 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The combined use of somatic cell count (SCC) and differential somatic cell count (DSCC), which is the ratio of neutrophils plus lymphocytes to total milk SCC, represents a novel approach to define cow’s udder health status, as it allows to identify healthy animals (those with low SCC and DSCC), cows susceptible to mastitis (those where an immune response has begun, so that there is an increase of neutrophils, i.e. DSCC, but not yet of total SCC), animals with a mastitic event in progress (those with high SCC and DSCC) and animals with possible chronic inflammation (those with high SCC and low DSCC, as macrophages prevail). We investigated the association of cow’s udder health status with milk yield, composition and coagulation properties in four cattle breeds. Results demonstrated that milk traits vary among cows with different udder health status, especially in terms of fat percentage, lactose percentage and coagulation ability. The most pronounced worsening in milk yield and coagulation ability was observed for animals with chronic inflammation. Our findings support the new approach based on the combined use of SCC and DSCC to screen for cow’s udder health, as it would allow to identify susceptible cows that will probably undergo a mastitic event and chronic cows that would possibly reduce the herd milk production and quality.
The combined use of SCC and DSCC is a novel approach to screen for udder health status.
Differences in milk yield, composition and coagulation exist among cows with different udder health status defined on the basis of combined SCC and DSCC.
The information about udder health status may be used to develop mastitis pre-screening protocols.
Highlights
Introduction
In the last years several efforts have been made to improve the udder health status of dairy cows, both improving hygienic conditions in the farm and enhancing cow’s resistance to mastitis by genetic selection. So far, milk somatic cell count (SCC) has been used as indicator of udder health and milk quality (Harmon Citation2001) and it has been included in selection indexes of different countries to reduce the susceptibility to mastitis (Weigel and Shook Citation2018). Nevertheless, a combination of different indirect indicators of mastitis could be a more successful predictor of udder health. Thus, alternative traits derived from milk SCC, like the presence of at least one test-day (TD) SCC above specific thresholds in the lactation (Urioste et al. Citation2010; Koeck et al. Citation2012; Bobbo et al. Citation2018), as well as blood serum proteins (Bobbo et al. Citation2017; Cecchinato et al. Citation2018) and cellular immune-associated traits (Denholm et al. Citation2017), have been explored as novel indicators of animal health. For instance, some of the new alternative SCC traits reported by Bobbo et al. (Citation2018) have already been incorporated into an aggregate selection index to reduce mastitis susceptibility in Italian Holsteins (Finocchiaro et al. Citation2018). In addition, the recent implementation of new advanced milk-testing technology has allowed measurement of the proportion of the different cell types in milk in a high-throughput manner (Damm et al. Citation2017). Milk somatic cells are mainly leukocytes, i.e. polymorphonuclear neutrophils, macrophages and lymphocytes. These three cell types play different roles in the immune response to mastitis and their proportion in milk varies according to the infection status of the mammary gland (Damm et al. Citation2017). Bobbo et al. (Citation2019) demonstrated that differential somatic cell count (DSCC), which is the ratio of neutrophils plus lymphocytes to total SCC (Damm et al. Citation2017), is potentially exploitable in breeding programmes aimed to reduce udder health problems. Furthermore, Zecconi et al. (Citation2019) established a correlation between DSCC and SCC, and defined the most accurate thresholds of DSCC to maximise the probability of correctly identifying mammary gland status in Italian Holsteins. Thresholds suggested by Zecconi et al. (Citation2019) are currently used by the Italian Breeders Association (Rome, Italy) in the frame of the monthly recording system, providing the farmers with information about a mastitis risk assessment defined on the basis of a combination of SCC and DSCC. The thresholds applied for grouping the cows are SCC of 200,000 cells/mL and DSCC of 66.3%, 69.2% and 69.3% for cows having days in milk (DIM) <100, 101–200 and >200, respectively. Combining SCC and DSCC thresholds is a novel and more precise approach to screen for mastitis (although bacteriology is still the gold standard for definition of intramammary infection), as it allows to distinguish between healthy and mastitic cows, to identify cows susceptible to the disease and to detect cows affected by chronic mastitis. In fact, in the early stages of infection neutrophil count increases faster than total SCC (Schwarz et al. Citation2011); thus cows with low SCC and high DSCC are prone to experience soon a mastitic event. On the other side, cows affected by chronic mastitis have high proportion of macrophages (low DSCC) in milk with high SCC (Leitner et al. Citation2000).
To the best of our knowledge, no information on the effect of udder health status (i.e. the combined effect of SCC and DSCC) on milk traits of different cattle breeds is currently available. Therefore, the aim of this study was to investigate the effect of udder health status, defined according to thresholds proposed by Zecconi et al. (Citation2019), on milk yield, composition and coagulation properties of four cattle breeds.
Materials and methods
Data collection and editing
The Breeders Association of Veneto Region (Padova, Italy) provided TD milk records collected from January 2018 to January 2020 during routine milk recording procedures. The data included milk yield (kg/d); fat, protein and lactose percentages, SCC (cells/mL) and DSCC (%) determined using the CombiFoss 7 DC (Foss, Hillerød, Denmark); and milk coagulation properties, namely rennet coagulation time (RCT, min), curd-firming rate to a curd firmness of 20 mm (k20, min) and curd firmness 30 min after rennet addition to milk (a30, mm) measured using the Formagraph (Foss). In this study, not all milk samples collected in the framework of the routine milk testing procedures of Veneto Region could be analysed for DSCC. Indeed, the laboratory of the Breeders Association of Veneto Region is currently equipped with 3 infra-red instruments to determine milk composition and SCC, but only one of them (CombiFoss 7 DC) provides also information on DSCC, meaning that approximately one third of the milk samples processed by the laboratory could be randomly analysed for this new phenotype. Moreover, the original dataset was edited to select Holstein Friesian, Brown Swiss, Simmental and Rendena cows between 5 and 305 DIM, whereas other breeds were excluded due to few observations available. As the performance range of the method includes SCC between 50,000 and 1,500,000 cells/mL due to accuracy and repeatability issues of the instrument (Damm et al. Citation2017), records with SCC > 1,500,000 cells/mL were discarded, whereas for records with SCC < 50,000 cells/mL DSCC were set to 45%, following the approach of Wall et al. (Citation2018) and Schwarz et al. (Citation2019). After editing, the data set included 291,527 TD records of 94,515 cows in 1128 herds. All previous editing steps explain why, although data were recorded over a 2-year period, the average number of TD per cow with DSCC information was low (approximately 3 TD/cow).
In accordance with what performed by the Italian Breeders Association, cow’s udder health status was classified using a predefined SCC threshold of 200,000 cells/mL (Dohoo and Leslie Citation1991), in combination with the DSCC thresholds specific for early (66.3%), middle (69.2%) or late (69.3%) lactation (Zecconi et al. Citation2019). Specifically, four classes obtained from the combination of SCC and DSCC thresholds were created:
Healthy = cows with SCC ≤ 200,000 cells/mL and DSCC ≤ cut-off.
Susceptible = cows with SCC ≤ 200,000 cells/mL and DSCC > cut-off.
Chronic = cows with SCC > 200,000 cells/mL and DSCC ≤ cut-off.
Mastitic = cows with SCC > 200,000 cells/mL and DSCC > cut-off.
Statistical analysis
Milk yield, composition and coagulation properties were analysed using the HPMIXED procedure of SAS version 9.4 (SAS Institute Inc., Cary, NC) according to the following linear mixed model:
where yijklmnop is the investigated trait (milk yield, fat percentage, protein percentage, lactose percentage, RCT, k20 or a30); μ is the overall mean; DIMi is the fixed effect of the ith class of stage of lactation (i = 10 classes of 30 d each, from 5 to 305 DIM); Parityj is the fixed effect of the jth parity (j = primiparous, pluriparous); Breedk is the fixed effect of the kth breed (k = Holstein Friesian, Brown Swiss, Simmental, Rendena); MYl is the fixed effect of the lth month-year of sampling (l = 24 classes, from January 2018 to January 2020); UHSm is the fixed effect of the mth udder health status (m = healthy, susceptible, chronic, mastitic); Cown is the random effect of the nth cow (n = 1–94,515) ∼ N(0, σ2cow); Herdo is the random effect of the oth herd (o = 1–1128) ∼ N(0, σ2herd); and eijklmnop is the random residual ∼ N(0, σ2e).
Results and discussion
Descriptive statistics of milk traits and classification of cow’s udder health status
Holstein Friesian was the most productive breed (33.3 kg/d) and milk of Brown Swiss cows was characterised by the greatest percentages of fat (4.06%) and protein (3.63%; Table ). Mean SCC and DSCC were slightly greater in Rendena than in the other breeds. These findings are in agreement with those of De Marchi et al. (Citation2007), who reported the best milk quality for Brown Swiss cattle and the greatest somatic cell score for Rendena. Our results slightly differed from those of Damm et al. (Citation2017), who reported DSCC from 72.68% to 76.12%, likely due to greater minimum value of DSCC (20%) in Damm et al. (Citation2017) compared with the minimum value (0.9%) of this study. Mean values of milk coagulation properties were comparable with those reported by De Marchi et al. (Citation2007) and Visentin et al. (Citation2015). Classification of cow’s udder health status resulted, on average, in 55.7% of healthy, 21.7% of susceptible, 19.0% of mastitic and 3.6% of chronic cows (Table ). Such classification, defined combining the information of SCC (below or above 200,000 cells/mL) and DSCC (below or above the specific threshold), was based on the assumption that macrophages are the predominant cell type (low DSCC) in uninfected udders with low SCC (Lee et al. Citation1980), whereas high proportions of macrophages in milk with elevated SCC could indicate udders affected by chronic mastitis (Leitner et al. Citation2000). In infected glands, neutrophils, which play a defense role against invading pathogens, can increase up to 95% of total SCC (Kehrli and Shuster Citation1994). Nevertheless, in the early stages of infection, neutrophils increase even faster than total SCC (Schwarz et al. Citation2011; Pilla et al. Citation2012), allowing the identification of cows with an ongoing inflammatory process. This classification represents an evolution of the grouping proposed by Wall et al. (Citation2018), who suggested a combination of high SCC (>200,000 cells/mL) and DSCC (lower or greater than 86%) to define mastitis in late or early stage. In this study, healthy primiparous cows ranged from 48.4% (Rendena) to 62.5% (Brown Swiss), and healthy pluriparae from 40.8% (Rendena) to 56.0% (Simmental; Table ). Regardless of the breed, the frequency of susceptible cows was lower and that of mastitic and chronic animals was greater for pluriparae than primiparae. Among breeds, Rendena showed the greatest percentage of susceptible and mastitic cows (Table ). Given that autochthonous cattle breeds, such as the Rendena, are known to be more robust and to have a lower prevalence of mastitis (Cremonesi et al. Citation2018), our frequencies could be biased by the use of a DSCC threshold developed for Italian Holsteins and possibly too low for Rendena, which showed grater mean DSCC than Holstein Friesian and the other two breeds (Table ). Nevertheless, in the early stages of DSCC exploration, a lower threshold is preferred in order to be more conservative in identifying cows possibly at risk of mastitis or with an ongoing mastic event.
Table 1. Mean and standard deviation (SD) of test-day milk yield, composition, somatic cell count (SCC), differential somatic cell count (DSCC) and milk coagulation properties of four cattle breeds.
Table 2. Classification of cow’s udder health status, combining the information of somatic cell count (SCC) and differential somatic cell count (DSCC), according to breed and parity.
Effects of udder health status on milk yield, composition and coagulation properties
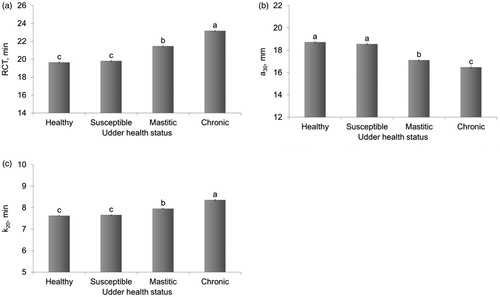
All the effects included in the statistical model were significant (p < .001) in explaining the variation of the investigated traits. In this study, we focussed on the effects of udder health status on milk traits, after adjusting for the other possible sources of variation. Healthy cows had an average milk production of 25.1 kg/d and their milk was characterised by 3.83% of fat and 3.43% of protein (Figure ). Cows susceptible to mastitis, i.e. those in the early stages of inflammation, had slightly greater milk production than healthy cows (25.5 kg/d; Figure ). This is in good agreement with previous studies (Oltenacu and Broom Citation2010), supporting the idea that high producing cows are at higher risk of production diseases, including metabolic stress and mastitis. In fact, high milk production was identified as a risk factor for mastitis (Rajala and Gröhn Citation1998). Susceptible cows produced milk with the lowest fat (3.80%) and protein percentage (3.42%), possibly as a result of proteolytic and lipolytic activity stimulated by the neutrophils recruitment (Le Maréchal et al. Citation2011). The lowest milk production was observed for cows classified as chronic (20.9 kg/d; Figure ), i.e. cows with high SCC, mostly macrophages (low DSCC). A state of chronic inflammation over a long period of time could possibly affect the development of milk-producing tissue. The greatest percentages of fat and protein in milk of chronic cows can be explained by a concentration effect due to reduced milk production, although data concerning the effect of mastitis on total protein and fat contents are controversial (Le Maréchal et al. Citation2011). Our results confirmed the known effect of mastitis on lactose content; indeed, a reduction of lactose percentage was observed in milk of mastitic (4.77%) and chronic cows (4.67%). Variation of lactose content due to mastitis can be explained both by increased milk barrier permeability and damage of the mammary tissue, with consequent impaired synthetic activity (Shuster et al. Citation1991). Results on milk coagulation properties (Figure ) corroborate previous findings about the impact of mastitis on milk coagulation (Leitner et al. Citation2011; Le Maréchal et al. Citation2011). Milk from mastitic cows was characterised by longer RCT (21.5 min) compared with milk of healthy animals (19.6 min), as well as by weaker a30 (17.1 mm vs 18.7 mm). Nevertheless, the greatest impairment in milk coagulation was observed for chronic cows (RCT = 23.2 min; Figure ). In the literature, only few studies investigated the effect of different milk somatic cell types on milk and dairy products, and most of them were focussed on neutrophils (Cooney et al. Citation2000; Wickström et al. Citation2009, Leitner et al. Citation2012). Nevertheless, Cohn (Citation1975) reported that macrophages with engulfing activity can produce five to six times more proteases than neutrophils, possibly explaining the worsening of milk coagulation ability of chronic animals in this study.
Figure 1. Least squares means (with standard errors) of (a) milk yield, (b) protein percentage, (c) fat percentage and (d) lactose percentage according to udder health status classification. Different letters mark statistical significance (p <.01).
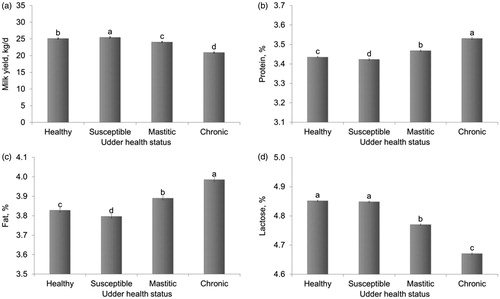
Figure 2. Least squares means (with standard errors) of (a) rennet coagulation time (RCT), (b) curd firmness 30 min after rennet addition to milk (a30), and (c) curd-firming rate to a curd firmness of 20 mm (k20) according to udder health status classification. Different letters mark statistical significance (p<.01).
Conclusions
Our findings highlighted that significant differences in milk production and quality exist among cows with different udder health status defined according to a combination of SCC and DSCC. This approach provides a more precise definition of the udder health status, helping farmers and veterinarians to identify and pay special attention to susceptible cows that could potentially undergo a mastitic event, and to chronic cows that could significantly impact on herd milk production and quality. The information about udder health status may be used to develop in the future mastitis pre-screening protocols, especially at the end of lactation, to optimise antimicrobial therapy.
Ethical approval
All authors declare that this study follows the principles of the Declaration of Helsinki.
Acknowledgments
The authors thank the Breeders Association of Veneto Region (Padova, Italy) for providing test-day milk records used in this study.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bobbo T, Fiore E, Gianesella M, Morgante M, Gallo L, Ruegg PL, Bittante G, Cecchinato A. 2017. Variation in blood serum proteins and association with somatic cell count in dairy cattle from multi-breed herds. Animal. 11(12):2309–2319.
- Bobbo T, Penasa M, Cassandro M. 2019. Short communication: genetic aspects of milk differential somatic cell count in Holstein cows: a preliminary analysis. J Dairy Sci. 102(5):4275–4279.
- Bobbo T, Penasa M, Finocchiaro R, Visentin G, Cassandro M. 2018. Alternative somatic cell count traits exploitable in genetic selection for mastitis resistance in Italian Holsteins. J Dairy Sci. 101(11):10001–10010.
- Cecchinato A, Bobbo T, Ruegg PL, Gallo L, Bittante G, Pegolo S. 2018. Genetic variation in serum protein pattern and blood β-hydroxybutyrate and their relationships with udder health traits, protein profile, and cheese-making properties in Holstein cows. J Dairy Sci. 101(12):11108–11119.
- Cohn ZA. 1975. The role of proteases in macrophage physiology. In: Proteases in biological control. Cold Spring Harbor, NY: Cold Spring Harbor Lab; p. 483.
- Cooney S, Tiernan D, Joyce P, Kelly AL. 2000. Effect of somatic cell count and polymorphonuclear leucocyte content of milk on composition and proteolysis during ripening of Swiss-type cheese. J Dairy Res. 67(2):301–307.
- Cremonesi P, Ceccarani C, Curone G, Severgnini M, Pollera C, Bronzo V, Riva F, Addis MF, Filipe J, Amadori M, et al. 2018. Milk microbiome diversity and bacterial group prevalence in a comparison between healthy Holstein Friesian and Rendena cows. PLoS One. 13(10):e0205054.
- Damm M, Holm C, Blaabjerg M, Bro MN, Schwarz D. 2017. Differential somatic cell count – a novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J Dairy Sci. 100(6):4926–4940.
- De Marchi M, Dal Zotto R, Cassandro M, Bittante G. 2007. Milk coagulation ability of five dairy cattle breeds. J Dairy Sci. 90(8):3986–3992.
- Denholm SJ, McNeilly TN, Banos G, Coffey MP, Russell GC, Bagnall A, Mitchell MC, Wall E. 2017. Estimating genetic and phenotypic parameters of cellular immune-associated traits in dairy cows. J Dairy Sci. 100(4):2850–2862.
- Dohoo IR, Leslie KE. 1991. Evaluation of changes in somatic cell counts as indicators of new intra-mammary infections. Prev Vet Med. 10(3):225–237.
- Finocchiaro R, Visentin G, Penasa M, van Kaam J, Marusi M, Civati G, Cassandro M. 2018. Alternative use of somatic cells counts in genetic selection for mastitis resistance: A new estimated breeding value for Italian Holstein breed. Interbull Bull. 53:31–33.
- Harmon RJ. 2001. Somatic cell counts: a primer. Proceedings of the 40th Annual Meeting of the National Mastitis Council; Feb 11–14; Reno, NV. p. 3–9.
- Kehrli ME, Shuster DE. 1994. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J Dairy Sci. 77(2):619–627.
- Koeck A, Miglior F, Kelton DF, Schenkel FS. 2012. Alternative somatic cell count traits to improve mastitis resistance in Canadian Holsteins. J Dairy Sci. 95(1):432–439.
- Le Maréchal C, Thiéry R, Vautor E, Le Loir Y. 2011. Mastitis impact on technological properties of milk and quality of milk products – a review. Dairy Sci Technol. 91(3):247–282.
- Lee CS, Wooding FBP, Kemp P. 1980. Identification, properties, and differential counts of cell populations using electron microscopy of dry cows secretions, colostrum and milk from normal cows. J Dairy Res. 47(1):39–50.
- Leitner G, Merin U, Krifucks O, Blum S, Rivas AL, Silanikove N. 2012. Effects of intra-mammary bacterial infection with coagulase negative staphylococci and stage of lactation on shedding of epithelial cells and infiltration of leukocytes into milk: comparison among cows, goats and sheep. Vet Immunol Immunopathol. 147(3–4):202–210.
- Leitner G, Merin U, Silanikove N. 2011. Effects of glandular bacterial infection and stage of lactation on milk quality: Comparison among cows, goats and sheep. Int Dairy J. 21(4):279–285.
- Leitner G, Shoshani E, Krifuck O, Chaffer M, Saran A. 2000. Milk leucocyte population patterns in bovine udder infection of different aetiology. J Vet Med Series B. 47(8):581–589.
- Oltenacu PA, Broom DM. 2010. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim Welf. 19:39–49.
- Pilla R, Schwarz D, König S, Piccinini R. 2012. Microscopic differential cell counting to identify inflammatory reactions in dairy cow quarter milk samples. J Dairy Sci. 95(8):4410–4420.
- Rajala PJ, Gröhn YT. 1998. Disease occurrence and risk factor analysis in Finnish Ayrshire cows. Acta Vet Scand. 39(1):1–13.
- Schwarz D, Diesterbeck US, König S, Brügemann K, Schlez K, Zschöck M, Wolter W, Czerny C-P. 2011. Microscopic differential cell counts in milk for the evaluation of inflammatory reactions in clinically healthy and subclinically infected bovine mammary glands. J Dairy Res. 78(4):448–455.
- Schwarz D, Lipkens Z, Piepers S, De Vliegher S. 2019. Investigation of differential somatic cell count as a potential new supplementary indicator to somatic cell count for identification of intramammary infection in dairy cows at the end of the lactation period. Prev Vet Med. 172:104803.
- Shuster DE, Harmon RJ, Jackson JA, Hemken RW. 1991. Suppression of milk production during endotoxin-induced mastitis. J Dairy Sci. 74(11):3763–3774.
- Urioste JI, Franzén J, Strandberg E. 2010. Phenotypic and genetic characterization of novel somatic cell count traits from weekly or monthly observations. J Dairy Sci. 93(12):5930–5941.
- Visentin G, Penasa M, Gottardo P, Niero G, Isaia M, Cassandro M, De Marchi M. 2015. Milk coagulation properties of cattle breeds reared in Alpine area. Poljoprivreda. 21(1 Suppl):237–240.
- Wall SK, Wellnitz O, Bruckmaier R, Schwarz D. 2018. Differential somatic cell count in milk before, during, and after artificially induced immune reactions of the mammary gland. J Dairy Sci. 101(6):5362–5373.
- Weigel KA, Shook GE. 2018. Genetic selection for mastitis resistance. Vet Clin North Am Food Anim Pract. 34(3):457–472.
- Wickström E, Persson-Waller K, Lindmark-Månsson H, Östensson K, Sternesjö Å. 2009. Relationship between somatic cell count, polymorphonuclear leucocyte count and quality parameters in bovine bulk tank milk. J Dairy Res. 76(2):195–201.
- Zecconi A, Vairani D, Cipolla M, Rizzi N, Zanini L. 2019. Assessment of subclinical mastitis diagnostic accuracy by differential cell count in individual cow milk. Ital J Anim Sci. 18(1):460–465.
