?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
With the aim of assessing bull fertility before or during its use for reproduction under tropical weather conditions where heat stress is present, and correlating it to herd parameters, BSE was carried out to determine which genetic groups of bulls have better reproductive performance. 223 bulls from different genetic groups, Zebu bulls (N = 72), European bulls (N = 58), and Crossbred Bulls (N = 93), were evaluated in situ over a two-year period, 2018–2019. Bull (age, corporal conditions, libido, and scrotal circumference), semen (concentration, motility and volume), herd (pregnancy rate and calving intervals) and environmental variables (THI, season, sampling month and spermatogenesis month) were analysed with One-Way ANOVA, as well as with factorial, multiple regression, and multivariate analysis. Zebu and European bulls have a higher libido than crossbreed bulls (p < .05), and it decreases with age (p < .05). High-libido bulls (>7) show high correlation (R = 0.70 p < .05) with herd parameters; BCS affects libido (p < .05). Sperm concentration is correlated to herd parameters (R = 0.82 p < .05). European bull sperm concentration and motility showed a decrease in autumn and winter (p < .05) in comparison to other genetic groups. Libido and semen variables are more associated with herd parameters than with other bull characteristics. Environmental variables are not associated with herd parameters (p > .05). Heat stress did not directly affect bull reproductive performance; Zebu bulls and crossbreeds showed better herd parameters (p < .05).
Bull breeding soundness examination and herd proficiency.
Herd proficiency of local genetic groups of bulls in tropical conditions.
Breeding soundness examination in tropical environment conditions.
Highlights
Introduction
Bulls have a decisive influence on the reproductive efficiency of herds, regardless of the reproductive programme used: natural, controlled mating or artificial insemination. Therefore, absence, or decrease, of bull fertility causes a reduction in productive parameters, with a possible decline in farm profitability, particularly in extensive livestock production systems. In some specific conditions, fertility does not only mean the characteristics of the seed but also the ability of the bull to obtain, even in natural mounts, a satisfactory number of pregnancies even in adverse environmental conditions. In tropical countries, breeding programmes for beef and dual-purpose cattle are characterised by extensive systems, where the aim is to increase calve numbers and selling weight (Martínez-González et al. Citation2017). Livestock production in Veracruz began since the first age of Spanish colonisation in XV century with the introduction of southern Spain’s animals (Bos taurus) that originated the Creole cattle population (Cañón et al. Citation2011); since the 50's, Bos Indicus cattle (Indo-Brazil, Brahman) from Brazil, as well as international cattle (Brown Swiss, Holstein, Charolais and Simmental), were introduced with the aim of introducing superior production performance through gradual adaptation to environmental conditions (Domínguez-Mancera et al. Citation2017; Ginja et al. Citation2019). In the full extensive farming system of the region, bulls are required to be extremely fertile in board sense to preserve farm profitability. Highly fertile bulls can achieve a pregnancy rate higher than 60% in females that show adequate reproductive activity; to achieve this, Bull Breeding Soundness Examination (BBSE) is necessary (Nichi et al. Citation2006; Páez-Barón and Corredor-Camargo Citation2014). BBSE is a procedure that reduces risk and improves strategic bull usage and herd fertility (Chenoweth Citation2002). Breeding Soundness is affected by several factors, such as age, nutrition (body condition), environmental conditions (seasons), management, breed (genetic group) and their interactions (Roberts et al. 2010; Menon et al. Citation2011; Lemma and Shemsu Citation2015). BBSE includes biometric measurements, mainly scrotal circumference (SC), semen analysis (sperm concentration, motility and morphology), but behaviour (libido) is not considered in it, although this parameter can be used to predict potential bull fertility (Lessard et al. Citation2011). SC is an indirect but trusty indicator of testicular weight and sperm production capacity (Chacon et al. Citation1999; Alexander Citation2008; Kumar et al. Citation2016). It has been extensively studied because of its favourable genetic correlations with age at puberty in males and females (Toelle and Robison Citation1985; Notter et al. Citation1993), and reproductive characteristics in females, such as heifer pregnancy (Van Melis et al. Citation2010; Santana et al. Citation2012). Sperm concentration, motility and morphology are key factors in BBSE, and, consequently, affect herd reproductive parameters in extensive systems (Burnett et al. Citation2018). Bull behaviour, especially libido, is another very important trait related to BBSE; bulls with high libido (>7 in scale 1–10) produce more ejaculates, have lesser reaction time and refractory period, and better semen quality (Ellis et al. Citation2005; Bury et al. Citation2011). In tropical conditions, where high ambient temperature and/or humidity are present, cattle reproduction can be affected by heat stress (Brito et al. Citation2002); this condition can have important effects on most aspects of reproduction (Hansen Citation2009). Semen characteristics are not immediately affected by changes in testicular temperature, because damaged spermatogenic cells do not enter ejaculates for some time after suffering from heat stress but other factors such as behaviour can be influenced early and indirectly affect global fertility (Lees et al. Citation2019). Regarding bulls, whose spermatogenesis takes about 61 days, alterations in semen occur about two weeks after heat stress, and it does not return to normal until up to eight weeks following the end of heat stress conditions (Meyerhoeffer et al. Citation1985; Paul et al. Citation2008), given the recent interest in the impact of climate change on the profitability and sustainability of cattle breeding, we present the following research with the aim of verifying the effect of environmental variables on global fertility parameters (BBSE) in bulls of different genetic types under the tropical conditions in Veracruz state, Mexico and its relationship with herd reproductive parameters.
Materials and methods
Ethical statement
All the procedures for handling, immobilisation and semen collection carried out on bulls within the farms by official veterinary services and were evaluated and approved with registration number COBIBA010/2017 by Bioethical Committee (School of Veterinary and Animal Sciences of Veracruz University). The welfare of the animal is paramount and stimulation was discontinued if either undue stress was being caused or physical injury to the bull might occur.
Animals
Bull breeding soundness examination (N = 223), carried out by the Animal Biology Reproduction Laboratory, and the Cell Biology Laboratory of Veracruz University, were used. The genetic groups to be evaluated were assigned as proposed by (Menegassi et al. Citation2015). Zebu bulls ‘Bos indicus’ (Gyr and Brahman), European bulls ‘Bos taurus’ (Brown Swiss, Holstein, Charolais and Simmental), Crossbreed bulls (Holstein × Zebu, Brown Swiss × Zebu, synthetic breed bulls × Ze; Beef Master, Brahman × Hereford × Shorthorn). All bulls were fed under an extensive grazing system grazed Cynodon nlemfuensis and Brachiaria humidicola grasses (Sollenberger Citation2008; Cruz et al. Citation2017), with 1–8 years of age, without any apparent health impairment at the time of evaluation. Animals and semen samples were examined in the farms where they were being kept during the 2018–2019 period.
The cows used to measure the fertility of bulls were clinically healthy and fertile at time when the Breeding Soundness Examination were performed, cows with reproductive abnormalities were excluded of the analysis. Palpation of the reproductive system by rectal examination or ultrasound using a Minitube Probe 6.5 MHz linear (Minitube, Verona, WI, USA) was used for rectal pregnancy and abnormalities detection. The reproductive records and clinical evaluation of the cow reproductive system (N = 6513) from different genetic groups. Zebu cows Bos indicus N = 2160 (Gyr n = 1258 and Brahman n = 902), European cows Bos taurus N = 1749 (Brown Swiss n = 612, Holstein n = 192, Charolais n = 334 and Simmental n = 612), Crossbreed cows N = 2603 (Holstein × Zebu n = 1093, Brown Swiss × Zebu n = 1511) with an age range of 4–9 years, mean calving interval of 914 ± 22 days (2.5 ± 0.07 years) and mean pregnancy percentage 35.6 ± 2.9%. The pregnancy rate was calculated as the total number of pregnant cows divide by the numbers of mated cows by bull examined. Calving interval, was calculated as the amount of time (days) between the birth of a calf and the birth of a subsequent calf, both from the same cow. This information was obtained from herd productive and reproductive records, considering the time each bull was in the herd (Perea-Ganchou et al. Citation2005; Galina et al. Citation2007). All cows were fed under an extensive grazing system grazed Cynodon nlemfuensis and Brachiaria humidicola grasses (Sollenberger Citation2008; Cruz et al. Citation2017).
Morphological and behavioural evaluation
BBSE was carried out following the indications of the Society for Theriogenology’s Manual for Breeding Soundness Examination in Bulls (Kennedy et al. Citation2010). Scrotal circumference was measured in each bull, using the technique described by Beggs et al. (Citation2013); body condition scores (scale 1–5) were measured using the technique described by Kunkle et al. (Citation1994). To evaluate bull libido and breeding ability, a test was performed in a small yard where bull and cow could be easily observed; the bull was allowed to be in contact a cow exhibiting behavioural oestrus during a 5-minute period. Libido was rated (0–10 scale) with the system proposed by Chenoweth (Citation1997, Chenoweth et al. Citation2010).
Semen quality evaluation
The semen samples were collected on a monthly basis, starting in January 2018 until December 2019, each of these were evaluated in the field immediately after being obtained (∼5 minutes). Semen was collected in a test tube with a 1–15 mL graduation, and volume, colour and density were evaluated macroscopically (Chenoweth Citation1983; Chenoweth Citation2002). Electro-ejaculation was attempted to procure a semen sample from each bull, using a three-electrode probe (Minitube, Verona, WI, USA; Ø: 2′’/5.08 cm; long: 33 cm). At each examination session, no more than one electro-ejaculation attempt was made to collect a semen sample from each bull (Furman et al. Citation1975). The semen sample was evaluated immediately for sperm motility, the materials that contact the sperm was at the same temperature as the sperm (to avoid temperature shock), clean, dry, and non-toxic. Individual motility was assessed in a sample diluted with warmed saline solution. A drop of diluted sperm was placed on a thermoplatin slide at 37 °C, covered with a coverslip and examined at 40X. The proportion of sperm that are moving progressively across the field of view was estimated by finding multiple groups of ∼10 sperm and estimating how many sperm are progressive versus how many are not (Moskovtsev and Librach Citation2013). Sperm concentration (×106/mL), spectrophotometric method was used to measure sperm concentration. Once sample was obtained, a drop of undiluted semen was taken and placed in the Micro-cuvette for SDM-1 (Minitube) with a capacity of 2 µl, then inserted into SDM-1 photometer (Minitube) calibrated for bovine, finally, we proceed to take the reading provided by screen equipment (Atiq et al. Citation2011; Minitube Citation2020). Semen density was categorised as: very good (creamy, 4), grainy consistency with 750–1000 × 106/mL, Good (milk-like, 3) with 450–750 × 106/mL, fair (skim milk-like, 2) with 250–450 × 106/mL, poor (translucent, 1) < 250 × 106/mL (Barth Citation2000).
Weather data collection and analysis
Weather data from 2018 to 2019, provided by the Gulf of Mexico forecast centre of the National Meteorological Service, were used (weather stations in closest proximity to evaluated farms ∼30kM). The Centre’s main office is located in the city of Veracruz. Livestock Weather Security Index (LWSI), commonly known as temperature humidity index (THI), was established by the following equation:
where: ‘T’ is the average daily temperature in °C, and RH is the relative humidity percentage (%) (National Research Council Citation1971). Hahn et al. (Citation1999), considers four categories of THI to evaluate thermal environmental conditions, and their impact associated with respirations per minute. In this sense, consider the increase in respiratory frequency as a proportional compensatory response to heat stress (Nienaber and Hahn Citation2007). Values of THI ≤ 74 were considered as the Comfort stage; 75–78 were considered as the Alert stage; 79–83 were considered as the Dangerous stage, and ≥84 were considered as the Emergency stage (Saizi et al. Citation2019). With the weather data, a brief description of the climatic conditions of the region where bulls grassing throughout the year was made, thereby showing the environmental effects in spermatozoa of bulls from different genetics groups.
Statistical analysis
STATISTICA V10 (2010) (StatSoft Citation2011) was used for all statistical analysis, and figures were edited in the Sigma Plot V11 (SigmaPlot Citation2008) software. First of all, normality was evaluated with the Shapiro–Wilk Test, and homoscedasticity was evaluated through Bartlett's test. One-Way analysis of variance (ANOVA), generalised linear model (GLM) were performed to evaluate main effects and combined effects; in addition linear mixed models (LMMs) as a method for analysing data that are non-independent of the following effects:
Intrinsic effects: age, body condition score, libido, genetic group, semen
Extrinsic effects: climatic factors (THI level at sampling day ‘0 day’ and day that the spermatogenesis cycle begins ‘−60 day’, year season, month sampling, month spermatogenesis), reproductive parameters (pregnancy rate and calving interval).
The fixed effects included in the models were genetic group, age, body condition score and random effects were month sampling, month spermatogenesis and year season.
Finally, Post-Hoc comparisons were evaluated as per Tukey (p < .05).
Simple linear relation was made with the following model:
where ‘Y’ is the dependent variable and ‘X’ is the independent variable.
Dependent variables: Sperm concentration, Sperm individual motility, Libido, Pregnancy Rate, Calving interval.
Independent variables: THI sampling day (0 day), THI spermatogenesis day (−60 day), Bull and semen characteristics.
In addition, were obtained the correlations (R), and determination values (R2).
Multiple correlation analysis was used to indicate the variables that were most associated with bull sexual behaviour (libido) and pregnancy rate. Multivariate analyses; Cluster analyses and Principal components & Classifications (Options for Multivariate and Exploratory Analysis from the Statistics Analysis Module, STATISTICA V10), Principal Components was the data analysis tool that was used to reduce the number of variables from a large number of interrelated variables while retaining as much of the information (variation) as possible and calculates an uncorrelated set of variables known as factors or principal components. For this reason, was used to obtain variables and the total variation of the model that best describes the bull and semen variables with climatic variables on pregnancy rate.
Results
BBSE descriptive analysis of local genetic groups
Table shows least squared means values and mean standard errors for each BBSE variable analysed, as well as differences (p < .05) of post-hoc comparisons through Tukey method for each genetic group.
Table 1. Breeding Soundness Examination (BBSE) descriptive analysis in each local genetic group showing average and standard mean error.
Regional climatologic analysis
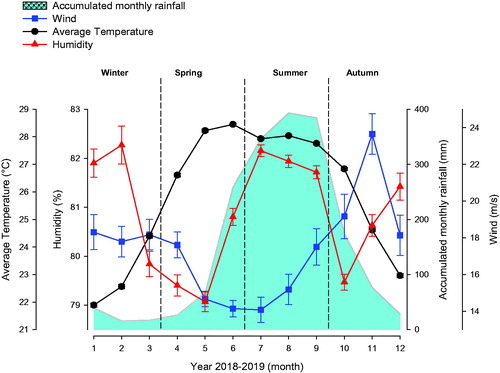
In a first preliminary analysis of the data from meteorological stations using daily data records was done. The regional data are shown in Figure plotted according a monthly distribution. Climatic variations can be appreciated throughout the year, with notable differences in accumulated monthly rainfall, dominant wind speed, relative humidity and average temperature for each season in central Veracruz State, Mexico.
Effects of heat stress on sperm concentration, individual motility and libido
Together with climatological analysis, temperature and humidity values were used to obtain the Livestock Weather Security Index (Temperature Humidity Index, THI), and, thereby, determine the effect of heat stress on bull sperm concentration and motility (Figure ) and Libido (Table ). Heat stress affects sperm concentration (p < .05); it was observed that sperm concentration decreases when semen samples are obtained on 2 or 3 months after high THI levels (alert and danger stages) day −60 (Table ). It must be taken into account that the sperm cycle takes 61 days, and, if this period elapses when THI is high (summer), it can affect sperm production and quality, as seen in Figure and Table , where an analysis was performed at −60 days before sampling. Bull libido was affected by year month, season and THI (p < .05), showing lower parameters when heat stress is present (Table ) represented by THI levels (danger and emergency stages). Another variable that must be taken into account is the quantity and quality of forage available for bull feeding in extensive systems. Forage decreases in the period between January to May; this period is known as dry season. Sperm concentration, Individual motility and Libido is low on dry season, and an increase is observed (p < .05) when bulls are sampled in rainy season. Sperm motility does not show changes (p > .05) related to heat stress effects; there are oscillatory changes in both the dry season, when bulls are in the Comfort stage, and the rainy season (june to december), when heat stress is high.
Figure 2. Concentration and sperm motility analysed according to Temperature Humidity Index (THI) and accumulated monthly rainfall. An average of 19 ± 3.75 standard error (EE) bulls were evaluated monthly. Sperm concentration (X106), red full triangle. Sperm motility (%), blue full diamond. THI, black full circle. Shaded area in bubbles indicates accumulated monthly rainfall. Horizontal short dashed red lines indicate THI threshold limit.
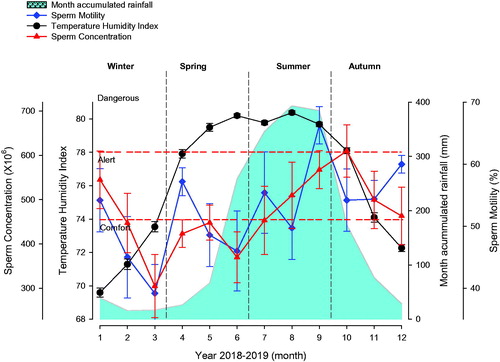
Table 2. Effect of extrinsic factors on Sperm concentration, individual motility and Libido of bull genetic groups in Veracruz, showing average and standard mean error.
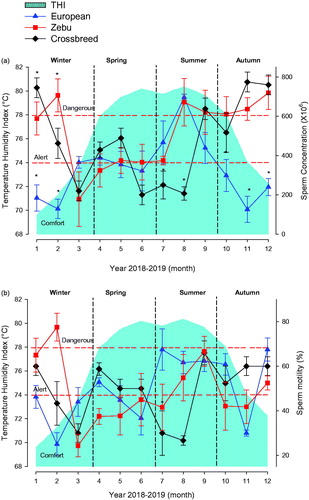
It was decided that one aspect to evaluate would be whether sperm concentration and individual sperm motility of different bull genetic groups present in the tropical regions of Veracruz were affected by heat stress condition (elevated THI); results are shown in Figure . European bulls show low sperm concentrations and individual motility in winter, when effects of heat stress are low (comfort), but also nutrients are low. Sperm concentration and motility increases in summer, when THI is high over the comfort threshold (alert and danger stages), and nutrients are also high due to rainfall, to descend in autumn, when THI is in the comfort stage, and nutrients decrease. Zebu bulls also show variations throughout the year, with increases in seasons when THI is at comfort stage. In spring and summer, when THI is at alert and danger level, sperm concentration values decrease, to increase in summer, when THI and nutrient intake are high. Crossbreed bulls show similar values to those found in Zebu bulls, with increases in seasons when THI is at comfort stage, and low sperm concentration with high THI. Sustained increases can be observed in rainy season (summer).
Figure 3. Effects of Temperature Humidity Index (THI) on sperm concentration (a) and sperm motility (b) of bull genetic groups in Veracruz. (a) Sperm concentration (X106) of the European genetic group (blue full triangle), Zebu (red full square) and crossbred (black full diamond) throughout the year. Grey shaded area indicates THI. (b) Sperm motility (%) of the international genetic group (blue full triangle), Zebu (red full square) and crossbred (black full diamond). *Indicate significant statistical differences (p < .05).
To determinate the THI value in which semen (sperm concentration and individual motility) and behaviour (libido) parameters change in each genetic group, a linear regression was performed with THI records at day sampling (day 0), to evaluate the effect of heat stress at the beginning of spermatogenesis; which was considered 60 days before sampling day, climatological records were obtained to determine the THI at the beginning of the spermatic cycle (−60 day), the results are showing in Figure and Table .
Figure 4. Linear fit of Temperature Humidity Index (THI) on sperm concentration, sperm motility and libido of bull genetic groups in Veracruz. (a, c, e) Linear fit with THI records at the sampling day (day 0). (b, d, f) Linear fit with THI records at the beginning spermatogenesis day (−60 day sampling).
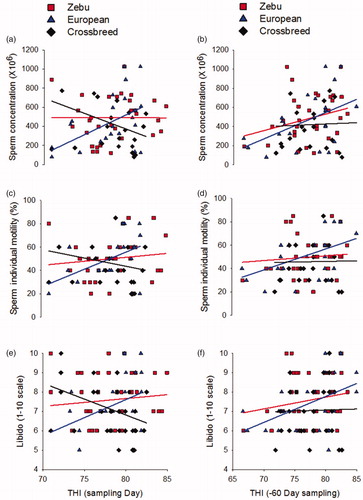
Table 3. Temperature Humidity Index (THI) correlation analysis on semen: sperm concentration and sperm individual motility and Behaviour: libido, of bull genetic groups in Veracruz.
Zebu bulls do not show changes in semen variables and libido when these variables were analysed with THI at the sampling day (day 0) or at the beginning of spermatogenesis (−60 day), Zebu bulls were not affected by heat stress. European bulls, show increments in these variables when THI is high (day 0) and the beginning of spermatogenesis (−60 day) probably due to increments in nutrients (Biomass) present in rainy season, when THI is high. Crossbreed bulls show decrease in sperm concentrations and libido at sampling day (day 0), probably just libido can be affected by heat stress in this genetic group, and not changes where found when THI is analysed at the beginning of spermatogenesis (−60 day). Others factors can be related in heat stress effect of bulls as: age, body condition, intensity and duration of THI at the beginning of spermatogenesis.
BBSE correlation analysis with herd parameters
After having carried out climatic variable analyses on some sperm variables and behaviour, BBSE correlations on herd parameters were obtained: Pregnancy Rate and Calving intervals. Results are shown in Table . Variables with high correlations on Pregnancy rate were: age (R = 0.630) and libido (R = 0.704); while low correlation variables were BCS (R = 0.383) and scrotal circumference (R = 0.207). Likewise, variables with medium to high associations for calving interval were: age (R = 0.506) and libido (R = 0.539), and those with low associations were BCS (R = 0.155) and scrotal circumference. When variables that characterise semen were used in association with pregnancy rate and calving interval, high correlations were observed with sperm concentration (R = 0.822) and density (R = 0.811); and medium to high correlations were observed with volume (R = 0.619) and motility (R = 0.686) on pregnancy rate. In the same sense, when the calving interval variable was correlated, medium to high correlations were observed with sperm concentration (R = 0.634) and density (R = 0.659), and medium correlations were observed with volume (R = 0.567) and motility (R = 0.600).
Table 4. Breeding Soundness Examination (BBSE) correlation analysis on herd parameters: pregnancy rate and Calving interval.
BBSE effect on herd reproductive parameter
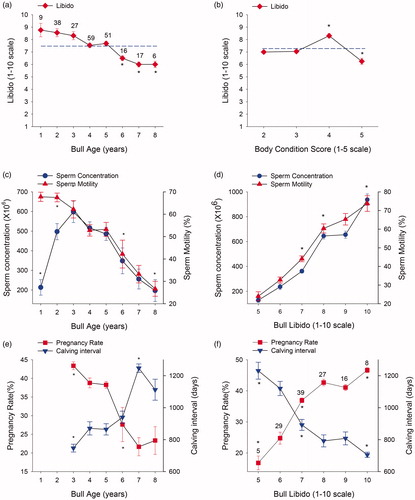
The main effects of ANOVA, and its’ interactions with pregnancy rate and calving interval were determined with the variables that best explain BBSE (age and BCS) and bull behaviour (libido) on herd reproductive performance (Figure ). The libido of bulls in the Veracruz tropical region decreases (p < .05) with age (Figure ); likewise, bulls with low BCS ≤ 3, and high BCS > 5 (1 − 5 scale), show decreased libido (p < .05). When sperm concentration and motility were analysed in relation to bull age (Figure ) and libido (Figure ), a decrease (p < .05) in sperm concentration and motility is observed in older bulls (age 6–8). Libido is associated with traits of concentration and motility, showing increases (p < .05) in the presence of higher libido values. It should be taken into account that young bulls (ages 1–2) show low sperm concentrations. Herd reproductive parameters, pregnancy rate and calving interval are affected (p < .05) by bull age (Figure ) and libido (Figure ). Herds with old bulls show low pregnancy rate and long calving intervals, herds with high libido bulls show high pregnancy rates and low calving intervals, in comparison to the average.
Figure 5. Breeding Soundness Examination (BBSE) main effect analysis (age, body condition score and libido) on sperm concentration and motility, as well as herd proficiency: pregnancy rate and calving interval. (a) Bull libido in different ages: blue short dashed line shows average libido, number indicate the amount of analysed bulls. (b) Effect of body condition on bull libido: blue short dashed line shows average libido. (c) Sperm concentration (left) and motility (right) in bulls of different ages (years). (d) Sperm concentration (left) and motility (right) in bulls with different libido values. (e, f) Herd reproductive parameters, pregnancy rate (left) and calving interval (right) in bulls of different age (e) and libido value (f). Numbers above the error bar show the number of analysed bulls. (*) indicates significant statistical differences (p < .05). Bulls 1 and 2 years old, no data on pregnancy rate and calving interval were reported.
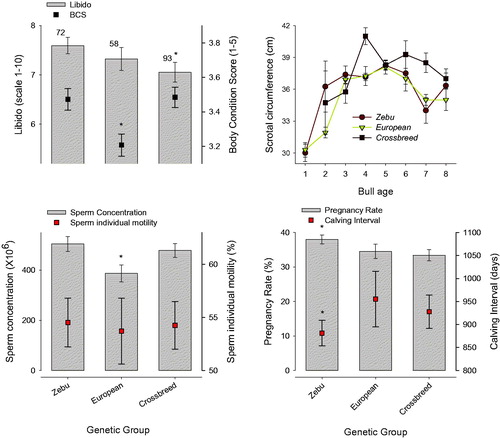
When different bull genetic groups were analysed (Figure ), it was observed that Zebu bulls have high libido values (Figure ) and BCS (p < .05). Testicular growth over the years (scrotal circumference) is similar (p > .05) in all genetic groups analysed (Figure ), with sustained growth to 5 − 6 years. Crossbreed bulls show lower values (p < .05) of sperm concentration and sperm individual motility than other genetic groups (Figure ). With respect to herd reproductive parameters values (pregnancy rate and calving interval), Zebu bulls showed the best results, Figure (p < .05).
Figure 6. Breeding Soundness Examination (BBSE) analysis in different genetic groups in the tropical central region of Veracruz. (a) Libido (left) and Body Condition Score (right). (b) Scrotal circumference with relation to bull age. (c) Sperm concentration (left) and motility (right). (d) Pregnancy rate (left) and Calving interval (right). Numbers above error bar show the amount of analysed bulls. (*) indicates significant statistical differences (p < .05).
BBSE multivariate analysis on herd proficiency
Finally, multiple linear regression and multivariate analysis (cluster, principal components & classification) were performed to determine mathematical models to describe variables associated with herd proficiency (reproductive parameters). First, Libido was analysed as a response variable, since this characteristic is associated with almost all BBSE variables, obtaining the model:
where ‘Y’ is Libido, a is the intercept, X1 is the age of bull, X2 is Body Condition Score, X3 is the genetic group and c is the standard error of estimate
Where age, BCS and genetic group were variables that forward methodology determined. Concerning herd reproductive variables, pregnancy rate was the one that was best associated with BBSE variables; the model obtained from this analysis was:
where ‘Y’ is pregnancy rate, a is the intercept, X1 is the scrotal circumference, X2 is the individual sperm motility, X3 is the sperm concentration and c is the standard error of estimate.where scrotal circumference, sperm individual motility, and sperm concentration were variables that forward methodology determined. Cluster analyses, whose different variables studied are shown in Figure . Libido is linked to clusters of bull characteristics, while pregnancy rate is linked to clusters of semen variables. Calving intervals are not directly associated with any study variable; its linkage distance is wide.
Figure 7. Breeding Soundness Examination (BBSE) Multivariate analysis on herd reproductive parameters. (a) Cluster analysis showing single linkage between bull and herd variables. (b) Principal component and classification analysis from bull and herd variables; in blue, herd parameters are shown.
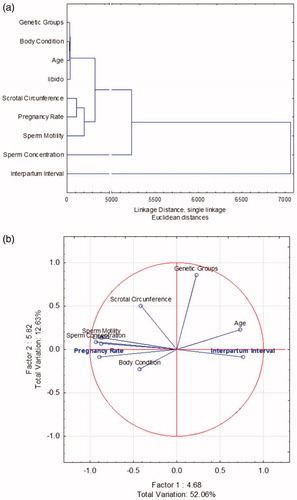
Principal components and classification analyses were performed to determine total variation from two main components (Factors, Eigenvalue 1 and 2) with bull and semen variables (Figure ). The total variation determined by analysis was 64.69% (Factor 1 = 52.06% and Factor 2 = 12.63%). Pregnancy rate clusters with libido, concentration and sperm individual motility; calving interval shows association with bull age, as seen in previous analyses, where bull age negatively affected calving interval.
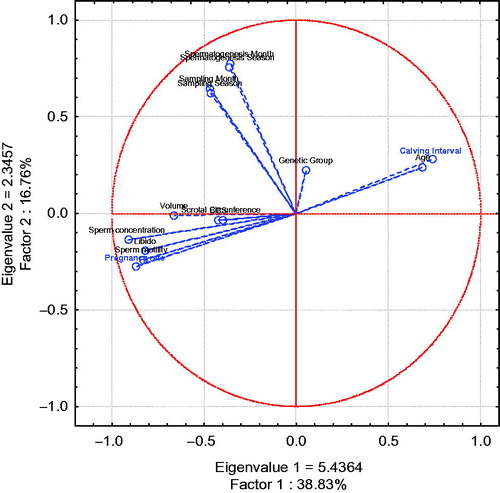
In Figure , Principal component & classification analyses were performed to determine total variation of two principal components (factors, eigenvalue 1 and 2) from bull, climatic variables and semen characteristics; the total variation determined by analysis was 55.59% (Factor 1 = 38.83% and Factor 2 = 16.76%). Pregnancy rate clusters with libido, concentration and sperm motility, volume, scrotal circumference and BCS; calving interval shows association with bull age. Climatic variables (season, month of sampling, month of spermatogenesis, season of spermatogenesis) do not show association with herd reproductive parameters.
Figure 8. Breeding Soundness Examination (BBSE) Multivariate analysis with climatic variables on herd reproductive parameters. Principal component and classification analysis from bulls, including climatic variables (season, month of sampling, month of spermatogenesis, and season of spermatogenesis) in herd variables; in blue, herd parameters are shown.
Discussion
Livestock from the central region of Veracruz State shows genetic group heterogeneity, and is used for meat and milk production, with significant differences in their reproductive parameters. Several researchers (Perea-Ganchou et al. Citation2005; Galina et al. Citation2007), confirm similar results as those obtained in this research, since typical region weather is coupled with pasture seasonality and herd management to make each genetic group behave differently. The tropical region climatology of Veracruz shows significant variations throughout the year, which can influence the reproductive performance of domestic species. Two constants that predominate are high temperature and humidity, which, when combined, cause animals to lose ability to dissipate the heat they produce, causing heat stress (Hahn et al. Citation1999; Domínguez-Mancera et al. Citation2017). The highest values of temperature and humidity are observed in the summer season, but monthly accumulated rain values are also high and, with them, there is an increase of biomass available for bulls in extensive systems (Casagrande et al. Citation2018).
Bull reproductive and genetic potential is influenced by several factors such as management, age, nutrition, environmental conditions and behaviour. A fundamental aspect in bull’s behaviour is the environment in which they perform; in tropical conditions, where temperatures exceed the ruminant thermo-neutral zone, research reports show that cows are more affected than bulls (Hansen Citation2009). Heat stress affects oestrus, as well as conception, and, consequently, low pregnancy rates are reported (Polsky et al. Citation2017). THI has been used as a tool to indicate the extent of the effect of climate on cattle (Hahn et al. Citation1999; Nienaber and Hahn Citation2007).
THI effects, has been demonstrated in relation to sperm quality semen, the increase can cause variations in the lipid conformation, reduces normal morphology and expression of the receptor lipoprotein mRNA very low density (VLDLr), thereby sperm motility and progressive speed can be affected (Sabés-Alsina et al. Citation2017, Citation2019). Bulls exposed to THI high levels (danger or emergency stages) semen samples shows alterations 3 weeks after; although alterations are not permanent, semen quality may take up to 3 months to return to normal (Rahman et al. Citation2018; Sabés-Alsina et al. Citation2019). In addition, sperm from bulls exposed to high temperatures do not show significant differences in morphology and DNA fragmentation compared to semen obtained from bulls without heat stress effect (Llamas Luceño et al. Citation2020). In this research, sperm concentration and motility show a slight decrease when THI is high, but it is not significant because it is found in months where the present biomass amount is lower (dry season). It should be noted that sperm cycle lasts 61 days, and semen samples taken at that time do not fully reflect the bull’s reproductive potential (Valeanu et al. Citation2015; Felton-Taylor et al. Citation2020). When these variables were analysed differently between genetic groups, it was observed that bulls from European origin are more affected at the end of the rainy season than Zebu bulls, thus showing that nutritional intake can be a key factor in sperm concentration and motility, instead of heat stress (Brito et al. Citation2002; Felton-Taylor et al. Citation2020). A positive relationship between food quality and sperm concentration has been described by other researchers (Dance et al. Citation2015; Bourgon et al. Citation2018), who demonstrated relationship between an adequate amount of nutrients from grass and spermatogenesis. In this research, Zebu bulls and their crossbreeds were not affected by THI, which shows that Zebu bulls in tropical conditions are more thermotolerant than European bulls (Jiménez-Severiano Citation2002; Deb et al. Citation2014; Khan et al. Citation2018; Rahman et al. Citation2018).
Bulls of European origin show lower reproductive performance than Zebu bulls and their crossbreeds (Polsky et al. Citation2017; Casagrande et al. Citation2018), in tropical conditions, as can be seen in the obtained results. Has been reported different results in genetic groups of bulls of European origin than Zebu bulls and their crossbreeds (Polsky et al. Citation2017), with emphasis in bull behaviour (libido), indicating that Zebu bulls have less libido than European bulls. In this research, there were no differences in libido between Zebu bulls and European bulls, just a decrease was observed in crossbred bulls. Bull behavioural characteristics involve two components: (1) their ability to identify females in oestrus, and (2) their capacity to mount them. Apparently, libido is not related to semen quality, nor is it related to scrotal circumference; thus, it is possible to obtain an excellent semen sample in bulls with low libido (Chenoweth Citation1997; Galina et al. Citation2007). Age plays a decisive role in BBSE; libido, sperm concentration and motility show their highest values in young and mature animals, as can be seen in the results. Others researchers (Jiménez-Severiano Citation2002; Khan et al. Citation2018) have reported similar results to those found in this research, and decreasing levels as the animal ages. Regardless of the genetic group analysed, once sexual maturity is reached, these indicators stabilise for a short period time (2 years), and when the bull ages, there is a continuous decrease of the indicators towards the last years of bull reproductive performance, causing decrease in herd proficiency (Brito et al. Citation2002). Bulls of 3–5 years of age show better performances in herd reproductive parameters than bulls more than 5 years old, which shows that the bulls have a useful life in extensive systems, regardless of their genetic group (Rawlings et al. Citation2008). The best associations of herd reproductive parameters (pregnancy rate) with BBSE variables were found in sperm concentration, motility and bull libido; calving interval was best associated with bull age. Although libido evaluation and mating ability are not sufficient to predict reproductive success, it is prudent to use bulls that passed all BBSE stages, including libido (Menegassi et al. Citation2015). Lastly, multivariate analysis has been used in the evaluation of the reproductive soundness examination of bulls in order to determine their inclusion and permanence in the herd, in the same way, has been analysed the effects of breed, age, year season, regions on sperm variables (Hancock et al. Citation2016; Felton-Taylor et al. Citation2020), this kind of analysis allows associating intrinsic and extrinsic factor that can affect the physiological performance of bull and with it, be able to explain effects as a whole that in simple analyzes could not be determined. The findings of this study will act as a guide for veterinary practitioners and dual-purpose cattle breeders in find bulls that can be expected to pass the BBSE test and libido based on a robust data set.
Conclusions
Environmental conditions do not have a direct effect on sperm concentration and motility in local bull genetic groups. Bulls that show the best performance on herd reproductive parameters are from Indian origin (Zebu, ‘Bos indicus’) and crossbreeds (Bos Tauris × Bos indicus); bulls more than 6 years old regardless of the genetic group should not be allowed to be in the herd, since there could be a decrease in herd proficiency. Libido is a characteristic that must be considered in BBSE, since it is highly correlated with herd reproductive parameters. Bull breeding soundness evaluation performed before or during breeding season reduces the risk of sub-fertile bulls in the herd.
Disclosure statement
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Alexander J. 2008. Bull breeding soundness evaluation: a practitioner's perspective. Theriogenology. 70:469–472.
- Atiq N, Ullah N, Andrabi S, Akhter S. 2011. Comparison of photometer with improved neubauer hemocytometer and makler counting chamber for sperm concentration measurement in cattle. Pak Vet J. 31:83–84.
- Barth AD. 2000. Bull breeding soundness evaluation. 2nd ed. Alberta: Western Canadian Association of Bovine practitioner Lacombe.
- Beggs D, Bertram J, Chenoweth P, Entwistle K, Fordyce G, Johnston H, Johnston P, McGowan M, Niethe G, Norman S. 2013. Veterinary bull breeding soundness evaluation. Brisbane, QLD, Australia: Australian Veterinary Association.
- Bourgon SL, Diel de Amorim M, Chenier T, Sargolzaei M, Miller SP, Martell JE, Montanholi YR. 2018. Relationships of nutritional plane and feed efficiency with sexual development and fertility related measures in young beef bulls. Anim Reprod Sci. 198:99–111.
- Brito LFC, Silva AEDF, Rodrigues LH, Vieira FV, Deragon LAG, Kastelic JP. 2002. Effect of age and genetic group on characteristics of the scrotum, testes and testicular vascular cones, and on sperm production and semen quality in AI bulls in Brazil. Theriogenology. 58:1175–1186.
- Burnett CR, Pratt SL, Long NM, Sell GS, Schrick FN. 2018. Assessment of semen quality and fertility in young growing beef bulls exposed to ergot alkaloids. Theriogenology. 118:219–224.
- Bury N, Stagnaro C, Mendez J, Yanez F, Moreno A. 2011. Sexual behavior of Criollo Limonero bulls. J Fac Agron. 28:505–513.
- Cañón J, García D, Delgado JV, Dunner S, Telo da Gama L, Landi V, Martín-Burriel I, Martínez A, Penedo C, Rodellar C, et al. 2011. Relative breed contributions to neutral genetic diversity of a comprehensive representation of Iberian native cattle. Animal. 5:1323–1334.
- Casagrande E, Recanati F, Melià P. 2018. Assessing the influence of vegetation on the water budget of tropical areas. IFAC-PapersOnLine. 51:1–6.
- Chacon J, Perez E, Müller E, Söderquist L, Rodriguez-Martinez H. 1999. Breeding soundness evaluation of extensively managed bulls in Costa Rica. Theriogenology. 52:221–231.
- Kennedy SP, Spitzer JC, Hopkins FM, Higdon HL, Bridges WC. 2010. Guidelines for using the bull breeding soundness evaluation form. Clinical Theriogenology. 58:947–950.
- Chenoweth PJ. 1983. Examination of bulls for libido and breeding ability. Vet Clin N Am Large Anim Pract. 5:59–74.
- Chenoweth PJ. 1997. Bull libido/serving capacity. Vet Clin North Am Food Anim Pract. 13:331–344.
- Chenoweth PJ. 2002. Semen quality assessment. Proceedings, The Applied Reproductive Strategies in Beef Cattle Workshop, September 5-6, Manhattan, Kansas.
- Chenoweth P, Hopkins F, Spitzer J, Larsen R. 2010. Guidelines for using the bull breeding soundness evaluation form. Clinical Theriogenology. 2:43–50.
- Cruz A, Hernández A, Chay AJ, Mendoza SI, Ramírez S, Rojas AR, Ventura J. 2017. Componentes del rendimiento y valor nutritivo de Brachiaria humidicola cv Chetumal a diferentes estrategias de pastoreo. Revista Mexicana de Ciencias Agrícolas. 8:599–610.
- Dance A, Thundathil J, Wilde R, Blondin P, Kastelic J. 2015. Enhanced early-life nutrition promotes hormone production and reproductive development in Holstein bulls. J Dairy Sci. 98:987–998.
- Deb R, Sajjanar B, Singh U, Kumar S, Singh R, Sengar G, Sharma A. 2014. Effect of heat stress on the expression profile of Hsp90 among Sahiwal (Bos indicus) and Frieswal (Bos indicus×Bos taurus) breed of cattle: a comparative study. Gene. 536:435–440.
- Domínguez-Mancera B, Hernández-Beltrán A, Rodríguez-Andrade A, Cervantes-Acosta P, Barrientos-Morales M, Pinos-Rodriguez JM. 2017. Changes in livestock weather security index (temperature humidity index, THI) during the period 1917–2016 in Veracruz, Mexico. Jour Anim Rese. 7:983–991.
- Ellis R, Rupp GP, Chenoweth P, Cundiff L, Lunstra D. 2005. Fertility of yearling beef bulls during mating. Theriogenology. 64:657–678.
- Felton-Taylor J, Prosser KA, Hernandez-Medrano JH, Gentili S, Copping KJ, Macrossan PE, Perry VE. 2020. Effect of breed, age, season and region on sperm morphology in 11,387 bulls submitted to breeding soundness evaluation in Australia. Theriogenology. 142:1–7.
- Furman J, Ball L, Seidel G. Jr 1975. Electroejaculation of bulls using pulse waves of variable frequency and length. J Anim Sci. 40:665–670.
- Galina C, Horn M, Molina R. 2007. Reproductive behaviour in bulls raised under tropical and subtropical conditions. Horm Behav. 52:26–31.
- Ginja C, Gama LT, Cortés O, Burriel IM, Vega-Pla JL, Penedo C, Sponenberg P, Cañón J, Sanz A, do Egito AA, et al.; BioBovis Consortium. 2019. The genetic ancestry of American Creole cattle inferred from uniparental and autosomal genetic markers. Sci Rep. 9:1–16.
- Hahn GL, Mader TL, Gaughan JB, Hu Q, Nehacer JA. 1999. Heat waves and their impacts on feedlot cattle. Proceedings of the 15th International Congress on Biometeorology and International Conference on Urban Climatology [ICB 131]; Nov 8–12; Sydney, Australia.
- Hansen PJ. 2009. Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond, B, Biol Sci. 364:3341–3350.
- Hancock AS, Younis PJ, Beggs DS, Mansell PD, Stevenson MA, Pyman MF. 2016. An assessment of dairy herd bulls in southern Australia: 2. Analysis of bull- and herd-level risk factors and their associations with pre- and postmating breeding soundness results. J Dairy Sci. 99:9998–10008.
- Jiménez-Severiano H. 2002. Sexual development of dairy bulls in the Mexican tropics. Theriogenology. 58:921–932.
- Kennedy SP, Spitzer JC, Hopkins FM, Higdon HL, Bridges WC. 2002. Breeding soundness evaluations of 3648 yearling beef bulls using the 1993 Society for Theriogenology guidelines. Theriogenology. 58:947–961.
- Khan IM, Khan RU, Qureshi MS, Usman T, Khan A, Ullah Z, Rehman H. 2018. Cross breeding promotes deterioration of semen quality in cattle bulls. PJZ. 50:97–103.
- Kumar A, Singh K, Honparkhe M, Ghuman S, Brar P. 2016. Physical conformation and ultrasonographic approaches for breeding soundness evaluation of high and low libido crossbred bulls. Indian J Anim Reprod. 37:19–22.
- Kunkle W, Sand R, Rae D. 1994. Effect of body condition on productivity in beef cattle. In: Factors affecting calf crop. 167–178. Florida (USA), Institute of Food and Agricultural Sciences, University of Florida.
- Lees AM, Sejian V, Wallage AL, Steel CC, Mader TL, Lees JC, Gaughan JB. 2019. The impact of heat load on cattle. Animals. 9:322.
- Lemma A, Shemsu T. 2015. Effect of age and breed on semen quality and breeding soundness evaluation of pre-service young bulls. Journal of Reproduction and Infertility. 6:35–40.
- Lessard C, Siqueira LG, D'Amours O, Sullivan R, Leclerc P, Palmer C. 2011. Infertility in a beef bull due to a failure in the capacitation process. Theriogenology. 76:891–899.
- Llamas Luceño N, de Souza Ramos Angrimani D, de Cássia Bicudo L, Szymańska KJ, Van Poucke M, Demeyere K, Meyer E, Peelman L, Mullaart E, Broekhuijse MLWJ, et al. 2020. Exposing dairy bulls to high temperature-humidity index during spermatogenesis compromises subsequent embryo development in vitro. Theriogenology. 141:16–25.
- Martínez-González JC, Castillo-Rodríguez SP, Villalobos-Cortés A, Hernández-Meléndez J. 2017. Sistemas de producción con rumiantes en México. Ciencia Agropecuaria. 132–152.
- Menegassi SRO, Barcellos JOJ, Peripolli V, Dias EA, Costa Junior JBG, Vieira MM, Moojen FG. 2015. Reproductive success or failure in four breed groups of beef bulls. R Bras Zootec. 44:240–247.
- Menon AG, Barkema HW, Wilde R, Kastelic JP, Thundathil JC. 2011. Associations between sperm abnormalities, breed, age, and scrotal circumference in beef bulls. Can J Vet Res. 75:241–247.
- Meyerhoeffer D, Wettemann R, Coleman S, Wells M. 1985. Reproductive criteria of beef bulls during and after exposure to increased ambient temperature. J Anim Sci. 60:352–357.
- Minitube. 2020. https://www.minitube.com/products/bovine/semen-analysis/photometer-sdm-1-calibrated-for-bovine
- Moskovtsev SI, Librach CL. 2013. Methods of sperm vitality assessment. In: Carrell D, Aston K, editors. Spermatogenesis. Totowa (NJ): Humana Press; p. 13–19.
- National Research Council (U.S.). 1971. Committee on Physiological Effects of Environmental Factors on Animals. A guide to environmental research on animals. Washington (DC): National Academy of Sciences.
- Nichi M, Bols P, Züge RM, Barnabe VH, Goovaerts I, Barnabe RC, Cortada CNM. 2006. Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology. 66:822–828.
- Nienaber J, Hahn G. 2007. Livestock production system management responses to thermal challenges. Int J Biometeorol. 52:149–157.
- Notter D, McFadden L, Bergmann J. 1993. Relationship between yearling scrotal circumference and measures of female reproduction in Angus cattle. Beff Improvement Federation. 25:180–184.
- Páez-Barón EM, Corredor-Camargo ES. 2014. Evaluación de la aptitud reproductiva del toro. Rev Cien Agri. 11:49–59.
- Paul C, Murray AA, Spears N, Saunders PT. 2008. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction. 136:73–84.
- Perea-Ganchou F, Belloso ES, Stagnaro CG, Castillo GS, Fonseca HH. 2005. Factors affecting fertility according to the postpartum period in crossbred dual-purpose suckling cows in the tropics. Trop Anim Health Prod. 37:559–572.
- Polsky LB, Madureira AM, Drago Filho EL, Soriano S, Sica AF, Vasconcelos JL, Cerri RL. 2017. Association between ambient temperature and humidity, vaginal temperature, and automatic activity monitoring on induced estrus in lactating cows. J Dairy Sci. 100:8590–8601.
- Rahman MB, Schellander K, Luceño NL, Van Soom A. 2018. Heat stress responses in spermatozoa: mechanisms and consequences for cattle fertility. Theriogenology. 113:102–112.
- Rawlings N, Evans A, Chandolia R, Bagu E. 2008. Sexual maturation in the bull. Reproduction in Domestic Animals. 43:295–301.
- Roberts CA, Geary TW, MacNeil MD, Waterman RC, Roberts AJ, Alexander LJ. 2010. Factors affecting spermatozoa morphology in beef bulls. Proceedings, Western Section, American Society of Animal Science. 61:122–125.
- Sabés-Alsina M, Johannisson A, Lundeheim N, Lopez-Bejar M, Morrell J. 2017. Effects of season on bull sperm quality in thawed samples in northern Spain. Vet Rec. 180:251–251.
- Sabés-Alsina M, Lundeheim N, Johannisson A, López-Béjar M, Morrell JM. 2019. Relationships between climate and sperm quality in dairy bull semen: a retrospective analysis. J Dairy Sci. 102:5623–5633.
- Saizi T, Mpayipheli M, Idowu P. 2019. Heat tolerance level in dairy herds: a review on coping strategies to heat stress and ways of measuring heat tolerance. Journal of Animal Behaviour and Biometeorology. 7:39–51.
- Santana M, Eler J, Ferraz J, Mattos E. 2012. Genetic relationship between growth and reproductive traits in Nellore cattle. Animal. 6:565–570.
- SigmaPlot. 2008. SigmaPlot version 11.0. San Jose (CA): Systat Software. www.systatsoftware.com
- StatSoft. 2011. STATISTICA (data analysis software system), version 10. www.statsoft.com
- Sollenberger LE. 2008. Sustainable production systems for Cynodon species in the subtropics and tropics. R Bras Zootec. 37:85–100.
- Toelle V, Robison O. 1985. Estimates of genetic correlations between testicular measurements and female reproductive traits in cattle. J Anim Sci. 60:89–100.
- Valeanu S, Johannisson A, Lundeheim N, Morrell JM. 2015. Seasonal variation in sperm quality parameters in Swedish red dairy bulls used for artificial insemination. Livestock Science. 173:111–118.
- Van Melis MH, Eler JP, Rosa G, Ferraz JBS, Figueiredo LGG, Mattos E, Oliveira HNd. 2010. Additive genetic relationships between scrotal circumference, heifer pregnancy, and stayability in Nellore cattle. J Anim Sci. 88:3809–3813.