fluorometer (Life Technologies, Carlsbad, CA). RNA integrity was also assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA).
Small RNA library construction and sequencing
Six small RNA libraries were constructed using the NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (New England Biolabs, Ipswich, MA) following the manufacturer’s instructions. Index codes were added so that sequences could be attributed to the proper sample. From each sample, 3 μg total RNA was used for library construction. The small RNAs were first ligated with Illumina 3t and 5a adapters. Then, first strand cDNAs were synthesised using M-MuLV Reverse Transcriptase (reverse ribonuclease H). Polymerase chain reaction (PCR) amplification was performed using the LongAmp Taq 2X Master Mix. PCR products were purified using 8% polyacrylamide gel (100 V, 80 min). DNA fragments of length 140–160 bp were recovered and dissolved in 8 μL elution buffer. Finally, library quality was assessed using the Agilent Bioanalyzer 2100 system using DNA High Sensitivity Chips. All small RNA libraries were sequenced with 50-bp paired end reads using the Illumina HiSeq2500 platform. The raw sequencing data have been deposited in the NCBI Sequence Read Archive (SRA) with accession number PRJNA515587.
Data processing
High-quality clean reads were obtained from raw data after removing reads with poly-N tails, 5i adapter contaminants, poly-A, -T, -G, or -C sequences, reads without 3a adapters or insert tags, and other low-quality reads. Clean reads were mapped to the Gallus_gallus-5.0 assembly of the chicken genome using Bowtie v. 1.2.2 (Langmead et al. Citation2009). After mapping, the small RNA reads were matched against known miRNAs in the miRBase 21.0 database (Ana and Sam Citation2014). Small RNA reads originating from protein-coding genes, repeat sequences, rRNA, tRNA, small nuclear RNA (snRNA), and small nucleolar RNA (snoRNA) were also identified by alignment with the publicly available Rfam (http://rfam.sanger.ac.uk) and RepBase (http://www.girinst.org/repbase/) databases. Potential novel miRNAs were predicted from the remaining unannotated clean reads using miREvo (Wen et al. Citation2012) and mirdeep2 (Friedländer et al. Citation2012) software. Small RNA tags were aligned with mature miRNA with an allowance of one positional mismatch to detect potential miRNA base edits. Additionally, the miFam.dat data file from miRBase (http://www.mirbase.org/ftp.shtml) was used to look for miRNA families, whereas searches for the families of novel miRNA precursors were performed using the Rfam database (http://rfam.sanger.ac.uk/search/).
Identification of differentially expressed miRNAs
Relative miRNA expression was estimated by transcript per million clean tags (TPM) as follows: normalised expression = (mapped readcount/total reads)×1,000,000 (Zhou et al. Citation2010). The expression levels of the BS and WS groups were compared using the DESeq2 package (v. 1.8.3) in R (Anders and Huber, Citation2010). Significance thresholds for differences in expression were set at p < .05 and |log2 (fold change)| >1.
Predicting target genes and functional annotation
To understand the functional role of miRNAs that were differentially expressed in skin melanogenesis for the two chicken groups, the target genes of these miRNAs were predicted using the RNAhybrid (Rehmsmeier et al. Citation2004), PITA (Kertesz et al. Citation2007), and miRanda (Betel et al. Citation2008) analysis tools. Gene ontology (GO) analysis of the target genes was then conducted using the GOseq method (Young et al. Citation2010). In this analysis, p < .001 represents significantly enriched GO terms. Pathway enrichment analysis based on the Kyoto Encyclopaedia of Genes and Genomes (KEGG) Pathway database (http://www.genome.jp/kegg/pathway.html) was carried out using KOBAS v. 2.0 (Mao et al. Citation2005).
Validation of candidate miRNA and their target genes expression using quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to detect miRNAs that were differentially expressed in the two chicken groups. The total RNA from the same samples used for RNA sequencing was reverse-transcribed to cDNA using the TransScript® miRNA First-Strand Synthesis Kit (Transgen, Beijing, China). Subsequently, miRNA and gene quantification were conducted using the Lightcycler Roche 480 system (Roche, Basel, Switzerland) with the LightCycler 480 SYBR Green I Master kit. Forward primers were designed using the mature miRNA sequences (Supplementary Table S2), and reverse primers were produced using a first-strand cDNA synthesis kit (Transgen, Beijing, China). The qRT-PCR primers of miRNA-target genes were listed in Supplementary Table S2. All reactions were performed in triplicate for each sample, and the chicken U6 small-nuclear RNA (U6 snRNA) and β-actin were used to normalise the qRT-PCR results for each miRNA and gene, respectively (Sun et al. Citation2016). Relative miRNA expression was calculated using the 2−ΔΔCT method (Livak and Schmittgen Citation2001).
Results
Phenotypic confirmation
TYR codes for tyrosinase, a multifunctional enzyme that is essential for melanin biosynthesis in melanocytes (Yu et al. Citation2019). TYR expression was used to confirm that the differences in skin colour had a genetic basis (Figure ). Our results revealed that TYR expression was significantly different (p < .01) between chickens with black and those with white skins (Figure ). Therefore, the chickens selected for the BS and WS groups were suitable candidates for screening the miRNAs involved in skin colouration.
Overview of miRNA sequencing
A total of 57.36 million and 59.76 million raw reads were obtained from the skins of BS and WS chickens, respectively. After filtering, we obtained more than 18 million clean reads for each library, with a mean clean read ratio of 99.09% (Supplementary Table S3). Most of the reads (>79%) were 21–23 nt in length, and the 22-nt small RNA was the most abundant (>51%). Almost all the small RNAs (>89%) mapped to the reference genome. The Q20 and Q30 values, which represent sequencing accuracy, were greater than 99% and 98% for each sample, respectively (Supplementary Table S3). These results indicate that small RNA sequences were reliably produced in our study, and the mapped reads were suitable miRNA candidates in the subsequent analyses.
Annotation of small RNA sequences
The mapped small RNA reads were annotated according to references in the miRBase21.0 database. Most small RNAs were categorised as miRNAs (mean 53.14%) or were unannotated (mean 54.98%; Supplementary Table S4). The percentages of small RNAs annotated as rRNA, tRNA, snRNA, snoRNA, repeat sequences, exons, and introns were relatively low, ranging from 3.24 to 8.47%.
Identification of known chicken miRNAs and candidates for novel miRNAs
To identify the miRNAs in chicken skin, the sequences of mapped reads were compared with those of known mature miRNAs and their precursors in miRBase v21.0. A total of 645 known miRNAs and 64 novel miRNAs were identified from the six chicken sequence libraries (Supplementary Tables S5 and S6). In terms of miRNA expression, the read numbers of the 10 most prolific miRNAs comprised more than 65% of the total reads for each sequence library. Our analysis of miRNA expression revealed that most miRNAs were expressed by a small proportion of the miRNA genes, consistent with other chicken studies (Wu et al. Citation2017a, Citation2017b). The miRNA gga-miR-143-3p had the highest expression level in both groups. Each miRNA was expressed at different levels among the six libraries (Supplementary Table S5). Most of the novel miRNAs were expressed at low levels compared to the known miRNAs. For example, the highest TPM of novel-353, a novel miRNA, were 25 and 492 in the BS and WS groups, respectively. Similar expression patterns have been reported in studies of chickens (Wu et al. Citation2017a) and other species (Zhiqiang et al. Citation2013; Wei et al. Citation2018). Additionally, results from the base bias analysis of known miRNAs in each library revealed that uracil (U) was the most common base at the first-nucleotide position in miRNAs with lengths of 20, 22, and 24 nt (Supplementary Figure S1). U was also most common at the last-nucleotide position in 22-nt miRNAs (Supplementary Figure S2).
Identification of miRNAs differentially expressed between black and white chickens
To identify candidate miRNAs associated with skin melanogenesis, relative expression was first calculated and normalised with reference to TPM. The miRNAs that were differentially expressed between the BS and WS groups were then identified using DESeq2. In total, 18 miRNAs had significantly different expression levels between the two groups, including 8 up-regulated and 8 down-regulated miRNAs (Table ).
Table 1. The microRNAs (miRNAs) that were differentially expressed between chickens with black and white skin.
Target gene prediction and gene functional annotation
Target gene prediction helps to determine the potential downstream biological functions of miRNAs. We predicted that the miRNAs that were differentially expressed between the two chicken groups target a total of 755 genes. Some of these predicted target genes and their related families, such as the solute carrier family, the Ras oncogene family, and the tumour necrosis factor receptor superfamily, are known to be involved in melanogenesis. Furthermore, we conducted GO annotation and KEGG pathway analysis to identify the potential functional roles of these differentially expressed miRNAs. From these analyses, 29 significantly enriched GO terms and 5 KEGG pathways were identified. In GO terms, the target genes were strongly involved with ‘cellular component’ and ‘biological process’ (Supplementary Table S7). Pathway analysis results revealed that the target genes were mainly involved with purine and pyrimidine metabolism, base excision repair, and glycosaminoglycan biosynthesis of chondroitin sulphate and RNA polymerase (Supplementary Table S7).
Validation of miRNAs differentially expressed and their target genes between black and white chickens
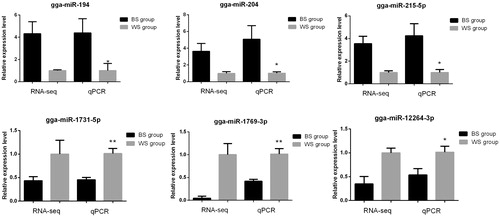
We used qRT-PCR to validate the expression levels of 6 miRNAs that were differentially expressed between the two groups. We selected 3 up-regulated miRNAs. i.e. gga-miR-194, gga-miR-204, and gga-miR-215-5p, and 3 down-regulated miRNAs, i.e. gga-miR-1731-5p, gga-miR-1769-3p, and gga-miR-12264-3p. The results from qRT-PCR matched those from our RNA-Seq analysis (Figure ), confirming that similar expression patterns were observed using both methods.
Figure 2. Validation of the microRNAs that were differentially expressed between chickens with black and white skin using quantitative real-time polymerase chain reaction (qPCR). Transcript per million clean tags values were used to calculate gene expression in RNA sequencing (RNA-seq) and to normalise gene expression in the WS group to the value of ‘1’. *p < .05, **p < .01. All data are presented as mean ± standard error. BS: chickens with black skin; WS: chickens with white skin.
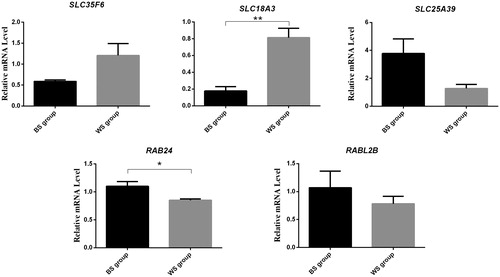
Five candidate genes: SLC35F6, SLC18A3, SLC25A39, RAB24 and RABL2B, were predicted to be the target genes of the miRNAs gga-miR-1454, gga-miR-204, gga-miR-12264-3p, gga-miR-6631-5p and gga-miR-1731-5p, respectively. Expression levels of these miRNA targets in the skin tissues of BS and WS were assessed by qRT-PCR. The results showed that the expression levels of SLC18A3 and RAB24 had significantly different between the BS and WS groups (Figure ).
Discussion
We investigated miRNA expression profiles in the skins of black and white phenotypes of the black-bone chicken to elucidate the regulation and roles of miRNAs in skin pigmentation, particularly melanogenesis. In total, 18 miRNAs were found to be differentially expressed between the skins of black and white chickens. These include gga-miR-204, which is a miRNA known to be associated with melanogenesis. The expression of gga-miR-204 is significantly different between black and white feather bulbs in ducks (Apopo et al. Citation2015), and is also associated with skin colour in fish (Yan et al. Citation2013). In our study, gga-miR-204 was up-regulated in chickens with black skin, similar to results from previous studies (Adijanto et al. Citation2012b; Apopo et al. Citation2015). ; In humans, the microphthalmia-associated transcription factor (MITF) gene and gga-miR-204 are necessary for the maintenance of retinal pigment epithelial (RPE) cells. MITF promotes the differentiation of RPE cells by regulating gga-miR-204 expression (Adijanto et al. Citation2012a, Citation2012b; Kutty et al. Citation2016), using the transcription factor PAX6 to up-regulate gga-miR-204 (Shaham et al. Citation2013). At the start of melanogenesis in the RPE, PAX6 also synergizes with MITF to activate the expression of the genes (TYR, TYRP1, and dopachrome tautomerase) involved in pigment biogenesis (Shaul et al. Citation2014). MITF has been reported to regulate PAX6 expression in chicken RPE cells as well (Mochii et al. Citation1998). Additionally, our previous research has revealed that MITF expression is associated with skin colour in the black-bone chicken (Wang et al. Citation2018). These findings suggest that gga-miR-204 may play an important role in melanogenesis in chicken skin. Further studies are required to elucidate the molecular mechanisms that link gga-miR-204 to skin colour in chickens.
MiRNAs are key regulators in the post-transcriptional modifications of several biological processes (Krol et al. Citation2010). The majority of miRNAs have several to several thousand gene targets (Shiwei et al. Citation2014). Thus, it is challenging to identify specific targets in the study of miRNA function. To elucidate the potential regulatory network that affects skin colour in chickens, we predicted the target genes of the miRNAs that were differentially expressed between the black and white chickens. SLC18A3 was predicted to be the target gene of the miRNA gga-miR-204. Expression levels of SLC18A3 in the skin tissues of BS and WS were assessed by qRT-PCR. The results showed that SLC18A3 had significantly lower expression levels in BS than in WS. In humans and other vertebrates (fish, mice, birds, horses, sheep, and Xenopus laevis), members of the SLC family such as SLC24A5, SLC24A4, SLC7A11, SLC2A11B, SLC36A1, and SLC45A2 have also been reported to be associated with melanin synthesis or pigmentation (Mariat et al. Citation2003; Gunnarsson et al. Citation2007; Ginger et al. Citation2008; Sturm Citation2009; Liu et al. Citation2011; He et al. Citation2012; Yuan et al. Citation2015). Results from our previous study revealed that five SLC members, SLC6A9, SLC38A4, SLC22A5, SLC35F3, and SLC16A3, were also associated with melanogenesis in the muscles of black-bone chickens (Yu et al. Citation2018). The SLC superfamily comprises a major group of membrane transport proteins that are present in various cells (Amber et al. Citation2009). However, only a few SLC members are known to be associated with pigmentation and melanogenesis. Our results indicate that gga-miR-204 and its SLC target gene may be important for pigmentation in chicken skin. Further research will help to clarify the relationship of gga-miR-204 and SLC18A3 with melanogenesis in chicken skin.
Ras-related proteins not only are critical regulators of cellular membrane trafficking (Pfeffer Citation2001) but also are involved in a variety of processes such as skin pigmentation (Wasmeier et al. Citation2006). To date, several Rab proteins such as RAB27A (Strom et al. Citation2002; Hume et al. Citation2007; Yoshida-Amano et al. Citation2012), RABRP1 (Fujikawa et al. Citation2002), RAB8 (Chabrillat et al. Citation2005), RAB11B (Tarafder et al. Citation2014), RAB29 (Yu et al. Citation2018), RAB7 (Jordens et al. Citation2006), RAB38 (Brooks et al. Citation2007), RAB9A (Mahanty et al. Citation2016), and RAB32 (Wasmeier et al. Citation2006) have been reported to have crucial roles in the pigmentation process, such as melanosome biogenesis, degradation, and transport. In this study, we predicted that gga-miR-6631-5p target RAB24, that belong to the Ras oncogene family. We have also found that RAB24 was expressed significantly higher in BS than that in WS. RAB27A can regulate the peripheral distribution of melanosomes in melanocytes, resulting in tissue colouration (Hume et al. Citation2001). Mutations in RAB27A can also disrupt melanosome transport, thereby affecting melanosome transfer to the surrounding tissues such as skin, feathers, or hair (Cieslak et al. Citation2011). Additionally, RAB32 and RAB38 are involved in the transport of melanogenic enzymes, including TYR and TYRP1, to melanosomes in melanocytes (Wasmeier et al. Citation2006; Marubashi et al. Citation2016). The expression of RAB29 mRNA was reported to be associated with muscle pigmentation in chickens as well (Yu et al. Citation2018). Therefore, we postulated that gga-miR-6631-5p may affect skin colour in the black-bone chicken by regulating the expression of its respective target gene (RAB24).
Conclusions
We used RNA-Seq to identify melanin-related miRNAs and their target genes in the black-bone chicken. In total, 18 differentially expressed miRNAs were identified between the skins of black and white chickens. MiR-204 and miR-6631-5p, were potential candidates influencing skin colour of chicken. Although additional studies are needed to characterise the functional roles of these miRNAs and their target genes in the regulation of skin melanogenesis, our results contribute an initial understanding of the molecular mechanisms governing the production of black or white skin in chickens.
Supplemental Material
Download MS Excel (11.8 KB)Supplemental Material
Download MS Excel (13.4 KB)Supplemental Material
Download MS Excel (49 KB)Supplemental Material
Download MS Word (14.9 KB)Supplemental Material
Download MS Word (14.7 KB)Supplemental Material
Download MS Word (16 KB)Supplemental Material
Download MS Word (14.2 KB)Supplemental Material
Download JPEG Image (848 KB)Supplemental Material
Download JPEG Image (666.4 KB)Disclosure statement
The authors have declared that no competing interests exist.
Additional information
Funding
References
- Adijanto J, Castorino JJ, Grunwald GB, Philp NJ. 2012a. MicroRNAs 204/211 promotes differentiation of human Retinal Pigment Epithelial (RPE) cells. Invest Ophthalmol Vis Sci. 53:1129.
- Adijanto J, Castorino JJ, Wang ZX, Maminishkis A, Grunwald GB, Philp NJ. 2012b. Microphthalmia-associated transcription factor (MITF) promotes differentiation of human retinal pigment epithelium (RPE) by regulating microRNAs-204/211 expression. J Biol Chem. 287:20491–20503.
- Amber D, Josh R, Hohmann JG, Joanne W. 2009. Expression profiling of the solute carrier gene family in the mouse brain. J Pharmacol Exp Ther. 329:558–570.
- Ana K, Sam GJ. 2014. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42:D68–D73.
- Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11(10)doi:10.1186/gb-2010-11-10-r106.
- Apopo S, Liu H, Jing L, Du X, Xie S, Gong Y, Xu R, Li S. 2015. Identification and profiling of microRNAs associated with white and black plumage pigmentation in the white and black feather bulbs of ducks by RNA sequencing. Anim Genet. 46:627–635.
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297.
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. 2008. The microRNA.org resource: targets and expression. Nucleic Acids Res. 36 :D149–D153.
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. 2003. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 113:25–36.
- Brooks BP, Larson DM, Chan CC, Kjellstrom S, Smith RS, Crawford MA, Lamoreux L, Huizing M, Hess R, Jiao X, et al. 2007. Analysis of ocular hypopigmentation in Rab38cht/cht mice. Invest Ophthalmol Vis Sci. 48:3905.
- Chabrillat ML, Wilhelm C, Wasmeier C, Sviderskaya EV, Louvard D, Coudrier E. 2005. Rab8 regulates the actin-based movement of melanosomes. Mol Biol Cell. 16:1640–1650.
- Cieslak M, Reissmann M, Hofreiter M, Ludwig A. 2011. Colours of domestication. Biol Rev Camb Philos Soc. 86:885–899.
- Dynoodt P, Mestdagh P, Peer GV, Vandesompele J, Goossens K, Peelman LJ, Geusens B, Speeckaert RM, Lambert JLW, Gele M. 2013. Identification of miR-145 as a key regulator of the pigmentary process. J Invest Dermatol. 133:201–209.
- Friedländer MR, Mackowiak SD, Li N, Chen W, Rajewsky N. 2012. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 40:37–52.
- Fujikawa K, Satoh AK, Kawamura S, Ozaki K. 2002. Molecular and functional characterization of a unique Rab protein, RABRP1, containing the WDIAGQE sequence in a GTPase motif. Zool Sci. 19:981–993.
- Ginger RS, Askew SE, Ogborne RM, Wilson S, Ferdinando D, Dadd T, Smith AM, Kazi S, Szerencsei RT, Winkfein RJ, et al. 2008. SLC24A5 encodes a trans-Golgi network protein with potassium-dependent sodium-calcium exchange activity that regulates human epidermal melanogenesis. J Biol Chem. 283:5486–5495.
- Gunnarsson U, Hellström AR, Tixier-Boichard M, Minvielle F, Bed'hom B, Ito S, Jensen P, Rattink A, Vereijken A, Andersson L. 2007. Mutations in SLC45A2 cause plumage color variation in chicken and Japanese quail. Genetics. 175:867–877.
- He X, Li H, Zhou Z, Zhao Z, Li W. 2012. Production of brown/yellow patches in the SLC7A11 transgenic sheep via testicular injection of transgene. J Genet Genomics. 39:281–285.
- Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. 2001. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 152:795–808.
- Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. 2007. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 120:3111–3122.
- Jordens I, Westbroek W, Marsman M, Rocha N, Mommaas M, Huizing M, Lambert J, Naeyaert JM, Neefjes J. 2006. Rab7 and Rab27a control two motor protein activities involved in melanosomal transport. Pigment Cell Res. 19:412–423.
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. 2007. The role of site accessibility in microRNA target recognition. Nat Genet. 39:1278–1284.
- Krol J, Loedige I, Filipowicz W. 2010. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 11:597–610.
- Kutty RK, Samuel W, Boyce K, Cherukuri A, Duncan T, Jaworski C, Nagineni CN, Redmond TM. 2016. Proinflammatory cytokines decrease the expression of genes critical for RPE function. Mol Vis. 22:1156–1168.
- Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10:R25.
- Liu XF, Luo J, Hu XX, Yang H, Lv XQ, Feng CG, Tong J, Wang YQ, Wang SH, Liu XJ. 2011. Repression of Slc24a5 can reduce pigmentation in chicken. Front Biosci. E3:158–165.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C(T)) method. Methods. 25:402–408.
- Mahanty S, Ravichandran K, Chitirala P, Prabha J, Jani RA, Setty S. 2016. Rab9A is required for delivery of cargo from recycling endosomes to melanosomes. Pigment Cell Melanoma Res. 29:43–59.
- Mao X, Cai T, Olyarchuk JG, Wei L. 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 21:3787–3793.
- Mariat D, Taourit S, Guérin G. 2003. A mutation in the MATP gene causes the cream coat colour in the horse. Genet Sel Evol. 35:15.
- Marubashi S, Shimada H, Fukuda M, Ohbayashi N. 2016. RUTBC1 functions as a GTPase-activating protein for Rab32/38 and regulates melanogenic enzyme trafficking in melanocytes. J Biol Chem. 291:1427–1440.
- Mione M, Bosserhoff A. 2015. MicroRNAs in melanocyte and melanoma biology. Pigment Cell Melanoma Res. 28:340–354.
- Mochii M, Mazaki Y, Mizuno N, Hayashi H, Eguchi G. 1998. Role of mitf in differentiation and transdifferentiation of chicken pigmented epithelial cell. Dev Biol. 193:47–62.
- Pfeffer SR. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487–491.
- Rehmsmeier M, Steffen P, Hochsmann M, Giegerich R. 2004. Fast and effective prediction of microRNA/target duplexes. RNA. 10:1507–1517.
- Shaham O, Gueta K, Mor E, Oren-Giladi P, Grinberg D, Xie Q, Cvekl A, Shomron N, Davis N, Keydar-Prizant M, et al. 2013. Pax6 regulates gene expression in the vertebrate lens through miR-204. PLoS Genet. 9:e1003357.
- Shaul R, Kapil B, Sigal RL, Yamit CT, Rachel S, Naveh E, Eran M, Alona Z, Maria I, Benjamin R. 2014. PAX6 regulates melanogenesis in the retinal pigmented epithelium through feed-forward regulatory interactions with MITF. PLoS Genet. 10:e1004360.
- Shenoy A, Blelloch RH. 2014. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 15:565–576.
- Shiwei D, Yunliang W, Hongwei W, Shufei W, Lindan J, Dongjun D, Danjie J, Xiaoxi Z, Qiang W. 2014. A novel PCR-based approach to discover miRNA target genes. Int J Med Sci. 11:1270–1274.
- Škrlep M, Čandek-Potokar. M. 2007. Pork color measurement as affected by bloom time and measurement location. J Muscle Foods. 18:78–87.
- Strom M, Hume AN, Tarafder AK, Barkagianni E, Seabra MC. 2002. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 277:25423–25430.
- Sturm RA. 2009. Molecular genetics of human pigmentation diversity. Hum Mol Genet. 18:9–17.
- Sun L, Dong H, Zhang Z, Liu J, Hu Y, Ni Y, Grossmann R, Zhao R. 2016. Activation of epithelial proliferation induced by Eimeria acervulina infection in the duodenum may be associated with cholesterol metabolism. Oncotarget. 7:27627–27640.
- Tarafder AK, Bolasco G, Correia MS, Pereira FJC, Iannone L, Hume AN, Kirkpatrick N, Picardo M, Torrisi MR, Rodrigues IP, et al. 2014. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J Invest Dermatol. 134:1056–1066.
- Wang G, Liao J, Tang M, Yu S. 2018. Genetic variation in the MITF promoter affects skin color and transcriptional activity in black-boned chickens. Br Poult Sci. 59:21–27.
- Wasmeier C, Romao M, Plowright L, Bennett DC, Raposo G, Seabra MC. 2006. Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol. 175:271–281.
- Wei W, Li B, Liu K, Jiang A, Dong C, Jia C, Chen J, Liu H, Wu W. 2018. Identification of key microRNAs affecting drip loss in porcine longissimus dorsi by RNA-Seq. Gene. 647:276–282.
- Wen M, Shen Y, Shi S, Tang T. 2012. miREvo: an integrative microRNA evolutionary analysis platform for next-generation sequencing experiments. BMC Bioinformatics. 13:140.
- Wu Z, Fu Y, Cao J, Yu M, Tang X, Zhao S. 2014. Identification of differentially expressed miRNAs between white and black hair follicles by RNA-sequencing in the goat (Capra hircus). Int J Mol Sci. 15:9531–9545.
- Wu N, Gaur U, Zhu Q, Chen B, Xu Z, Zhao X, Yang M, Li D. 2017a. Expressed microRNA associated with high rate of egg production in chicken ovarian follicles. Anim Genet. 48:205–216.
- Wu N, Zhu Q, Chen B, Gao J, Xu Z, Li D. 2017b. High-throughput sequencing of pituitary and hypothalamic microRNA transcriptome associated with high rate of egg production. BMC Genomics. 18:255.
- Xue T, Jiang J, Fan R, Wang H, Meng X, He X, He J, Li H, Geng J, Yu X, et al. 2012. Identification and characterization of microRNAs in white and brown alpaca skin. BMC Genomics. 13:555.
- Yan B, Liu B, Zhu CD, Li KL, Yue LJ, Zhao JL, Gong XL, Wang CH. 2013. microRNA regulation of skin pigmentation in fish. J Cell Sci. 126:3401–3408.
- Yoshida-Amano Y, Hachiya A, Ohuchi A, Kobinger GP, Kitahara T, Takema Y, Fukuda M. 2012. Essential role of RAB27A in determining constitutive human skin color. PLoS One. 7:e41160.
- Young MD, Wakefield MJ, Smyth GK, Oshlack A. 2010. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11:R14.
- Yu S, Wang G, Liao J, Tang M. 2019. Five alternative splicing variants of the TYR gene and their different roles in melanogenesis in the Muchuan black-boned chicken. Br Poult Sci. 60:8–14.
- Yu S, Wang G, Liao L, Tang M. 2018. Transcriptome profile analysis identifies candidate genes for the melanin pigmentation of breast muscle in Muchuan black-boned chicken. Poult Sci. 97:3446–3455.
- Yuan L, Mikki B, William B, Kuan Y, Manfred S, Walter RB. 2015. Molecular genetic response of Xiphophorus maculatus-X. couchianus interspecies hybrid skin to UVB exposure. Comp Biochem Physiol C Toxicol Pharmacol. 178:86–92.
- Yu S, Liao J, Tang M, Wang Y, Wei X, Mao L, Zeng C, Wang G. 2017. A functional single nucleotide polymorphism in the tyrosinase gene promoter affects skin color and transcription activity in the black-boned chicken. Poult Sci. 96:4061–4067.
- Zhang J, Liu F, Cao J, Liu X. 2015a. Skin transcriptome profiles associated with skin color in chickens. PLoS One. 10:e0127301.
- Zhang XD, Wang HH, Zhang CX, Li QH, Chen XH, Lou LF. 2015b. Analysis of skin color change and related gene expression after crossing of Dongxiang black chicken and ISA layer. Genet Mol Res. 14:11551–11561.
- Zhiqiang X, Jiaping C, Xuguang L, Jiachun G, Jianlin P, Xiaofeng X. 2013. Identification and characterization of microRNAs in channel catfish (Ictalurus punctatus) by using Solexa sequencing technology. PLoS One. 8:e54174.
- Zhou L, Chen J, Li Z, Li X, Hu X, Huang Y, Zhao X, Liang C, Wang Y, Sun L, et al. 2010. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS One. 5:e15224.