Abstract
The purpose of this study was to examine the effect of adding an essential fatty acid mixture (EOM; Eucalyptus globulus Labill, thymus vulgaris, Cymbopogon nardus, and Syzygium aromaticum) to the drinking water in the heat stress broilers on the adipokine (Apelin, BDNF) response, the histopathologic changes in liver and intestines, and some biochemical parameters. This study lasted for a total of 42 days, including the physical exercise period (7 days) and the fattening period (35 days). A total of 400 Ross 308 male broilers (1-d-old) were randomly divided into 8 groups (50 animals per group), each of which was exposed to various conditions of temperature (C: 22 °C and SC: 36 °C) and treatment dose (C, 250, 500 and 750 mL/1000 L). Each group was divided into 5 subgroups, each comprising 10 animals. In the stress-free groups, whereas the Apelin level linearly decreased, the BDNF level linearly increased. In histopathology, the liver tissue was found to be normal in all groups whereas the duodenum villi length was found to increase in the group of 750 mL/1000 L. No statistically significant difference was found between the stressed groups and the non-stressed groups in terms of VLDL, Glucose, Total bilirubin, ALT, and TG (p > .05). While Apelin level increased in the stressed groups, the BDNF level increased in the group of 250 mL/1000 L. In the histopathological examination, a small amount of coagulation necrosis was detected in hepatocyte, a diffuse hydropic degeneration was observed in hepatocytes, and finally a dilatation and hyperaemia were seen in sinusoid in the groups of EOM-500 mL/1000 L and EOM-750 m/1000 L compared to the control group. Whereas there was no difference between the group of EOM-250 mL/1000 L and the control group in terms of duodenum villa length, there was a significant decrease in other groups (p < .00). In conclusion, this study showed that adding 250 mL/1000 L of EOM to the drinking water had a positive effect on the serum levels of Apelin and p-BDNF in the groups exposed to stress (p <.05).
Heat stress results in serious annual economic losses in the poultry industry.
Essential oil fatty mix acids have positive effects on poultry health.
Apelin and BDNF may be a protective factor against deleterious consequences of chronic exposition to heat stress
HIGHLIGHTS
Introduction
Stress is a physiological and psychological reaction of the body against deterioration of homeostasis and the stressors causing neurochemical and neurobiological changes in the brain. Heat stress is poultry causes endocrinological changes by influencing metabolism and is an important stressor affecting vital organs. Poultry animals are homoeothermic animals, keeping body temperature within a certain interval, independent of ambient temperature. Whenever the temperature rises above this level, the animals are stressed. Due to the absence of sweat glands as well as their fast metabolism in poultry animals, stress is a common problem leading to delay in growth, increasing mortality rates as well as physiological problems because of immunosuppressive effects (Naslund et al. Citation2002; Moraes et al. Citation2003; Bayraktar and Tekce Citation2018).
Adipose tissue is a metabolically active endocrine organ that secretes hormones known as adipokines and plays a role in energy homeostasis, maintaining body temperature and adaptive thermogenesis (Medina-Gómez, Citation2012; Demirci and Cennet, Citation2017; Bayraktar and Tekce, Citation2018). Apelin is an adipokine secreted from a fatty tissue that controls thermogenesis (Bertrand et al. Citation2015) and reaction to stres via hypothalamohypophyseal-adrenal axis (Newson et al. Citation2009), prevention of oxidative stress (Than et al. Citation2014), energy metabolism (Bertrand et al. Citation2015) and immune functions and is vital in many physiological processes (Tatemoto et al. Citation2001; Matsuzawa Citation2005). Apelin has several isoforms such as 12, 13, 17, and 36. The biological activity of Apelin-13 is eight times stronger than that of Apelin-17 and 60 times stronger than that of Apelin-36 (Tatemoto et al. Citation1998). Apelin is a new enteric peptide and stress increases the apelin content in the gut (Wang et al. Citation2004). It has a regulatory effect on gastrointestinal function (Wang et al. Citation2004) and plays a role in intestinal contraction to control glucose metabolism through the brain (Fournel et al. Citation2017). Apelin is reported to be used as a promising therapeutic agent in liver diseases due to its effect on liver regeneration (Principe et al. Citation2008; Fausto et al. Citation2012).
Stress can severely affect brain functions and neuronal damage due to glucocorticoid release (Hüther, Citation1996). BDNF supports the development of noradrenergic and serotoninergic neurons and protects them from stress and toxic injuries (Horch, Citation2004; Habib et al. Citation2001). BDNF is an adipokine with a neuroprotective effect against neuron development and differentiation as well as stress and ischaemia in the brain (Zigova et al. Citation1998; Benraiss et al. Citation2001; Kertes et al. Citation2017) . Plasma BDNF (p-BDNF) is defined as a sensitive marker indicating the BDNF level in the brain (Lommatzsch et al). Its role in BDNF hepatocyte metabolism leads to the activation of catabolic pathways such as fatty acid oxidation. In addition, while gluconeogenesis is inhibited, it provides stimulation of glycogen storage (Genzer et al. Citation2017). BDNF has been reported to protect the intestinal mucosal barrier function and modify the gut microbiota (Lin et al., 2018). BDNF has a role in regulating stress response by binding to Tropomyosin-related kinase B (TrkB) with high affinity (Shirayama et al. Citation2015; Sosanya et al. Citation2019).
In the studies in recent years, various products have been used in order to eliminate the effects of stress on the organism. Medicinal aromatic plants are the foremost of these products possessing no risk of accumulation in animal tissues without causing any drug resistance and no risks for human health dose-dependently (Daroui-Mokaddem et al. Citation2010; Feizi et al. Citation2013). In the studies made on the effects of EOM (Eucalyptus globulus Labill, Thymus vulgaris, Cymbopogon nardus ve Syzgium aromaticum) mixture on the plants, they were reported to have antioxidant (Vollmayr et al. Citation2001), anti-microbial (Karpouhtsis et al. Citation1998; Dorman and Deans Citation2000; Ultee et al. Citation2002), anti-inflammatory (Bishop Citation1995; Guimarães et al. Citation2013), anti-viral (Al-Ja’fari et al., Citation2011; Silva et al. Citation2011), anti-tumoral (Al-Ja’fari et al., Citation2011), anti-fungal (Fu et al. Citation2007; Monzote et al. Citation2007), antiparasitic (Chowdhury et al. Citation2018) and positive effects on growth performance by having effects on intestinal villuses via digestional enzymes (Jang et al. Citation2007). In addition, positive effects of Essantial oil supplementation in broiler chickens exposed to heat stress has been confirmed by other authors (Parvar et al., Citation2013; Gopi et al., Citation2014; Akbarian et al. Citation2015; Petrolli et al., Citation2019).
In this experimental trial we conducted, dose-dependent administration of the Essential Oil Mixture (EOM) (Eucalyptus globulus Labill, Thymus vulgaris, Cymbopogon nardus ve Syzygium aromaticum)added at different amounts into the drinking water of the broiler hens under heat stress (22 and 36 °C, respectively), was studied. The animals’ some blood parameters histopathological changes developed in the liver and small intestine tissues, plasma BDNF and serum apelin levels were investigated.
Materials and methods
Animals
Ethical approval was obtained from the Local Ethics Committee of Bayburt University Local Ethics Committee, Turkey (Date and number of the decision is 02.07.2018- 2018/16). In this study, a total of 400 one-day old Ross 308 broiler male chicks were used. The study was conducted in line with ethical principles and rules, protecting animal welfare and rights.
Experimental design
Four-hundred 1-day-old male Ross-308 broiler chicks were used in the study. During 7-day of exercise and 35-day of fattening period of the trial, broiler chicks were kept in 110 × 110 × 100 cm cages, 10 animal each, at Bayburt University Food, Agriculture and Livestock Application and Research Centre Unit (AOAC Citation2005). On d 7 of the experiment, the animals were randomly assigned to 8 groups, each composed of 50 animals of equal body weight and groups each containing five subgroups. The research groups were designed according to the two temperature measurements, 22 °C (C) and 36 °C (SC) and four treatments levels in drinking water as control, 250, 500 and 750 mL/1000 L. Therefore, the control, EOM-250, EOM-500, EOM-750, SEOM-250, SEOM-500 and SEOM-750 groups were used during the research period.
Feed
The chicks feeds used in the experiment (starter, grower and finisher feed) were prepared by a private company operating in Turkey (Erzurum). Basal diet feed, with the ration content shown in (Table ) was given at the same time every day (Around 08:00 h). After the feed remaining from the previous day was taken back and weighed. Nutrient analyses of the feeds used throughout the research were performed in accordance with the methods described by A.O.A.C. (AOAC, Citation2005). Broiler chicks were given fresh drinking water ad libitum. The remaining drinking water was also taken back and freshwater supplemented with EOM was given at the same time every day (Around 08:00 h). EOM was included in the water at rates of 250, 500, 750 mL/1000 L (Bayraktar and Tekce Citation2019; Tekce et al. Citation2020) and was given to all groups at the same time every day (Around 08:00 h) except for control groups.
Table 1. Basal diet: nutrient content and chemical analysis (g/kg).
Poultry house heat moisture and illumination
The general temperature in the pen was kept constant at 32–28 °C for the first 2 days and at 27–28 °C for the next 5 days of the experiment. Trial groups are divided into stress and stress-free starting from 7 days. Heating of the cluster was provided by means of 36 ± 1 °C sensitive thermostat appliances (TURKEY) connected to the central heating system for 7–42 days. Temperature and humidity values were measured with daily digital temperature-humidity metre (TFA Dostmann, GERMANY) thermometers placed at 4 different points of the coop to control the temperature in the coop. The room temperature of stress-free groups is designed to be 22 °C.
Contents of the EOM mixture
The contents of the EOM mixture were analysed using GC (gas chromatography) (Agilent 5977B, GC/MSD, GERMANY) in the Central Research Laboratories of Bayburt University (Özek et al. Citation2010). The EOM mixture added to the drinking water was provided from a commercial company (Ankara, Turkey). In the content, 26.70% Durenol, 23.89% Eugenol, 16.49% Gamma terpinene, 8.35% Heptaethylene glycol, 6.42% Hexaethylene glycol,3.31% Cymene, 3.08% Pentaethylene glycol, 2.87% Caryophyllene, 2.30% D-Limonene, 2.18% Beta Pinene and 0.95% Eucalyptol were determined.
Collection of plasma and serum samples
At the end of the trial, cervical dislocation was performed to 80 animals in total, with 10 animals randomly selected from each group. Blood samples were collected into 10 mL biochemistry tubes (VACUETTE® TUBE 9 mL Z Serum Clot Activator) and 5 mL EDTA hemogram tubes (Becton Dickinson Co. Brea, CA, USA). Serum samples were then centrifuged at 4,100 xg for 12 min at 4° C (NF 1200 R, Nüve, Ankara, Turkey), and the serum was transferred to eppendorf tubes (Tekce and Gül Citation2015).
Biochemical analysis
Serum levels of glucose, alanine aminotransaminase (ALT), triglycerides (TG), very low density lipoprotein cholesterol (VLDL) were assayed at the Erzurum Technical Chemistry and Medical Laboratories using a Cobas-8000 auto-analyzer, which is a closed spectrophotometric system, with Roche kits (Mannheim, Germany).
Measurement of serum Apelin-13 levels and plasma BDNF levels
Serum Apelin and Plasma Brain-derived neurotrophic factor (BDNF) were measured using ELISA (R&D Systems, Minneapolis, MN, USA) and parameters were read with ELISA reader (Mindray MR- 96 A, CHİNA). The minimum detectable concentration used to measure the apelin level in blood serum obtained from the research was < 18.75 pg/mL. An ELISA kit type-specific for chicken apelin (FineTest, Product code: ECH0078, China) from 31.25–2000.00 pg/mL, an intra-assay coefficient 8.0%, and an inter-assay coefficient of 10.0% was utilised in accordance with the manufacturer’s protocol. The results were evaluated by reading absorption values at 450 nm in accordance with the procedure reported in the kit (Apelin Citation2018).
The minimum detectable concentration used to measure the BDNF level in blood plasma obtained from the research was 8 pg/mL. An ELISA kit type-specific for chicken Birds Brain Derived Neurotrophic Factor (BDNF) (SinoGeneClon, ELISA Kit Product code: SG-82023, China) from 35–2000 pg/mL, an intra-assay coefficient 8.0%, and an inter-assay coefficient of 10.0% was utilised in accordance with the manufacturer’s protocol. The results were evaluated by reading absorption values at 450 nm in accordance with the procedure reported in the kit (BDNF. Citation2018).
Histopathological analysis
At the end of the trial, servical dislocation was performed in totally 80 animals, randomly selected as 10 out of each group, for histopathological assessment and necropsy. Intestinal and liver tissue samples taken for histopathological assessment were then fixed for 48 hours in 10% formalin solution. They were embedded in paraffin blocks according to the routine histological follow-up procedures. Cross sections were taken from each block at 4 µm thickness (Leica Citation2018). All sections were microscopically examined (Bancroft et al. Citation2012).
Statistical analysis
The parameters were all normally distributed, and the data expressed by means and standard errors. The effect of temperature and processed essential oil mixture (EOM) in treatment on apelin, VLDL, glucose, total bilirubin, Alt and TG values were analysed using factorial ANOVA with GLM procedures of JMP 7.0 statistical software (SAS. Citation1999). The used statistical model was: Yijk = µ + Di + Tj+ (D × T)ij + eijk, where: Yijk = an observation, µ = overall mean, Di = Treatment effect, Ti = Temperature effect, (D × T)ij=the interaction effect and eijk = experimental error. The least significance differences among treatments and interactions were detected using Tukey post hoc test (p < .05). The polynomial contrast option was also computed to determine nature of response to increasing levels of essential oil mixture.
Results
Apelin, BDNF, and some blood parameters
The effects of adding various EOM concentrations (250, 500, and 750 mL/1000 L) to the drinking water of the broilers which were fed under different temperature stress conditions (22 °C and 36 °C) on Apelin, BDNF, and some blood parameters were analysed in the Table . No statistically significant difference was found between the stressed groups and the non-stressed groups in terms of VLDL, Glucose, Total bilirubin, ALT, and TG (p > .05). In the non-stressed groups, whereas the Apelin level linearly decreased, the BDNF level linearly increased. On the other hand, in the stressed groups, while the Apelin level increased in all groups; the BDNF level decreased in the groups of 500 and 750 mL/1000 L, but increased in the group of 250 mL/1000 L.
Table 2. Effect of essential oil mixture (EOM) on Apelin, BDNF, VLDL, glucose, total bilirubin and TG values added to drinking water of groups fed in stress conditions.
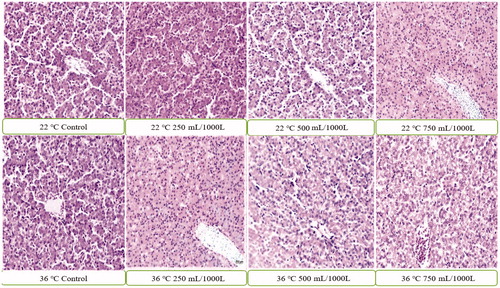
Intestinal and liver histopathology
Histopathological findings analysing the effects of stress and EOM on liver tissues and intestinal villuses (Table ) are displayed in figures and 3. In the histopathological analysis of the liver tissues of the 22 °C no-stress control group and EOM-250 mL/1000 L, EOM-500 mL/1000 L groups, parenchyma tissue and its serosa was in normal appearance, histological appearance of the liver tissues of EOM-750 mL/1000 L group displayed mild dilatation and hyperaemia in the sinusoids (Figure ). Analysing the intestinal tissues histopathologically, villus lengths were determined to have a considerable and dose-dependant increase in control, EOM-250 mL/1000 L, EOM-500 mL/1000 L, EOM-750 mL/1000 L groups (Figure ). Histopathological examination in 36 °C groups revealed dilatation and hyperaemia in EOM-500 mL/1000 L and EOM-750 mL/1000 L sinusoids, diffuse hydropic degeneration in hepatocytes and coagulation necrosis in a small number of hepatocytes compared to the control group (Figure ). There was no difference in EOM-250 mL/1000 L dose on duodenum villa length compared to the control group, whereas there was a significant decrease in other groups (Figure ).
Figure 1. Histopathology of the small intestine (duodenum) belongs to stressful and stress-free groups.
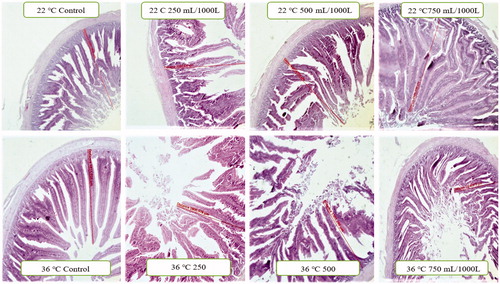
Table 3. Intestinal villus lenght of the experimental groups (7–42 days).
Discussion
Apelin, BDNF, and some blood parameters
Apelin is an important neuropeptide that has a physiological role in the neuroendocrine response to stress. Under stress, an increase is observed in the apelin secretion from the adipocyte. (Newson et al. Citation2013). As a result of our study, when the serum levels of apelin were examined depending on the increase in the dose of EOM applied to the experimental groups, it was found that the serum level of apelin linearly decreased in the groups exposed to 22 °C whereas a linear increase was observed in the experimental groups exposed to a heat stress at 36 °C (p < .01). In this regards, the findings of our study were in line with the studies which reported an increase in the apelin levels caused by stress (Li et al. Citation2016; Izgut-Uysal et al. Citation2018). The fact that the serum levels of apelin were found to increase in the experimental groups exposed to a heat stress at 36 °C confirmed the literature information indicating that apelin has an antidepressant-like effect on the regulation of neuroendocrine response to stress.
The basic endocrine reaction seen as the response to stress is the activation of the HPA (Hypothalamic – pituitary – adrenal) axis. Thus, stress causes the brain to stimulate the endocrine system. BDNF not only regulates an endocrine response to stress, but it is also an adipokine hormone that promotes the neuron development and differentiation and has a neuroprotective effect against ischaemia (Zigova et al. Citation1998; Kertes et al. Citation2017). Plasma BDNF (p-BDNF) is a sensitive marker indicating the level of brain BDNF (Lommatzsch et al). Whereas the BDNF level was found to increase linearly in the non-stressed groups; in the stressed groups, it decreased in the groups exposed to 500 and 750 mL/1000 L doses, but increased in the group exposed to 250 mL/1000 L dose (p < .05). To the best of our knowledge, in the literature, there is no other similar study conducted using the substances similar to what we used in this study as a supplement to the drinking water of broilers. It is thought that the increase in the p-BDNF level of the group exposed to 250 mL/1000 L dose was due to the response to cellular stress and the neuroprotective effect of BDNF (Lommatzsch et al).
Furthermore, the findings of this study show that p-BNDF can be used as a biomarker in evaluating the endocrine response to stress in the brain. In the literature review, it was found that there have been limited number of studies examining the effect of the EOM application in various doses to the drinking water of the broilers exposed to heat stress on the serum levels of VLDL, Glucose, Total bilirubin, and TG. In our study, the EOM application was found to have no effect on the serum levels of VLDL, Glucose, Total bilirubin, and TG (Table ) in both experimental groups (stressed and non-stresses groups) (p > .05). According to the trend analysis, the cubic effect was found to be significant for glucose and total bilirubin. The findings of our study were in line with some studies conducted on some medicinal aromatic plants in mice (Hou et al. Citation2010; Wang et al. Citation2010; Zotti et al. Citation2013; Younesi Citation2014; Motaghinejad et al. Citation2017), but different from some others (Silva et al. Citation2009; Máthé and de Sales Silva Citation2018). This discrepancy may be attributed to using different plants containing different flavonoids, administration routes, and experimental materials.
Intestinal and hepatic histopathology
The villus length and crypt depth are directly related to the digestive system health in birds and the absorption capacity of the intestinal mucosa (Awad et al. Citation2008). An increase in villus length helps to effectively digest the consumed foods (Awad et al. Citation2008). However, the decrease in villi length in the intestine due to stress not only impairs the metabolism and digestive function but also decreases the growth rate, body weight, and feed consumption (Salehifar et al. Citation2017). In the previous studies, it was reported that the addition of the essential oils of medicinal aromatic plants to the broiler rations in stress-free environments increased the villus height and decreased the epithelial thickness, thus enhancing the performance of the intestinal morphology by improving the use of feed. In our study, it was found that there was a linear increase in duodenum villus length in the non-stressed groups due to the increase in the dose of EOM, compared to the control group (22 °C). In this regard, our study was consistent with some of the information in the literature (Garcia et al. Citation2007; Hong et al. Citation2012; Akbarian et al. Citation2013; Du et al. Citation2016; Yang et al. Citation2018), but not at all with some others (Hong et al. Citation2012; Akbarian et al. Citation2013). It was reported that the heat stress negatively affected the intestinal epithelium in the birds and caused a decrease in villous length (Yamauchi et al. Citation2006; Burkholder et al. Citation2008). In addition, in the chronic heat-exposed broilers, the development of digestive organs and intestinal digestive enzymes was adversely affected by the activities; as a result, the cell apoptosis increased and the intestinal epithelial cells were found to be damaged (Tekce and Gul Citation2016; He et al. Citation2018). In the previous studies, it was stated that EOM had a positive effect on the intestinal villi length in the broilers fed under heat stress (Hajati et al. Citation2015; Hosseini et al. Citation2016). On the other hand, some studies stated that EOM did not have an effect on villi length (Shen et al. Citation2007; Du et al. Citation2016). In our study, although the villus lengths of the group of 250 mL/1000 L were similar to the control group, it was determined that the addition of essential oil mixture to the drinking water did not have an effect on the villi length in all groups. As a matter of fact, the data obtained were found to be in parallel with the data of live weight and feed use. According to the obtained data, it was found that the addition of different oil mixtures to the drinking water had no effect on the heat stress. Our study was in line with some of the information in the literature (Shen et al. Citation2007; Yang et al. Citation2018), but not at all in line with some others (Hajati et al. Citation2015; Hosseini et al. Citation2016). The reason for this difference is thought to be the differences in the essential oils or mixtures used and their composition. The liver damage caused by stress leads to a damage to the liver cell (parenchymal and sinusoidal cells). After perfusion, the Kupffer cell releases free radicals and proinflammatory cytokines. These cytokines, along with increased expression of adhesion molecules by sinusoidal endothelial cells, cause neutrophil infiltration of the liver and damage to tissues (Rauen et al. Citation1994; Ozcelik et al. Citation2014). It is stated that Medicinal Aromatic Plants increase the antioxidant enzyme activation and protect the liver tissue by reducing the neutrophil infiltration (Rauen et al. Citation1994; Kapakin et al. Citation2012; Li et al. Citation2014; Khodadadi et al. Citation2016).
In this study, the addition of EOM mixture to the drinking water of broilers fed under heat stress did not have a pathological effect on the group exposed to 22 °C compared to the control group; however, mild dilatation and hyperaemia were detected in sinusoids in the group of 750 mL/1000 L. In the groups exposed to a heat stress at 36 °C, there was no difference between the group of 250 mL/1000 L and the control group; however, a small amount of coagulation necrosis was detected in hepatocyte, a diffuse hydropic degeneration was observed in hepatocytes, and finally a dilatation and hyperaemia were seen in sinusoid in the groups of EOM-500 mL/1000 L and EOM-750 m/1000 L compared to the control group. The results of our study were consistent with some literature information (Fatemi et al. Citation2010; Kapakin et al. Citation2012), but not with some other literature information (SAS. Citation1999; Li et al. Citation2014; Iqbal et al. Citation2015). This difference is thought to be caused by the use of different doses of herbal extracts and the different methods or combinations used in administering these herbal extracts.
Conclusions
In this study, the EOM application was found to have no effect on the serum levels of VLDL, Glucose, Total bilirubin, TG (Table ) in both experimental groups (stressed and non-stresses groups) (p > .05). As a result, this study showed that the EOM addition in the dose of 250 mL/1000 L to the drinking water had a positive effect on Apelin and BDNF in the stressed and non-stressed groups (p < .05). Whereas the EOM addition in the dose of 750 mL/1000 L to the drinking water had a positive effect on the villus length in the non-stressed groups, the EOM addition to the drinking water had no effect on the villus length in the stressed groups compared to the control group. We are of the opinion that the apelin and BDNF hormones will make an important contribution to the literature as they provide data to the future studies in terms of the elucidation of adipokine hormone physiology and the biomarkers used for evaluating the stress response and certain diseases. To the best of our knowledge, this is the first study in this field in terms of the substances used as a supplement to the drinking water. This study does, however, have some limitations due to not involving a clinical study. In order to overcome the limitations, there is a need for increasing the experimental and prospective studies to determine the clinical significance of our findings.
Ethical approval
The experimental method was approved by Bayburt University Local Ethics Committee (Date and number of the decision is 02.07.2018- 2018/16).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Akbarian A, Golian A, Kermanshahi H, De Smet S, Michiels J. 2015. Antioxidant enzyme activities, plasma hormone levels and serum metabolites of finishing broiler chickens reared under high ambient temperature and fed lemon and orange peel extracts and Curcuma xanthorrhiza essential oil. J Anim Physiol Anim Nutr (Berl)). 99(1):150–162.
- Akbarian A, Golian A, Kermanshahi H, Farhoosh R, Raji AR, De Smet S, Michiels J. 2013. Growth performance and gut health parameters of finishing broilers supplemented with plant extracts and exposed to daily increased temperature. Span J Agric Res. 11(1):109–119.
- Al-Ja'fari A-H, Vila R, Freixa B, Tomi F, Casanova J, Costa J, Cañigueral S. 2011. Composition and antifungal activity of the essential oil from the rhizome and roots of Ferula hermonis. Phytochemistry. 72(11–12):1406–1413.
- AOAC. 2005. Official Methods of Analysis of AOAC International. 18th ed. Rockville, MD, USA: Association of Official Analytical Chemists.
- Apelin. 2018. Enzyme Lınked Immunosorbent Assay (ELISA) kits (Apelin, Sunred, Product code: 201-16-3073, China).
- Awad W, Ghareeb K, Böhm J. 2008. Intestinal structure and function of broiler chickens on diets supplemented with a synbiotic containing Enterococcus faecium and oligosaccharides. Int J Mol. 9(11):2205–2216.
- Bancroft JD, Suvarna K, Layton C. 2012. Bancroft’s theory and practice of histological techniques. 7th ed. London: Churchill Livingstone. 2012 E book ISBN: 978-0-7020-5032-9.
- Bayraktar B, Tekce E. 2018. Deneysel Olarak Sıcaklık Stresi Oluşturulan Broilerde Farklı Oranlarda Kullanılan Bazı Bitkisel Ekstrelerin Serum Demir Seviyesine Etkisinin İncelenmesi. J Tradit Complem Med. 1(2):50–55.
- Bayraktar B, Tekce E. 2019. Effects of varying essential oil mixture concentrations applied underconditions of different temperature stress on cardiac markers and other blood parameters. Braz J Poultry Scı. 21:1–9.
- BDNF. 2018. Enzyme Lınked Immunosorbent Assay (ELISA) kits (BDNF, S., Product code: 201-16-1172, China).
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. 2001. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 21(17):6718–6731.
- Bertrand C, Valet P, Castan-Laurell I. 2015. Apelin and energy metabolism. Front Physiol. 6:115–120.
- Bishop CD. 1995. Antiviral activity of the essential oil of Melaleuca alternifolia (maiden amp; Betche) Cheel (tea tree) against tobacco mosaic virus. J. Essent. Oil Res. 7(6):641–644.
- Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA. 2008. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult Sci. 87(9):1734–1741.
- Chowdhury S, Mandal GP, Patra AK, Kumar P, Samanta I, Pradhan S, Samanta AK. 2018. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim Feed Sci Technol. 236:39–47.
- Daroui-Mokaddem H, Kabouche A, Bouacha M, Soumat B, El-Azzouny A, Bruneau C, Kabouche Z. 2010. GC/MS analysis and antimicrobial activity of the essential oil of fresh leaves of Eucalytus globulus, and leaves and stems of Smyrnium olusatrum from Constantine (Algeria). Nat Prod Commun. 5(10):1669–1672.
- Demirci Ş, GÜN, C. 2019. Adipoz doku ve adipoz dokudan salgılanan bazı proteinler. MAKÜ Sag. Bil. Enst. Derg. 5(2):155–179.
- Dorman HJD, Deans SG. 2000. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 88(2):308–316.
- Du E, Wang W, Gan L, Li Z, Guo S, Guo Y. 2016. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J Anim Sci Biotechnol. 7:19.
- Fatemi F, Allameh A, Khalafi H, Ashrafihelan J. 2010. Hepatoprotective effects of gamma-irradiated caraway essential oils in experimental sepsis . Appl Radiat Isot. 68(2):280–285.
- Fausto N, Campbell JS, Riehle KJ. 2012. Liver regeneration. J Hepatol. 57(3):692–694.
- Feizi A, Bijanzad P, Kaboli K. 2013. Effects of thyme volatile oils on performance of broiler chickens. Eur J Exp Biol. 3:250–254.
- Fournel A, Drougard A, Duparc T, Marlin A, Brierley SM, Castro J, Le-Gonidec S, Masri B, Colom A, Lucas A, et al. 2017. Apelin targets gut contraction to control glucose metabolism via the brain. Gut. 66(2):258–269.,
- Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S, Efferth T. 2007. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res. 21(10):989–994.
- Garcia V, Catala-Gregori P, Hernandez F, Megias MD, Madrid J. 2007. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J Appl Poult Res. 16(4):555–562.
- Genzer Y, Chapnik N, Froy O. 2017. Effect of brain-derived neurotrophic factor (BDNF) on hepatocyte metabolism. Int J Biochem Cell Biol. 88:69–74.
- Gopi M, Karthik K, Manjunathachar HV, Tamilmahan P, Kesavan M, Dashprakash M, Purushothaman MR. 2014. Essential oils as a feed additive in poultry nutrition. Adv Anim Vet Sci. 2(1):1–7.
- Guimarães AG, Quintans JS, Quintans-Júnior LJ. 2013. Monoterpenes with analgesic activity-a systematic review. Phytother Res. 27(1):1–15.
- Habib KE, Gold PW, Chrousos GP. 2001. Neuroendocrinology of stress. J. Clin. Endocr. Metab. 30(3):695–728.
- Hajati H, Hassanabadi A, Golian A, Nassiri-Moghaddam H, Nassiri MR. 2015. The effect of grape seed extract and vitamin C feed supplementation on some blood parameters and HSP70 gene expression of broiler chickens suffering from chronic heat stress. Ital. J. Anim. Sci. 14:3273.
- He X, Lu Z, Ma B, Zhang L, Li J, Jiang Y, Zhou G, Gao F. 2018. Chronic heat stress damages small intestinal epithelium cells associated with the adenosine 5′-Monophosphate-Activated Protein Kinase Pathway in Broilers . J Agric Food Chem. 66(28):7301–7309.
- Hong JC, Steiner T, Aufy A, Lien TF. 2012. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 144(3):253–262.
- Horch HW. 2004. Local effects of BDNF on dendritic growth. Rev Neuroscıence. 15(2):117–130.
- Hosseini SM, Nazarizadeh H, Ahani S, Vakili Azghandi M. 2016. Effects of mannan oligosaccharide and Curcuma xanthorrhiza essential oil on the intestinal morphologyand stress indicators of broilers subjected to cyclic heat stress. Arch Anim Breed. 59(2):285–291.
- Hou Y, Aboukhatwa MA, Lei DL, Manaye K, Khan I, Luo Y. 2010. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 58(6):911–920.
- Hüther G. 1996. The central adaptation syndrome: psychosocial stress as a trigger for adaptive modifications of brain structure and brain function. Prog Neurobıol. 48(6):569–612.
- Iqbal Z, Kamran Z, Sultan JI, Ali A, Ahmad S, Shahzad MI, Ahsan U, Ashraf S, Sohail MU. 2015. Replacement effect of vitamin E with grape polyphenols on antioxidant status, immune, and organs histopathological responses in broilers from 1-to 35-d age. J Appl Poult Res. 24(2):127–134.
- Izgut-Uysal VN, Acar N, Birsen I, Ozcan F, Ozbey O, Soylu H, Avci S, Tepekoy F, Akkoyunlu G, Yucel G, et al. 2018. Apelin-APJ system is responsible for stress-induced increase in atrial natriuretic peptide expression in rat heart. Tissue Cell. 51:91–96.
- Jang IS, Ko YH, Kang SY, Lee CY. 2007. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 134(3–4):304–315.
- Kapakin KAT, Gümüş R, Imik H, Kapakin S, Sağlam YS. 2012. Effects of ascorbic and α-lipoic acid on secretion of HSP-70 and apoptosis in liver and kidneys of broilers exposed to heat stress. Ankara Univ Vet Fak Derg. 59:279–287.
- Karpouhtsis I, Pardali E, Feggou E, Kokkini S, Scouras ZG, Mavragani-Tsipidou P. 1998. Insecticidal and genotoxic activities of oregano essential oils. J Agric Food Chem. 46(3):1111–1115.
- Kertes DA, Bhatt SS, Kamin HS, Hughes DA, Rodney NC, Mulligan CJ. 2017. BNDF methylation in mothers and newborns is associated with maternal exposure to war trauma. Clin Epigenetics. 9:68–80.
- Khodadadi M, Mousavinasab SS, Khamesipour F, Katsande S. 2016. The effect of Cichorium intybus L. ethanol extraction on the pathological and biomedical indexes of the liver and kidney of broilers reared under heat stress. Rev Bras Cienc Avic. 18(3):407–412.
- Leica DM. 2018. The preparations for histopathologic examination were stained with hematoxylin-eosin (HE) and examined under the light microscope (Leica DM 1000).
- Li E, Deng H, Wang B, Fu W, You Y, Tian S. 2016. Apelin-13 exerts antidepressant-like and recognition memory improving activities in stressed rats. Eur Neuropsychopharmacol. 26(3):420–430.
- Li J, Zhang X, Huang H. 2014. Protective effect of linalool against lipopolysaccharide/D-galactosamine-induced liver injury in mice. Int Immunopharmacol. 23(2):523–529.
- Lin H, Decuypere E, Buyse J. 2008. Effect of thyroid hormones on the redox balance of broiler chickens. Asian Australas J Anim Sci. 21(6):794–800.
- Máthé Á, de Sales Silva JC. 2018. Introduction to medicinal and aromatic plants in Brazil. In Medicinal and Aromatic Plants of South America Springer, Dordrecht. 47–69.
- Matsuzawa Y. 2005. Adipocytokines and metabolic syndrome. Semin Vasc Med. 5(1):34–38.
- Medina-Gómez G. 2012. Mitochondria and endocrine function of adipose tissue. Best Pract Res Clin Endocrinol Metab. 26(6):791–804.
- Monzote L, García M, Montalvo AM, Scull R, Miranda M, Abreu J. 2007. In vitro activity of an essential oil against Leishmania donovani. Phytother Res. 21(11):1055–1058.
- Moraes VMB, Malheiros RD, Bruggeman V, Collin A, Tona K, Van As P, Onagbesan OM, Buyse J, Decuypere E, Macari M. 2003. Effect of thermal conditioning during embryonic development on aspects of physiological responses of broilers to heat stress. J. Therm. Biol. 28(2):133–140.
- Motaghinejad M, Motevalian M, Fatima S, Faraji F, Mozaffari S. 2017. Mozaffari, S. The neuroprotective effect of curcumin against nicotine-induced neurotoxicity is mediated by CREB–BDNF signaling pathway. Neurochem Res. 42(10):2921–2932.
- Naslund E, Ehrstrom M, Ma J, Hellstrom PM, Kirchgessner AL. 2002. Localization and effects of orexin on fasting motility in the rat duodenum. Am J Physiol Gastrointest Liver Physiol. 282(3):G470–G479.
- Newson MJF, Pope GR, Roberts EM, Lolait SJ, O’Carroll A-M. 2013. Stress-dependent and gender-specific neuroregulatory roles of the apelin receptor in the hypothalamic-pituitary-adrenal axis response to acute stress. J Endocrinol. 216(1):99–109.
- Newson MJF, Roberts EM, Pope GR, Lolait SJ, O'Carroll A-M. 2009. The effects of apelin on hypothalamic-pituitary-adrenal axis neuroendocrine function are mediated through corticotrophin-releasing factor- and vasopressin-dependent mechanisms . J Endocrinol. 202(1):123–129.
- Ozcelik M, Simsek UG, Ceribasi S, Ciftci M. 2014. Effects of different doses of rosemary oil (Rosmarinus officinalis L.) on oxidative stress and apoptosis of liver of heat stressed quails. Europ. Poult. Sci. 78:2014–2032.
- Özek G, Demirci F, Özek T, Tabanca N, Wedge DE, Khan SI, Başer KHC, Duran A, Hamzaoglu E. 2010. Gas chromatographic–mass spectrometric analysis of volatiles obtained by four different techniques from Salvia rosifolia Sm., and evaluation for biological activity. J Chromatogr A. 1217(5):741–748.
- Parvar R, Khosravinia H, Azarfar A. 2013. Effect of supplementation of Satureja essential oils in drinking water on immune performance of broiler chickens reared under heat stress. J Cell Anim Biol. 7(10):121–124.
- Petrolli TG, Sutille MA, Petrolli OJ, Stefani LM, Simionatto AT, Tavernari FDC, Girardini LK. 2019. Eucalyptus oil to mitigate heat stress in broilers. Rev Bras Zootecn. 48:1–8.
- Principe A, Melgar-Lesmes P, Fernández-Varo G, del Arbol LR, Ros J, Morales-Ruiz M, Bernardi M, Arroyo V, Jiménez W. 2008. The hepatic apelin system: a new therapeutic target for liver disease. Hepatology. 48(4):1193–1201.
- Rauen U, Viebahn R, Lauchart W, de Groot H. 1994. The potential role of reactive oxygen species in liver ischemia/reperfusion injury following liver surgery. Hepatogastroenterology. 41(4):333–336.
- Salehifar E, Abbasi M, Bahari-Kashani R. 2017. Effects of Myrtle (Myrtus communis) essential oil on growth performance, carcass characteristics, intestinal morphology, immune response and blood parameters in broiler chickens. J Livest. Sci. 8:63–71.
- SAS. 1999. SAS User’s Guide. Cary (NC): SAS Institute Inc.
- Shen SQ, Zhang Y, Xiang JJ, Xiong CL. 2007. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol. 13(13):1953–1961.
- Shirayama Y, Yang C, Zhang JC, Ren Q, Yao W, Hashimoto K. 2015. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur Neuropsychopharmacol. 25(12):2449–2458.
- Silva CF, Moura FC, Mendes MF, Pessoa F. 2011. Extraction of citronella (Cymbopogon nardus) essential oil using supercritical CO2: experimental data and mathematical modeling. Braz J Chem Eng. 28(2):343–350.
- Silva M. A d, Pessotti B. M d S, Zanini SF, Colnago GL, Rodrigues MRA, Nunes L. d C, Zanini MS, Martins IVF. 2009. Intestinal mucosa structure of broiler chickens infected experimentally with Eimeria tenella and treated with essential oil of oregano. Cienc Rural. 39(5):1471–1477.
- Sosanya NM, Garza TH, Stacey W, Crimmins SL, Christy RJ, Cheppudira BP. 2019. Involvement of brain-derived neurotrophic factor (BDNF) in chronic intermittent stress-induced enhanced mechanical allodynia in a rat model of burn pain. BMC Neuroscı. 20:1–18.
- Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou M-X, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, et al. 1998. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Bioph. Res. Co. 251(2):471–476.,
- Tatemoto K, Takayama K, Zou MX, Kumakı I, Zhang W, Kumano W, Fujımıya M. 2001. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 99(2-3):87–92.
- Tekce E, Çınar K, Bayraktar B, Takma Ç, Gül M. 2020. Effects of an essential oil mixture added to drinking water for temperature-stressed broilers: performance, meat quality, and thiobarbituric acid-reactive substances. J Appl Poultry Res. 29(1):77–84.
- Tekce E, Gül M. 2015. Sıcaklık Stresi Altında Beslenen Etçi Piliçlerde Origanum Syriacum Uçucu Yağının performans Antioksidan Potansiyel Lipid Profili Bağırsak Mikroflorası ve Et Kalitesine Etkisi. Doktora Tezi, AÜ Sağlık Bilimleri Enstitüsü, Erzurum.
- Tekce E, Gul M. 2016. Effects of Origanum syriacum essential oil added in different levels to the diet of broilers under heat stress on performance and intestinal histology. Europ Poult Sci. 80:1–11.
- Than A, Zhang X, Leow MKS, Poh CL, Chong SK, Chen P. 2014. Apelin attenuates oxidative stress in human adipocytes. J Biol Chem. 289(6):3763–3774.
- Ultee A, Bennik MHJ, Moezelaar R. 2002. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 68(4):1561–1568.
- Vollmayr B, Faust H, Lewicka S, Henn FA. 2001. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 6(4):471–474.
- Wang G, Anini Y, Wei W, Qi X, OCarroll A-M, Mochizuki T, Wang H-Q, Hellmich MR, Englander EW, Greeley GH. 2004. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 145(3):1342–1348.
- Wang R, Li Y-H, Xu Y, Li Y-B, Wu H-L, Guo H, Zhang J-Z, Zhang J-J, Pan X-Y, Li X-J. 2010. Curcumin produces neuroprotective effects via activating brain-derived neurotrophic factor/TrkB-dependent MAPK and PI-3K cascades in rodent cortical neurons. Prog Neuropsychopharmacol Biol Psychiatry. 34(1):147–153.
- Yamauchi K, Buwjoom T, Koge K, Ebashi T. 2006. Histological intestinal recovery in chickens refed dietary sugar cane extract. Poult. Sci. 85(4):645–651.
- Yang X, Xin H, Yang C, Yang X. 2018. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim Nutr. 4(4):388–393.
- Younesi E. 2014. Evidence-based modeling of mode-of-action for functional ingredients influencing Alzheimer’s disease through neurotrophin pathway. Funct Food Health Dis. 4(8):362–369.
- Zigova T, Pencea V, Wiegand SJ, Luskin MB. 1998. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol Cell Neurosci. 11(4):234–245.
- Zotti M, Colaianna M, Morgese M, Tucci P, Schiavone S, Avato P, Trabace L. 2013. Carvacrol: from ancient flavoring to neuromodulatory agent. Molecules. 18(6):6161–6172.