Abstract
In view of the important progress made in veterinary science and the increasing availability of new reproductive technologies in the 21st century, demand for canine artificial insemination (CAI) for medical and/or breeding management reasons is growing worldwide resulting in raising some legal and ethical concerns. Currently, imported chilled and frozen semen from European countries can be sold to the final consumer with no strings attached. To compound this problem, the importation in Europe has no bio-security procedures. Given the potential role of this canine semen’s movements in spreading of bacterial (i.e. leptospirosis), parasitic (i.e. leishmaniasis), viral (i.e. canine herpesvirus) or genetic (i.e. progressive retinal atrophy) diseases from one state to another, the authors propose the application of regulatory measures regarding importation and exportation of semen in order to secure animal welfare and health. They underline that the interests and welfare of puppies that will be born as well as the health and welfare of bitches and sperm donors should be respected. For these reasons, ethical implications related to the use of canine semen should be taken into account.
Canine artificial insemination is growing worldwide together with interest in the international semen shipment.
Canine semen’s movements could introduce disease agents or genetic diseases from one state to another.
There are several different regulatory frameworks governing the use of artificial insemination in dogs.
We suggest that the application of regulatory measures regarding importation and exportation of semen should be included in the EU legislation.
Highlights
Introduction
Canine artificial insemination (CAI) is a method that become progressively common over the past several decades that involves placing semen—fresh, chilled or frozen/thawed previously prepared in the laboratory (Table ), in the female internal genital tract without sexual contact (Payan-Carreira et al. Citation2011).
Table 1. Canine artificial insemination (CAI) schedules on the basis of the type of semen used.
Payan-Carreira et al. (Citation2011), Linde-Forsberg (Citation2005) and Mason (Citation2018) report that in the CAI could be requested for several reasons: (i) failure to mate naturally (only use dogs able to reproduce naturally); (ii) inability to achieve a mating as such as for conformational of the penis or vagina (acquired anatomical defects or pathological causes); (iii) request of breeder, for example, in order to facilitate genetic improvement or to allow a rapid method of breeding (such as using two stud dog simultaneously on the same bitch at the last mating); (iv) reduce the infectious disease risks; (v) geographical distance separating the mating pair; (vi) preserve the stud dog from any traumatic lesions; and (vii) little experience of the stud dog.
Obtaining successful pregnancies and adequate number of offspring per litter is important that the procedure be performed at the appropriate stage of the bitch’s cycle (timing of insemination), that the semen of adequate quality is used (types of semen used), and that the techniques of artificial insemination (AI) by fresh, chilled and frozen semen are performed appropriately with a good understanding of the success rates of the different procedures used.
Considering that CAI with chilled and frozen–thawed semen has become services available to dog owners worldwide increasing the interest amongst breeders and veterinarians, the demand for services to freeze semen is progressively increased (Thomassen and Farstad Citation2009). When it comes to planning a litter, most breeders they want to increase conception rates, maximise litter size and produce quality puppies, all with the safety of the bitch of the utmost importance. While natural breeding may be preferred, it is not always an option. Although using fresh or fresh-chilled semen allows for the most convenient and less technical breeding’s, frozen semen can allow for the use of superior genetics from dogs that are no longer able to be collected. CAI has been done for many years, but the ability to freeze semen and chill and ship semen has dramatically changed the ability to do planned breeding with incredible success. CAI are now the standard for many kennels, and it is now possible to successfully breed with semen from almost anywhere in the world reducing stress for the bitch and travel costs for the breeder. For these reasons, some legislative and ethical aspects will be discussed.
Risk of disease transmission via AI of specific pathogens originating from donor
Some diseases may be transmitted via AI, given that some organisms may infect the male reproductive system and may pass into semen. However, not all organisms that may infect the reproductive system may pass into semen and not all organisms shed into semen remain viable in semen.
Diseases of greatest concern are viral (canine herpesvirus and rabies), bacterial (leptospirosis and brucellosis) and parasitic (leishmaniasis and toxoplasmosis)
Rabies
Rabies is an acute fatal viral illness of the central nervous system that infects domestic and wild animals. It is also a viral zoonosis and dogs are the major vectors (Drew Citation2004; Fekadu Citation1993). The disease is cosmopolitan except for some countries that have eradicated it or have remained free of rabies due to the natural protection as islands or enforcing rigorous quarantine regulation (Mucheru et al. Citation2014) or vaccination programmes. Transmission of rabies virus usually begins when infected saliva of a host (i.e. a rabid dog) is passed to an uninfected organism (i.e. a human being; Debbie Citation1974). To date, the most common mode of rabies virus transmission is through the bite and virus-containing saliva of an infected host (Fekadu et al. Citation1982). Other transmission routes exist. These include contamination of mucous membranes (i.e. eyes, nose and mouth), aerosol transmission, and corneal and organ transplantations. However, these alternative transmission routes have been rarely documented (Jackson and Wunner Citation2007; Kumar Citation2009). There is only one report of rabies transmission following a mating with a male dog with a history of routine vaccination with local rabies containing live attenuated rabies viruses (Offiong et al. Citation2014).
Brucellosis
Infection with Brucella canis is common in Central and South America and in southern USA (Lucero et al. Citation2008) and has also been reported from Canada (Bosu and Prescott Citation1980; Brennan et al. Citation2008), Japan (Hayashi and Isayama Citation1977; Saegusa et al. Citation1978), India (Srinivasan et al. Citation1992), Philippines (Baluyut and Duguies Citation1977), Korea (Park et al. Citation2001), China (Jiang Citation1989), Malaysia (Joseph et al. Citation1983), Taiwan (Tsai et al. Citation1983) and Nigeria (Adesiyun et al. Citation1986).
In Europe, infection has also reported from Austria (SchäFer-Somi and Hofer Citation2011), Great Britain (Taylor Citation1980), Poland (Kopczewski et al. Citation1995), Hungary (Gyuranecz et al. Citation2011), Spain (Mateu-de-Antonio et al. Citation1994), Italy (Corrente et al. Citation2010; Ebani et al. Citation2003), Germany (Nockler et al. Citation2003; Weber and Schliesser Citation1975, Citation1978) and Czechoslovakia (Sebek et al. Citation1976).
B. canis
It can be detected from the prostate, epididymis and semen, or in female dogs in vaginal secretion. Dogs are most commonly infected by contact with vaginal discharges at oestrous or after abortions, at mating or via AI (Carmichael et al. Citation1984; Keid et al. Citation2007a,Citationb). The concentration of bacteria in semen is highest from 1 to 4–6 months after infection (Carmichael and Joubert Citation1988).
Diagnosis is made by culture, polymerase chain reaction (PCR) or serology. Urinary cultures can be positive even if a blood culture is negative up to 8–12 weeks after infection, with a higher concentration in urine in male dogs than in females (Carmichael and Joubert Citation1988). PCR represents a more sensitive method than bacterial culture because it detects not only viable but also dead bacteria and moreover the method is not influenced by contamination with other bacteria; PCR can be done directly on the sperm (Bricker Citation2002; Kim et al. Citation2006; Keid et al. Citation2007). Serological tests, such as the commonly used rapid slide agglutination test, RSAT, have the disadvantage of false positives. Enzyme-linked immunosorbent assays have been developed (de Oliveira et al. Citation2011) and a lateral flow immune-chromatographic assay (LFIA) has recently been described (Corde et al. Citation2011). A combination of different serological tests is often recommended.
Dogs from endemic areas should be kept isolated until tested free of B. canis to avoid further spread of the disease. This is recommended for natural mating, AI with fresh, chilled or frozen semen.
Serologic tests can be negative up to 4 weeks after infection, and at least 12 weeks must pass to be sure of detecting antibodies in an infected animal (Carmichael et al. Citation1984). Therefore, two negative tests 4–6 weeks apart are needed in case the dog is incubating the disease (Hollett Citation2006). At least one of the sampling occasions should be no earlier than 12 weeks after suspected contact with an infected animal. Chronically infected male dogs can also be difficult to detect, as they too can be serologically negative. In addition to serologic methods, bacterial culture or PCR analysis can be performed to detect the bacterium. Only dogs tested free from B. canis should be used for breeding, and recommended tests depend on the level of risk.
Leptospirosis
Canine leptospirosis is an important zoonotic disease of worldwide significance and distribution (Levett Citation2001; Ward Citation2002) caused by pathogenic spirochaetes (Leptospira interrogans sensu lato, of which eight serovars are most important to dogs).
Clinically, canine leptospirosis can be acute (septicaemia, hepatitis and nephritis), chronic (abortion, stillbirth and infertility) or, in its most common form, asymptomatic.
Leptospira interrogans
It has been identified in semen of experimentally infected dogs and leptospires do colonise reproductive tissue in naturally infected dogs (Kim et al. Citation2006). Venereal transmission is reported and organisms have been cultured from semen samples held up to 48 h at 37 °C (Kim et al. Citation2006).
Although there are not studies documenting viability of leptospires in extended chilled or frozen semen, in some countries could be required testing for dogs from whom semen is collected for shipment (Linde-Forsberg Citation2001; Levy and Fontbonne Citation2007).
Canine herpesvirus
Canine herpesvirus (CHV-1) causes neonatal deaths as well as infertility due to embryonal death, abortion and stillbirths in breeding kennels (Nöthling et al. Citation2008). In newborn puppies up to 2 weeks old cause widespread focal necrosis in parenchymatous organs, while is of low pathogenicity in puppies older than 5 weeks (Appel et al. Citation1969) and may cause tracheobronchitis in adult dogs (Karpas et al. Citation1968). Apart from the important disease in newborn puppies, CHV-1 also affects reproduction of dogs in other ways: the virus may cause vesicular lesions in the vestibulum and vagina of the bitch, as well as on the penis and the preputial mucosa of dogs (Poste and King Citation1971). To date, no specific studies have been conducted on the transmission of CHV through semen.
CHV-1 is relatively unstable outside the host, so close contact is required for transmission. Transmission usually occurs by contact between susceptible individuals and the infected oral, nasal or vaginal secretions of shedding dogs. Many dogs shedding virus exhibit no clinical signs. Immunologically naive pregnant bitches are at risk of acute infection, which may be transmitted to foetuses or neonatal pups; previously infected bitches are unlikely to transmit infection (Decaro et al. Citation2008).
A vaccine is available commercially in Europe and the vaccine is not licenced for the United States, for active immunisation of bitches to prevent mortality, clinical signs and lesions in puppies resulting from canine herpes virus infections acquired in the first few days of life (Poulet et al. Citation2001). The vaccine must be administered by subcutaneous route twice during the pregnancy period: the first injection must be done during heat or 7–10 days after the presumed date of mating and the second one 1–2 weeks before the expected date of whelping. The bitches need to be revaccinated only during the pregnancy, according to same schedule.
Toxoplasmosis
Toxoplasmosis is caused by the globally distributed intracellular protozoan parasite Toxoplasma gondii. The disease has a complex epidemiology; the parasite is capable of infecting virtually all warm-blooded animals, and has a two-host life cycle. Domestic cats and other felids are the definitive hosts. All non-feline animals, including dogs and humans, are intermediate hosts (Dubey Citation2010). Clinical cases of toxoplasmosis are much more frequent in cats than in dogs (Dubey Citation2010; Dubey et al. Citation2009), which mostly suffer from neosporosis (Dubey et al. Citation2017). Dogs rarely suffer from toxoplasmosis as a primary disease, and, in most cases, the disease is linked to immunosuppression and absence of vaccination against canine distemper virus.
Neurological disease, with signs of seizures, cranial nerve deficits, tremors, ataxia, and paresis or paralysis within encephalomyelitis (Patitucci et al. Citation1997), may be seen. Most of infections in dogs and cats occur horizontally, but infectivity by the oral route depends on the parasite stage ingested (Dubey Citation2010). By contrast, the aspect of congenital infections in dogs and cats has not been reviewed in depth in the literature. In dogs, T. gondii was isolated from pups from a seropositive bitch in Australia; however, no clinical signs were seen in any of the animals (Al-Qassab et al. Citation2009). T. gondii DNA was detected in fresh semen from five out of eleven seropositive naturally infected healthy dogs in Brazil, and in vitro isolation of the parasite was achieved (Koch et al. Citation2016). However, venereal transmission had been demonstrated earlier, when fresh semen, collected from experimentally infected dogs that tested positive for T. gondii, was used for AI of four naïve bitches. Seroconversion was observed in all females seven days after AI, and reabsorption occurred in two of the dogs. The remaining bitches sustained full-term gestations, and T. gondii cysts were detected in brains of four offspring (Arantes et al. Citation2009).
Leishmaniasis
Visceral leishmaniasis (VL) is a major zoonotic disease in several parts of the world (Moreno and Alvar Citation2002; Diniz et al. Citation2008). The classical clinical manifestation of VL in dogs includes a chronic emaciating disease associated with lymphadenopathy, splenomegaly and hepatomegaly (Slappendel and Greene Citation1990; Moreno and Alvar Citation2002). Importantly, naturally infected dogs often develop genital lesions associated with the presence of amastigotes, particularly in the epididymis, prepuce, glans penis and prostate (Diniz et al. Citation2005; Mir et al. Citation2012). Furthermore, these genital lesions are associated with shedding of Leishmania in the semen of a large proportion of infected dogs (Diniz et al. Citation2005). Conversely, no specific lesions were observed in the genital tract of naturally infected bitches (Silva et al. Citation2008), in spite of reports of vertical transmission in pregnant bitches (Dubey et al. Citation2005; Masucci et al. Citation2003; Rosypal et al. Citation2005). Silva et al. (Citation2009) has demonstrated for the first time that venereal transmission of canine VL can occur in the absence of the natural vector.
Risk of transmission of genetic diseases by AI and medical viewpoint
Most modern dog breeds can inherit defects and diseases. Almost 700 hereditary diseases are registered in dogs according to Online Mendelian Inheritance in Animals (OMIA Citation2020), where information on single-locus traits and genes in 239 animal species are collected, Canine Inherited Disorders Database (UPEI Citation2020) and Inherited Diseases in Dogs (Sargan Citation2004; Nicholas et al. Citation2011; Farstad Citation2018).
The developing new breed of companion animals, the presence of an underlying unaware congenital problem in a puppy born through AI and, consequently the possibility of hereditary transmission of diseases with AI rises ethical and welfare issues that demand for ruling out clinical reasons for this technique.
This concern is previewed in the Fédération Cynologique Internationale (FCI) breeding rules, so strategies to monitor and improve the genetic health of the dog have been brought for years to the attention of the FCI of the Federation of European Animal Veterinary Associations (FECAVA), and of the Kennel Clubs (Hedhammar Citation1997, Citation1999, Citation2005; Hedhammar and Indrebø Citation2011; Hedhammar et al. Citation2011).
Indubitably genetic health is the most valid strategy in breeding dog breeds, this highlights the need to develop and implement national and international rules, regulations and strategies for the breeding and reproduction of healthy dogs.
The control of hereditary pathologies is of great importance, but too strict rules and regulations could also cause negative results especially in those breeds with limited diffusion and with a certain degree of inbreeding. Rules that are too rigid could too intense selection, thus leading to an increase in inbreeding and the reduction of genetic pools, or to an increase in the number of unregistered dogs, therefore without pedigree and any official health control.
If the genetic condition of a breed is not widely known, too strict rules can also cause the emergence or increase of other diseases that cannot be detected through genetic tests, not subjected to eradication programmes (Indrebø Citation2008).
The Code of Breeding Ethics (FCI Standing Orders, Article 122) states that breeding and the development of dog breeds must be based on long-term objectives and sound principles so that the breeding does not result in diseases, bad temperament or lack of working skills. Breeding must serve the objective of preserving and preferably extending the genetic diversity (polygenicity) of the breed.
Any dog used for the AI it must be screened for inherited diseases if a DNA-test for the disease/functional disability is available, the breeding stock should be tested in order to avoid mating of two carriers. Screening results (positive or negative) for phenotypic appearance of polygenetic diseases should be available in open registries. The results should be used to aid the selection and combination of breeding dogs. Breeding values based on screening results should when possible be computerised to facilitate selection of the breeding stock not only on the phenotypic appearance but also by indicated genotype. As a general rule the estimated breeding value for a combination should be better than the average for the breed. Screening should only be recommended for diseases and breeds where the disease has major impact on the dogs’ functional health. Results from DNA tests for inherited diseases should primarily be used to avoid breeding diseased dogs. For diseases with autosomal recessive inheritance a carrier may only be used for breeding if mated to a partner with DNA test result clear (genetically free of the mutation). Functionally healthy dogs that are homozygous for the disease mutation (DNA test result affected) must be mated to a partner with status clear to avoid compromising the welfare of the offspring. The breeder must follow rules and regulations regarding breeding restrictions to reduce the prevalence of inherited diseases and functional disabilities. Any dog used for breeding must fulfil all breed specific demands (e.g. radiographic screening, genetic testing). Health issues that cannot be diagnosed by DNA-tests or screening programmes should have equal impact in the breed specific breeding programmes.
Therefore, the International Breeding Regulations of the FCI are binding on all member countries and contract partners. The dogs with congenital and genetic faults such as, for example, congenital deafness or blindness, hare-lip, cleft palate, substantial dental defects or jaw anomalies, eyes disease, heart disease, epilepsy, cryptorchidism, albinism, improper coat colours or diagnosed severe hip and elbow dysplasia, luxating patellas may not be reproduced.
For example, in Italy it is mandatory (Technical Standards of the dog stud book, Ministerial Decree of 8 March 2005 no. 21203, Article 8) the storage and conservation (DNA-deposit) of biological sample in the following cases (ENCI Citation2020):
Dogs allowed for the selected reproduction.
Stud dogs that have produced more than 5 litters.
Stud dogs used in AI.
Foreign stud dogs in Italy at the Stud Service.
National, International and Foreign Champions of beauty and/or work.
Moreover, from 1 January 2019 (Ministerial Decree of 13 November 2019, no. 31369, Article 8), even for dogs that mate with them. Storage and conservation of biological samples is mandatory at one of the laboratory is accredited by the International Society for Animal Genetics (ISAG) and National Agency of the Italian Kennel Club (ENCI).
Once in the laboratory, the biological sample kept and remained available to the ENCI for 10 years for possible analysis of parentage performed on DNA extracted from the blood of the animal. This analysis serves to verify that the parenthood of a puppy is actually that declared. For this reason, the analysis involves comparing the genetic profiles submitted by the father and the mother, which must always be compatible with those of the puppy. The genetic profile includes evaluation of 21 microsatellites (plus amelogenin for confirmation of sex) according to profiles ISAG for the canine species. Microsatellites used are: AHT121, FH2054, AHTk253, AHTk211, CXX279, INRA21, REN162C04, AHTh171, AHTh260 REN105L03 REN54P11, REN64E19 REN169O18 AHTH130, AHT137, AMELOGENIN, REN169D01, FH2848, REN247M23, INU005, INU030, INU055 (ISAG Citation2020).
The knowledge of the underpinnings and epidemiology regarding the canine genetic disorders remain of great importance for veterinary care, for organisations such as kennel clubs, breed clubs, and for dog registries that establish guidelines for sustainable breeding practices (Donner et al. Citation2018). Nonetheless, the reduction of the spread of genetic diseases is mainly based on ethic of the breeder and of the owner but also linked to their specific knowledge and training. The large number of FCI Member Countries, the relative cultural diversity, the different means and screening programmes to control animal health, the education of breeders, the type of open databases with pedigrees and health outcomes, can make this hard. The Kennel and Breed Clubs are already making an important effort in the formation and sensitisation of the members about the topic of the risk for transmission of genetic diseases. For example, the ENCI, since 2012, offers a ‘Master dog breeder’, consisting of three sections, one of which deals with hereditary diseases and how to manage them in breeding practices. Furthermore, the Breed Clubs organise every year also meetings regarding hereditary genetic diseases and the all related available tests, with the aim also of encouraging the members to perform genetic screenings on their dogs through advantageous agreements with laboratories. In conclusion to what has been said above, the membership of breeders and owners to the Kennel and Breed Clubs has become increasingly important for them to have greater competence and ability to optimise the matching for a healthy breeding. The ENCI established, under indication of the Breed Clubs, a special and more prestigious pedigree, called ‘Selected Reproducer’, that can be obtained only by the dogs that have registered good placements in dog shows and working tests and that are not affected by hereditary genetic diseases. In addition, another way to improve genetic screenings would be to make them compulsory, in order to achieve the title of beauty and work Champion. For example, the Italian Retrievers Club decided that to formalise the Champion title the dog must be within the normal limits for Hip and Elbow dysplasia. In this way, more and more owners and breeders will look for puppies clear by parentage and will carry out combability checks of the stud dog and the bitch, in order to produce more healthy litters.
Legal measures and recommendations
In several countries of the world there are no legislative provisions regarding AI and the use of chilled and/or frozen–thawed dog semen. In the absence of a specific national regulation, most Kennel Clubs follows FCI determinations for AI, transposed to the FCI International Regulation for Breeding.
FCI recommends that AI should only be done in healthy dogs with proven fertility (article 13). In addition, in the introductory section of this regulation, FCI specifically limit the use of dogs presenting diseases possible to be transmitted to following generations and those presenting major, eliminatory defects in regard to the breed standard.
Considering the potential risk of disease transmission with the semen, from one region to another, it is necessary to secure dog health.
Imported semen carrying disease agents can come into European countries and be sold to the final consumer/owner, well before any disease would become apparent. On the basis of these considerations, put forward the following proposals:
A list of third world countries or parts thereof, from which Member States are authorised to import chilled and/or frozen semen in the Community, should be established.
It is necessary to lay down specific animal health conditions and model certificates for those third countries, taking into account the animal health situation of the third country concerned and of the canine semen to be imported, in order to prevent the introduction of disease agents that could cause significant impact to the inseminated bitches in the Community. Attention should be paid to emerging diseases and diseases that are unusual to the Community.
Sampling and testing methods used for the detection of diseases transmitted by the semen must be in accordance with those laid down in the International Office of Epizootics (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.
Finally, a public education programme on the risks to imported semen, with emphasis on responsible pet ownership, breeder and veterinarian, should be implemented.
Particularly, we recommend the points below:
Equipment used for collection, processing and storage of semen must be new or sanitised and free from contamination.
The donor dog must have been resident in the country for at least 2 months prior to the semen collection.
The donor dog must not be under any quarantine restrictions during and between the first and last semen collections.
The donor dog must not have mated naturally in the period between the blood sampling and the semen collection.
The bitches inseminated with the imported semen must not mate with another dog in the same oestrus period. When an abortion should occur, the veterinarian must be immediately notified.
The country of export must have been free from rabies for at least 12 months, and the donor dog must be vaccinated against rabies with an inactivated vaccine of at least one antigenic unit per dose (recommendation from the World Health Organisation) or a recombinant vaccine expressing the immunising glycoprotein of the rabies virus in a live virus vector at least 30 days and not more than 12 months before the collection of semen as for example in Denmark and New Zealand.
The donor dog must be tested with a negative result for B. canis using either a rapid slide agglutination test, a tube agglutination test or indirect fluorescent antibody test, on a blood sample collected between 30 and 45 days following the last collection of semen in the export consignment (such as in Sweden).
The donor dog must be tested with a negative result (less than 50% agglutination) at a serum dilution of 1:100 using a microscopic agglutination test for leptospirosis (usually Leptospira canicola and l. ichterohaemorrhagica) on a blood sample collected between 30 and 45 days following the last collection of semen in the export consignment; or be fully vaccinated against leptospirosis at least 14 days before the first collection of semen in the export consignment and the vaccination must be current at the time of the last collection of semen in the export consignment.
The donor dog must be tested with a negative result for Leishmania infantum using either indirect fluorescent antibody test or enzyme linked immunosorbent assay, on a blood sample collected between 30 and 45 days following the last collection of semen in the export consignment.
The veterinarian is responsible for ensuring that the donor is healthy and free clinical evidence of infectious diseases transmissible in semen on the day (s) of semen collection.
For this motive, each international shipment of canine semen must be accompanied by the following documents:
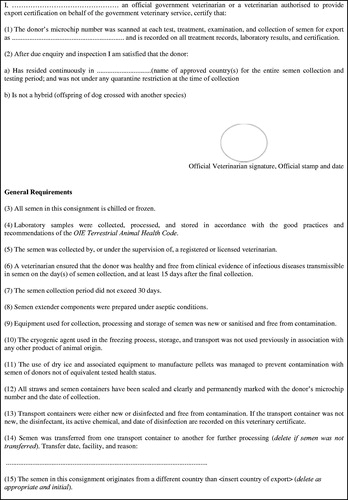
An animal health certificate, including an identification document (containing information relating to species, breed, date of birth, sex, and colour of the dog, as well as the alphanumeric code of the microchip), a statement that the dog has a normal testicular status and is free from contaminating pathogenic microorganisms, and the blood test report (B. canis, L. infantum and, if not vaccine, L. canicola) and a certificate with the results of the sperm evaluation after collection (Figure ).
Figure 1. Model of veterinary certificate.
It must be completed and issued by a veterinarian. When semen is imported from a third country, the veterinary certificate must be issued by an official veterinarian, or by an authorised veterinarian and subsequently endorsed by the competent authority. It could be used the model veterinary certificate for international trade in oocytes, embryos or semen as referred to in article 5.10.3 of the Terrestrial Animal Health Code (OIE Citation2020). A semen quality assessment form, and in the case of frozen semen also thawing instructions should always accompany the sperm when transported.
All documents must:
Be original, unless otherwise stated.
Accompany the imported goods.
Be drawn up in at least one of the official languages of the country of destination and in English.
Be endorsed on every page by the Veterinarian and/or Official Veterinarian with their original stamp, signature and date on every page or be endorsed in the space allocated and all pages have paper-based alternative security features.
The microchip number of the donor animal must be recorded on all the records, laboratory reports, certification and semen containers. In particular, all semen containers must be sealed and clearly and permanently marked with the donor’s microchip number and the date of collection.
The quantity of semen must be reported, as well as name and contact details of the importer (consignee) and exporter (consignor).
The donor dog must be examined by veterinarian and found to be healthy and free from infectious or contagious diseases within 30–45 days after the last semen collection for the export consignment (See above-mentioned points 6–8).
Relating to shipment of chilled and frozen dog semen, legislative and regulatory provisions differ from country to country. Therefore, before buying dog semen, firstly the bitch owner and the veterinarian should contact the competent authority and/or the Kennel Club in the importing country to receive information about the latest set of rules and regulations, and so that all the necessary health certificates and blood tests can be made in accordance with the requirements.
Along the same lines of Australian Government (Australian Government—Department of Agriculture and Water Resources) and of the United Kingdom’s Government (GOVUK 2020), in order to understand if a country is or no eligible for import frozen canine semen we propose a categorisation of the countries according to a satisfactory animal health status depending on the recognised rabies status of the country of export:
Category 1—no risk countries, as there is no indigenous rabies in terrestrial animals (i.e. Australia, Austria, Balearic Islands, Belgium, Bermuda, Canary Islands, Corsica, Cyprus, Czech Republic, Denmark, Estonia, Finland, Formentera, France, Germany, Gibraltar, Greece, Iceland, Ireland, Italy, Jamaica, Japan, Luxembourg, Malta, Monaco, the Netherlands, New Zeeland, Norway, Portugal, San Marino, Spain, Sweden, Switzerland, the United Kingdom, etc.).
Category 2—low risk countries, where rabies occurs in wild animals but not in companion animals (i.e. Bulgaria, Canada, Chile, Croatia, Czech Republic within 50 km border Poland/Slovakia, Grenada, Hungary, Latvia, Slovakia, Slovenia, United Arab Emirates, United States of America, etc.).
Category 3—high risk countries, where the rabies occurs in wild and companion animals (i.e. Afghanistan, Albania, Algeria, Argentina, Armenia, Azerbaijan, Bosnia and Herzegovina, Brazil, Cambodia, Central African Republic, China, Congo, Costa Rica, Cuba, Egypt, Guinea, India, Indonesia, Israel, Jordan, Kenya, Korea, Kosovo, Lithuania, Malaysia, Mexico, Montenegro, Morocco, Nepal, Peru, Poland, Romania, Russian Federation, Serbia, Turkey, Ukraine, etc.).
Good governance, ensuring transparency in disease reporting, efficiency in disease management and reliability in veterinary certification, is fundamental to the relationship of trust between an exporting and an importing country.
To comply with the requirements from most countries the semen-containing tubes, straws, or vials should always be marked with the following information:
The breed.
The dog’s registered name.
The dog’s registration number and/or chip number.
The date of semen collection (obligatory when blood tests and veterinary certificates are required).
The location where the semen was collected/processed.
Ethical aspects
Although the use of AI could have a positive ethical impact (i.e. disease control), there may be ethical concerns such as those associated with the surgical procedure of AI. Indeed, in order to respect the welfare especially of the bitch, each choice of intervening must be carried out firstly in her best interests and secondly considering benefits and risks of the surgical procedure.
In some European countries (i.e. Norway, Sweden and UK), where is applied a precautionary approach (England and Ponzio Citation1996), canine surgical AI has been banned because it is considered an invasive technique interfering with animal welfare (Linde-Forsberg Citation2005; Loeb Citation2019). In particular, in England this type of procedure is prohibited by animal welfare laws and amended Section 27.30 of the supporting guidance to the Code of Professional Conduct (RCVS Citation2020).
Another ethical concern on the use of AI may be the inbreeding of dogs that may endanger the health of following generations (England and Ponzio Citation1996), as it will be described in detail in Section 3, due an underlying and exiting unaware problem (congenital or behavioural). In fact, according to the FCI breeding rules (FCI Citation2020a), AI should not be performed in dogs not having at least one previous litter registered from natural service. Exceptions can be made by the national canine organisations to improve the health of the breed, for the welfare of the bitch—or to preserve or increase the genetic pool within the breed (Hedhammar and Indrebø Citation2011).
Additional considerations
The use of canine semen may represent a risk in terms of infection. Obviously, the pathogens may origin from the donor animal. Due to well-established, globally accepted sanitary measures during handling, the risk of pathogen transmission may be significantly reduced. In order to reduce the possible contaminations, the donor should be selected from countries, regions that have been shown to be free of specific pathogens, and tested individually.
The breeding of dogs and the interest of genetic disorders have progressively increased and companion animal veterinarians are faced with health and welfare problems in their patients that are breed related and have a strong genetic backstory. For these reasons they should help raise awareness and ensure breed-related health and welfare problems are not considered only as typical for the breed.
Veterinarians should not perform AI ‘to overcome physical inabilities of the dog’ and consider it an accepted necessity for certain breeds. ‘Any dog should be able to mate naturally’ (FCI breeding strategies Article 6; FCI Citation2020b).
As above-said in the paragraph relating to ethical aspects, CAI to be a recognisable breeding technique must be carried out by a veterinarian or a specifically recognisable technician. They need to have a variety of skills to offer the best care possible to their patients. Especially these practitioners should acquire specific knowledge of reproductive physiology and pathology, of collecting semen and inseminating the female without risking animal welfare.
The veterinary profession has an important role to play and should call for all stakeholders to join forces. Good collaboration and communication between all stakeholders is essential to joint actions in order to protect and look to the animal health and welfare.
Therefore, in conclusion, professional veterinary organisations could work together with national and international breeding organisations, veterinary universities and other stakeholders to set up relevant pre-breeding health screening programmes and to promote responsible pet ownership.
Ethical approval
Ethics approval was not required for this study
Author contributions
Conceptualisation by A.P. and M.Q.; literature review by M.Q., V.B., L.L. and A.P.; writing original draft preparation by M.Q., V.B., L.L. and A.P.; writing-review and editing by M.Q., L.L. and A.P.; supervision by A.P. All the authors gave final approval to the manuscript and any revised version submitted.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adesiyun AA, Abdullahi SU, Adeyanju JB. 1986. Prevalence of Brucella abortus and Brucella canis antibodies in dogs in Nigeria. J Small Anim Pract. 27(1):31–37.
- Al-Qassab S, Reichel MP, Su C, Jenkins D, Hall C, Windsor PA, Dubey JP, Ellis J. 2009. Isolation of Toxoplasma gondii from the brain of a dog in Australia and its biological and molecular characterization. Vet Parasitol. 164(2–4):335–339.
- Appel MJG, Menegus M, Parsonson IM, Carmichael LE. 1969. Pathogenesis of canine herpesvirus in specific-pathogen-free dogs: 5- to 12-week-old pups. Am J Vet Res. 30(12):2067–2073.
- Arantes TP, Lopes WD, Ferreira RM, Pieroni JS, Pinto VM, Sakamoto CA, Costa AJ. 2009. Toxoplasma gondii: evidence for the transmission by semen in dogs. Exp Parasitol. 123(2):190–194.
- Baluyut CS, Duguies MV. 1977. Serological survey for Brucella canis agglutinins in dogs using macroscopic tube agglutination test. Philippine J Vet Med A. 16:93–101.
- Bosu WT, Prescott JF. 1980. A serological survey of dogs for Brucella canis in southwestern Ontario. Can Vet J. 21(7):198–200.
- Brennan SJ, Ngeleka M, Philibert HM, Forbes LB, Allen AL. 2008. Canine brucellosis in a Saskatchewan kennel. Can Vet J. 49:703–708.
- Bricker BJ. 2002. PCR as a diagnostic tool for brucellosis. Vet Microbiol. 90(1–4):435–446.
- Carmichael LE, Joubert JC. 1988. Transmission of Brucella canis by contact exposure. Cornell Vet. 78(1):63–73.
- Carmichael LE, Zoha SJ, Flores-Castro R. 1984. Problems in the serodiagnosis of canine brucellosis: dog responses to cell wall and internal antigens of Brucella canis. Dev Biol Stand. 56:371–383.
- Corde Y, Drapeau A, Albert D, Garin-Bastuji B. 2011. Assessment of a lateral flow immuno-chromatographic assay for the serological diagnosis of canine brucellosis due to Brucella canis. losis International Research Conference Including the 64th Brucellosis Research Conference, September 21–23, Buenos Aires, Argentina, p. 139, 123 (abstract).
- Corrente M, Franchini D, Decaro N, Greco G, D’Abramo M, Greco MF, Latronico F, Crovace A, Martella V. 2010. Detection of Brucella canis in a dog in Italy. New Microbiol. 33:337–341.
- de Oliveira MZ, Vale V, Keid L, Freire SM, Meyer R, Portela RW, Barrouin-Melo SM. 2011. Validation of an ELISA method for the serological diagnosis of canine brucellosis due to Brucella canis. Res Vet Sci. 90(3):425–431.
- Debbie JG. 1974. Rabies. Prog Med Virol. 18(0):241–256.
- Decaro N, Martella V, Buonavoglia C. 2008. Canine adenoviruses and herpesvirus. Vet Clin North Am Small Anim Pract. 38(4):799–814.
- Diniz SA, Melo MS, Borges AM, Bueno R, Reis BP, Tafuri WL, Nascimento EF, Santos RL. 2005. Genital lesions associated with visceral leishmaniasis and shedding of Leishmania sp. in the semen of naturally infected dogs. Vet Pathol. 42(5):650–658.
- Diniz SA, Silva FL, Carvalho Neta AV, Bueno R, Guerra R, Abreu-Silva AL, Santos RL. 2008. Animal reservoirs for visceral leishmaniasis in densely populated urban areas. J Infect Developing Countries. 2:24–33.
- Donner J, Anderson H, Davison S, Hughes AM, Bouirmane J, Lindqvist J, Lytle KM, Ganesan B, Ottka C, Ruotanen P, et al. 2018. Frequency and distribution of 152 genetic disease variants in over 100,000 mixed breed and purebred dogs. PLoS Genet. 14(4):e1007361.
- Drew WL. 2004. Rabies. In: Ryan KJ, Ray CG, editors. Sherris Medical Microbiology. 4th ed. New York (NY): McGraw Hill; p. 597–600.
- Dubey JP. 2010. Toxoplasmosis of Animals and Humans. 2nd ed. Boca Raton (FL): CRC Press.
- Dubey JP, Hemphill A, Calero-Bernal R, Schares G. 2017. Neosporosis in Animals. Boca Raton (FL): CRC Press.
- Dubey JP, Lindsay DS, Lappin MR. 2009. Toxoplasmosis and other intestinal coccidial infections in cats and dogs. Vet Clin North Am Small Anim Pract. 39(6):1009–1034.
- Dubey JP, Rosypal AC, Pierce V, Scheinberg SN, Lindsay DS. 2005. Placentitis associated with leishmaniasis in a dog. J Am Vet Med Assoc. 227(8):1266–1269.
- Ebani VV, Cerri D, Fratini F, Bey RF, Andreani E. 2003. Serological diagnosis of brucellosis caused by Brucella canis. New Microbiol. 26(1):65–73.
- ENCI 2020. Ente Nazionale della Cinofilia Italiana; [accessed 2020 March 31]. https://www.google.com/search?client=firefox-b-d&q=enci+codice+etico
- England GCW, Ponzio P. 1996. Comparison of the quality of frozen-thawed and cooled-rewarmed dog semen. Theriogenology. 46(1):165–171.
- Farstad W. 2018. Ethics in animal breeding. Reprod Dom Anim. 53(Suppl. 3):4–13.
- FCI 2020a. Federation Cynologique Internationale; [accessed 30 March 2020]. http://www.fci.be/circulaires/102-2010-annex-fr.pdf.
- FCI 2020b. Federation Cynologique Internationale; [accessed 30 March 2020]. http://www.fci.be/en/Breeding-42.html.
- Fekadu M. 1993. Canine Rabies. Onderstepoort J Vet Res. 60(4):421–427.
- Fekadu M, Shaddock JH, Baer GM. 1982. Excretion of rabies virus in the saliva of dogs. J Infect Dis. 145(5):715–719.
- Gyuranecz M, Szeredi L, Ronai Z, Denes B, Dencso L, Dan A, Palmai N, Hauser Z, Lami E, Makrai L, et al. 2011. Detection of Brucella canis-induced reproductive diseases in a kennel. J Vet Diagn Invest. 23(1):143–147.
- Hayashi TT, Isayama Y. 1977. Detection of Brucella canis infection in dogs in Hokkaido. Microbiol Immunol. 21(5):295–298.
- Hedhammar Å. 1997. FCI strategies to enhance genetic health of dogs. FCI Magazine. 25:5–8.
- Hedhammar Å. 1999. European strategies to enhance canine genetic health. Eur J Companion Anim Pract. 9:93.
- Hedhammar Å. 2005. Actions by FCI and WSAVA to promote canine genetic health. Eur J Companion Anim Pract. 5:22–25.
- Hedhammar A, Indrebø A. 2011. Rules, regulations, strategies and activities within the Fédération Cynologique Internationale (FCI) to promote canine genetic health. Vet J. 189(2):141–146.
- Hedhammar Å, Malm S, Bonnett B. 2011. International and collaborative strategies to enhance genetic health in purebred dogs. Vet J. 189(2):189–196.
- Hollett RB. 2006. Canine brucellosis: outbreaks and compliance. Theriogenology. 66(3):575–587.
- Indrebø A. 2008. Animal welfare in modern dog breeding. Acta Vet Scand. 50(S1):10–14.
- ISAG 2020. International Society for Animal Genetics; [accessed 31 March 2020]. https://www.isag.us/news_archive.asp
- Jackson AC, Wunner WH. 2007. Rabies: scientific basis of the disease and its management. London: Academic Press by Elsevier.
- Jiang FX. 1989. A survey on canine brucellosis in Wusu County. Chinese J Vet Sci Technol. 1:18–19.
- Joseph PG, Mahmud ZBH, Sirimanne ES. 1983. Canine brucellosis in Malaysia: a first report. Kajian Veterinar. 15:17–22.
- Karpas A, Garcia FG, Calvo F, Cross RE. 1968. Experimental production of canine tracheobronchitis (kennel cough) with canine herpesvirus isolated from naturally infected dogs. Am J Vet Res. 29(6):1251–1257.
- Keid LB, Soares RM, Vasconcellos SA, Chiebao DP, Megid J, Salgado VR, Richtzenhain LJ. 2007a. A polymerase chain reaction for the detection of Brucella canis in semen of naturally infected dogs. Theriogenology. 67(7):1203–1210.
- Keid LB, Soares RM, Vasconcellos SA, Chiebao DP, Salgado VR, Megid J, Richtzenhain LJ. 2007b. A polymerase chain reaction for detection of Brucella canis in vaginal swabs of naturally infected bitches. Theriogenology. 68(9):1260–1270.
- Kim S, Lee DS, Suzuki H, Watarai M. 2006. Detection of Brucella canis and Leptospira interrogans in canine semen by multiplex nested PCR. J Vet Med Sci. 68(6):615–618.
- Koch MO, Weiss RR, Cruz AA, Soccol VT, Gonçalves KA, Bertol M, Beltrame OC, Dittrich RL. 2016. Detection and isolation of Toxoplasma gondii from fresh semen of naturally infected dogs in Southern Brazil. Reprod Domest Anim. 51(4):550–554.
- Kopczewski A, Krolak M, Arent Z, Rudnicki K. 1995. A case of brucellosis in a male dog Przypadekbrucelozy u psa. Zycie Weterynaryjne. 70:230–231.
- Kumar PD. 2009. Rabies. Westport (CT): Greenwood Press.
- Levett P. 2001. Leptospirosis. Clin Microbiol Rev. 14(2):296–326.
- Levy X, Fontbonne A. 2007. Canine semen banking: sanitary and ethical aspects. Legislation Rev Bras Reprod Anim Belo Horizonte. 31:92–107.
- Linde-Forsberg C. 2001. Regulations and recommendations for international shipment of chilled and frozen semen. In: Concannon PW, England G, Verstegen J, Linde-Forsberg C, editors. Recent Advances in Small Animal Reproduction. Ithaca (NY):International Veterinary Information Service. No A1209.501
- Linde-Forsberg C. 2005. Regulations and recommendations for international shipment of chilled and frozen canine semen. In SAVS-EVSSAR Course Reproduction in Companion, Exotic and Laboratory Animal; September 12–17; Nantes.
- Loeb J. 2019. Canine surgical AI now a prohibited procedure. Vet Record. 184(7):20.
- Lucero NE, Ayala SM, Escobar GI, Jacob NR. 2008. Brucella isolated in humans and animals in Latin America from 1968 to 2006. Epidemiol Infect. 136(4):496–503.
- Mason SJ. 2018. Current review of artificial insemination in dogs. Vet Clin North Am Small Anim Pract. 48(4):567–580.
- Masucci M, De Majo M, Contarino RB, Borruto G, Vitale F, Pennisi MG. 2003. Canine leishmaniasis in the newborn puppy. Vet Res Commun. 27:771–774.
- Mateu-de-Antonio EM, Martin M, Casal J. 1994. Comparison of serologic tests used in canine brucellosis diagnosis. J Vet Diagn Invest. 6(2):257–259.
- Mir F, Fontaine E, Reyes-Gomez E, Carlus M, Fontbonne A. 2012. Subclinical leishmaniasis associated with infertility and chronic prostatitis in a dog. J Small Anim Pract. 53(7):419–422.
- Moreno J, Alvar J. 2002. Canine leishmaniasis: epidemiological risk and the experimental model. Trends Parasitol. 18(9):399–405.
- Mucheru GM, Kikuvi GM, Amwayi SA. 2014. Knowledge and practices towards rabies and determinants of dog rabies vaccination in households: a cross sectional study in an area with high dog bite incidents in Kakamega County, Kenya. Pan Afr Med J. 19:255.
- Nicholas FW, Crook A, Sargan DR. 2011. Internet resources cataloguing inherited disorders in dogs. Vet J. 189(2):132–135.
- Nockler K, Kutzer P, Reif S, Rosenberger N, Draeger A, Bahn P, Gollner C, Erlbeck C. 2003. Canine brucellosis—a case report. Berl. Munch. Tierarztl. Wochenschr. 116(9–10):368–372.
- Nöthling JO, Hüssy D, Steckler D, Ackermann M. 2008. Seroprevalence of canine herpesvirus in breeding kennels in the Gauteng Province of South Africa. Theriogenology. 69(3):276–282.
- Offiong EEA, Essien CA, Otoh AJ, Eyo GD, Habib M, Obioku OE. 2014. Transmission of rabies to a dog through the semen. Int J Dev Sustain. 3:956–958.
- OIE 2020. World Organisation for Animal Health; [accessed 30 March 2020]. http://www.oie.int/index.php?id=169&L=0&htmfile=chapitre_certif_live_animals.htm.
- OMIA 2020. Online Mendelian Inheritance in Animals; [accessed 30 March 2020]. http://omia.org/
- Park C, Oh J, Park CK, Oh JY. 2001. Bacteriological and serological investigation of Brucella canis infection of dogs in Taegu city. Korea. Korean J Vet Res. 41:67–71.
- Patitucci AN, Alley MR, Jones BR, Charleston W. 1997. Protozoal encephalomyelitis of dogs involving Neospora caninum and Toxoplasma gondii in New Zealand. N Z Vet J. 45(6):231–235.
- Payan-Carreira R, Miranda S, NiżAński W, 2011. Artificial insemination in dogs. In: Manafi M. editor. Artificial insemination in farm animals. Rieka: InTech; p. 51–78.
- Poste G, King N. 1971. Isolation of a herpesvirus from the canine genital tract: association with infertility, abortion and stillbirths. Vet Rec. 88(9):229–233.
- Poulet H, Guigal PM, Soulier M, Leroy V, Fayet G, Minke J, Chappuis Merial G. 2001. Protection of puppies against canine herpesvirus by vaccination of the dams. Vet Rec. 148 (22):691–695.
- RCVS 2020. Royal College of Veterinary Surgeons; [accessed 30 March 2020]. https://www.rcvs.org.uk/setting-standards/advice-and-guidance/code-of-professional-conduct-for-veterinary-surgeons/supporting-guidance/miscellaneous/
- Rosypal AC, Troy GC, Zajac AM, Frank G, Lindsay DS. 2005. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J Parasitol. 91(4):970–972.
- Saegusa J, Ueda K, Goto Y, Fujiwara K. 1978. A survey of Brucella canis infection in dogs from Tokyo area. Nippon Juigaku Zasshi. 40(1):75–80.
- Sargan DR. 2004. IDID: inherited diseases in dogs: web-based information for canine inherited disease genetics. Mamm Genome. 15(6):503–506.
- SchäFer-Somi S, Hofer E. 2011. Brucella canis—ein wenig beachteter Zoonoseerreger, aktuelle Fälle. Austrian Vet J. 1:20–24.
- Sebek Z, Sykora I, Holda J, Komarek J. 1976. Serological demontsration of Brucella canis in the breeding of laboratory dogs of the beagle breed in Czechoslovakia. Cesk Epidemiol Mikrobiol Imunol. 25(3):129–136.
- Silva FL, Oliveira RG, Silva TMA, Xavier MN, Nascimento EF, Santos RL. 2009. Venereal transmission of canine visceral leishmaniasis. Vet Parasitol. 160(1–2):55–59.
- Silva FL, Rodrigues AAM, Rego IOP, Santos RLH, Oliveira RG, Silva TMA, Xavier MN, Nascimento EF, Santos RL. 2008. Genital lesions and distribution of amastigotes in bitches naturally infected with Leishmania chagasi. Vet Parasitol. 151(1):86–90.
- Slappendel RJ, Greene CE. 1990. Leishmaniasis. In: Greene CE., editor. Infectious diseases of the dog and cat. Philadelphia (PA): Saunders Company; p. 769–777.
- Srinivasan VK, Nedunchelliyan S, Venkataraman KS. 1992. Seroepidemiology of canine brucellosis in Madras city. Indian Vet J. 69:978–980.
- Taylor DJ. 1980. Serological evidence for the presence of Brucella canis infection in dogs in Britain. Vet Rec. 106(5):102–104.
- Thomassen R, Farstad W. 2009. Artificial insemination in canids: a useful tool in breeding and conservation. Theriogenology. 71(1):190–199.
- Tsai IS, Lu YS, Isayama Y, Sasahara J. 1983. Serological survey for Brucella canis infection in dogs in Taiwan and the isolation and identification of B. canis. Taiwan J Vet Med Anim Husb. 42:91–98.
- UPEI 2020. University of Prince Edward Island. Canine Inherited Disorder Database; [accessed 30 March 2020]. https://cidd.discoveryspace.ca
- Ward MP. 2002. Clustering of reported cases of leptospirosis among dogs in the United States and Canada. Prev Vet Med. 56(3):215–226.
- Weber A, Schliesser T. 1975. Serological and cultural determination of Brucella canis in beagles in laboratory kennels. Zentralblatt Veterinarmedizin Reihe B. 22(5):403–410.
- Weber A, Schliesser T. 1978. The occurrence of antibodies to Brucella canis in domestic dogs in the Federal Republic of Germany. Berl Munch Tierarztl Wochenschr. 91(2):28–30.